Abstract
Calcium functions as a trigger for the switch between epithelial cell growth and differentiation. We report here that the calcium/calmodulin-dependent phosphatase calcineurin is involved in this process. Treatment of primary mouse keratinocytes with cyclosporin A, an inhibitor of calcineurin activity, suppresses the expression of terminal differentiation markers and of p21WAF1/Cip1 and p27KIP1, two cyclin-dependent kinase inhibitors that are usually induced with differentiation. In parallel with down-modulation of the endogenous genes, suppression of calcineurin function blocks induction of the promoters for the p21WAF1/Cip1 and loricrin differentiation marker genes, whereas activity of these promoters is enhanced by calcineurin overexpression. The calcineurin- responsive region of the p21 promoter maps to a 78-bp Sp1/Sp3-binding sequence next to the TATA box, and calcineurin induces activity of the p21 promoter through Sp1/Sp3-dependent transcription. We find that the endogenous NFAT-1 and -2 transcription factors, major downstream targets of calcineurin, associate with Sp1 in keratinocytes in a calcineurin-dependent manner, and calcineurin up-regulates Sp1/Sp3-dependent transcription and p21 promoter activity in synergism with NFAT1/2. Thus, our study reveals an important role for calcineurin in control of keratinocyte differentiation and p21 expression, and points to a so-far-unsuspected interconnection among this phosphatase, NFATs, and Sp1/Sp3-dependent transcription.
Primary mouse keratinocytes provide a well established system to study control of epithelial cell growth and differentiation. Addition of calcium to these cultures induces a terminal differentiation program similar to that observed in the upper epidermal layers, including specific structural changes, cell cycle withdrawal, and induction of differentiation-related genes (1). Intracellularly, induction of tyrosine phosphorylation and phospholipase C activation occur as early and specific events in keratinocyte differentiation, together with phosphatidylinositol turnover and increase of intracellular calcium, probably by mobilization from intracellular stores (1). A direct influx of extracellular calcium through voltage-independent channels also has been reported, but it may occur as a relatively late event, which could account for a second and sustained increase in intracellular calcium levels (2). Increased intracellular calcium could in turn activate several calcium-dependent pathways, such as calcium/calmodulin-dependent kinases and phosphatases (3). Surprisingly little is known about the involvement of any of these enzymes in further transduction of the keratinocyte differentiation signal(s).
The calcium/calmodulin-dependent phosphatase calcineurin (PP2B) is the only serine/threonine phosphatase under calcium/calmodulin control and its molecular structure is unique within the phosphatase family, because it consists of two subunits with catalytic and regulatory activity, respectively (4). The calcineurin A subunit contains an amino-terminal catalytic region and a carboxy-terminal regulatory region. The regulatory region can be further subdivided in three distinct domains: a calcineurin B-binding site, an autoinhibitory region, and a calmodulin-binding domain. Deletion of the autoinhibitory and calmodulin-binding domains results in a constitutively active form of the enzyme, which still depends on its binding to the calcineurin B subunit for activity (4). The calcineurin B subunit belongs to the family of EF-hand calcium-binding proteins, and contains four sites that bind calcium with different affinity (4).
Calcineurin has a relatively narrow substrate specificity. Among the proteins that are dephosphorylated as a consequence of calcineurin activation are transcription factors such as Elk-1 and NFAT, although a wide range of other substrates has been discovered (4). The dephosphorylation of NFATs by calcineurin unmasks the nuclear localization signal of NFAT, allowing its import into the nucleus and induction of specific genes carrying NFAT-responsive elements (5). After NFAT dephosphorylation, cytosolic calcineurin can also translocate to the nucleus in the form of a complex with NFAT itself (6). Calcineurin suppresses export of NFAT from the nucleus, masking the nuclear export signal of this molecule by a noncatalytic mechanism (6).
Studies on the biological function of calcineurin have been greatly facilitated by the use of the inhibitory drugs cyclosporin A (CsA) and FK506, which bind and suppress calcineurin activity as a complex with cyclophilins and FK-binding protein, respectively (7, 8). The immunosuppressive effects of these drugs have been studied in great detail in T cells and can be attributed, at least in part, to inhibition of calcineurin-dependent activity of NFAT transcription factors (9). CsA also inhibits the expression of myogenic markers of commitment to differentiation, which can be induced by increased calcineurin expression (10). Similarly, during erythropoiesis, CsA treatment inhibits the commitment to differentiation by relieving the calcineurin-dependent down-modulation of the c-fos and egr-1 transcription factors (11).
CsA and FK506 are widely used for therapy of a variety of dermatological diseases, including psoriasis, alopecia areata, and ichthyosis (12). The effectiveness of these drugs generally has been attributed to inhibition of T cell function. However, a common side effect of both CsA and FK506 in the skin is induction of hair growth. This side effect is probably independent of the immune system, because it occurs also in T cell-deficient nude mice (13, 14). Moreover, to which extent CsA treatment can directly affect keratinocyte growth/differentiation control and the role that calcineurin plays in this process have not been investigated in any detail. The present study was designed to address this question.
Experimental Procedures
Cell Culture.
Primary keratinocytes were prepared from newborn Sencar mice and grown as described (15). The Drosophila cell line SL2 was a gift of K. White (Harvard Medical School, Boston) and grown at room temperature in Drosophila serum-free medium (GIBCO/BRL).
Plasmids.
Luciferase reporter plasmids for the human p21 promoter were described (16). Expression plasmids for constitutive active mouse calcineurin Aα (CNA) and the calcineurin B subunit (CNB) were described (17, 18). The reporter plasmid for the loricrin promoter was obtained by PCR amplification of a 2.6-kb fragment of the mouse loricrin promoter region (19) with the oligonucleotide 5′-AGCTGTCTCTTTTGAGACTATCCCGGGGCCTAG-3′ as forward primer and 5′-TGTTTAAGGAGAAGGAAGCTTCTGGAAGAAGAG-3′ as reverse primer. Expression plasmids for the human Sp1 and Sp3 cDNAs (20, 21), the Sp1 and Sp3-Gal4 fusion proteins (22), the pcDNA3-GFP-NFAT4 construct (6), the mammalian expression vectors for the human NFAT1 and NFAT2 (23, 24), and the eukaryotic expression vector GFP-VIVIT and the GFP control vector (25) have been described previously.
Antibodies.
Affinity-purified rabbit antisera against K1, K5, loricrin, and filaggrin were raised against the published protein sequence (26–28). Rabbit antiserum against involucrin was purchased from Babco (Richmond, CA). Goat polyclonal antibodies against Sp1, mouse monoclonal antibodies against Sp1 and p21, and rabbit polyclonal antibodies against cdk2 were purchased from Santa Cruz Biotechnology. Monoclonal M2 anti-FLAG was purchased from Sigma, and the monoclonal anti NFAT2 was a gift of G. R. Crabtree (Howard Hughes Medical Institute, Stanford, CA). Monoclonal antibodies against E-cadherin and p27 were purchased from Transduction Laboratories (Lexington, KY).
Coimmunoprecipitation Experiments.
At 48 h after transfection, 293 cells were collected and lysed in Nonidet P-40 lysis buffer (29, 30). Same protein amounts (1 mg) were incubated overnight at 4°C with 5 μg of mouse monoclonal antibodies against Sp1 or affinity-purified mouse IgGs followed by incubation with protein-G Sepharose beads for 1 h at 4°C. For coimmunoprecipitation of the endogenous proteins, keratinocytes were lysed with Nonidet P-40 buffer. Two milligrams of proteins were immunoprecipitated with mouse monoclonal antibodies against NFAT2 or affinity-purified mouse IgGs for 1 h at 4°C, followed by incubation with protein-G Sepharose beads for 1 h at 4°C.
Results
Calcineurin-Dependent NFAT Transcription Is Induced in Differentiating Keratinocytes.
Primary mouse keratinocytes provide a well defined system to study the switch between epithelial cell growth and differentiation (1). In an initial set of experiments we tested whether the activity of a well characterized family of calcineurin-responsive transcription factors, NFAT (5), increases in differentiating keratinocytes, and whether this increase can be blocked by CsA, a specific inhibitor of calcineurin activity (7). Primary mouse keratinocytes were transfected with a luciferase reporter plasmid with a minimal NFAT-responsive promoter (pGL3-NFAT/AP1). As expected, the activity of this promoter was induced in keratinocytes by the concomitant expression of exogenous calcineurin A and B, and this increase was blocked by CsA treatment (Fig. 1A). Activity of the NFAT reporter was also substantially increased in keratinocytes induced to differentiate by exposure to high extracellular calcium (2 mM), and this increase was blocked by treatment of these cells with CsA (Fig. 1B). Keratinocytes were transfected with an expression vector for the NFAT4 isoform fused to green fluorescent protein (GFP) (6). Along with NFAT promoter activation in response to calcium treatment, GFP-NFAT4 was found in the cytoplasm of growing keratinocytes but exhibited nuclear localization in differentiating cells (Fig. 1C).
Figure 1.
Calcineurin-dependent NFAT transcriptional activity in differentiating mouse keratinocytes. (A) Primary keratinocytes were transfected with a luciferase reporter plasmid (1 μg) carrying a minimal TK promoter linked to four tandemly repeated NFAT-binding sites (pGL3-NFAT/AP1), plus/minus expression vectors for a constitutively active form of the CNA and CNB subunits (2 μg). Promoter activity was measured at 72 h after transfection in cells untreated or treated with increasing concentrations of CsA for the last 24 h of the experiment. Control cells were cotransfected with the reporter plasmid plus empty vector control DNA. Each condition was tested in triplicate wells, and the results are representative of three independent experiments. (B) Keratinocytes were transfected with the pGL3-NFAT/AP1 reporter alone, and maintained in low-calcium conditions (0.05 mM) or switched to high-calcium concentration (2 mM) for 48 h before termination of the experiment. Cells were treated with CsA at the indicated concentrations 2 h before addition of calcium. (C) Keratinocytes were transfected with an expression vector for the NFAT4 isoform fused to GFP (6). Cells were either kept in low-calcium conditions or treated with calcium for 48 h. Samples were counterstained with To-pro-3-Iodide for nuclear identification and analyzed by confocal microscopy. Green (GFP-NFAT4) and red (nuclei) images were superimposed, so that sites of overlap are visualized as yellow. The photographs are representative of 20 independent fields with an average of 5–10 GFP-positive cells in each field.
Suppression of Calcineurin Activity by CsA Interferes with Induction of Terminal Differentiation Markers and Cell Cycle Inhibitors.
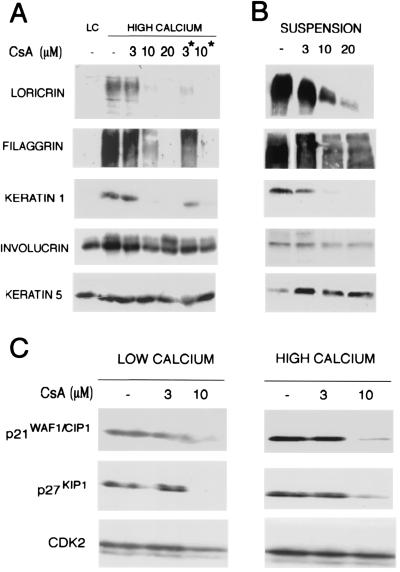
The results above indicate that calcium-induced keratinocyte differentiation is associated with an increase in calcineurin-dependent NFAT transcription. To test whether suppression of calcineurin function can directly affect differentiation, primary keratinocytes were pretreated with increasing concentrations of CsA followed by induction of differentiation by increased extracellular calcium. The establishment of cadherin-mediated intercellular junctions is one of the earliest structural changes induced by increased extracellular calcium (31, 32). Immunofluorescence analysis with anti-E-cadherin antibodies indicated that inhibition of calcineurin activity by CsA treatment exerted no effect on the establishment of keratinocyte cell/cell adhesion (data not shown). By contrast, immunoblotting with antibodies against biochemical markers of differentiation revealed that induction of the keratin 1 and loricrin markers by high calcium exposure is severely reduced by CsA treatment in a dose- and time-dependent manner (Fig. 2A). Relative to these markers, filaggrin expression was suppressed to a lesser extent by CsA treatment, whereas involucrin expression was not significantly affected. Expression of keratin 5, which is a marker of the basal epidermal layers constitutively expressed in cultured mouse keratinocytes, was also unaffected (Fig. 2A). Similar suppression of terminal differentiation markers also was observed after treatment of keratinocytes with the unrelated calcineurin inhibitor FK506 (data not shown).
Figure 2.
Inhibition of calcineurin activity by CsA suppresses biochemical markers of differentiation, as well as p21 WAF1/CIP1 and p27KIP1 expression. (A) Keratinocytes were pretreated with increasing concentrations of CsA for either 24 h or 48 h (asterisk) before induction of differentiation by calcium for 12 h. The same amount of total cell extracts (35 μg) was analyzed by SDS/7.5% polyacrylamide gel and immunoblotted with antibodies specific for the indicated differentiation markers. Filaggrin is synthesized as a high-molecular-weight precursor, profilaggrin, which is subsequently processed. The diffused bands correspond to the multiple products of this processing. LC, control cells in low-calcium conditions. (B) Keratinocytes were treated for 24 h with different CsA concentrations. The spontaneously detached cell populations were collected at the end of the treatment and analyzed by immunoblotting with antibodies against the indicated differentiation markers. (C) Keratinocytes were treated with CsA for 24 h at increasing concentrations and further incubated in medium at low- or high-calcium concentration for 12 h. Total cell extracts (25 μg) were analyzed by SDS/12% polyacrylamide gel and immunoblotted with antibodies against p21 and p27.
In addition to exposure to extracellular calcium, expression of terminal differentiation markers is strongly enhanced in keratinocytes that spontaneously detach from the dish in cultures under low-calcium conditions (33). Even in this case, keratinocyte differentiation is associated with increases in intracellular calcium concentrations (33). CsA treatment exerted inhibitory effects on terminal differentiation marker expression in the spontaneously detached keratinocyte population similar to the attached calcium-treated cells (Fig. 2B).
Besides biochemical markers of differentiation, the onset of keratinocyte differentiation is associated with the induction of the cyclin-dependent kinase inhibitors p21WAF1/CIP1 and p27KIP1, which contribute to growth arrest of these cells (15, 34). Immunoblotting with antibodies against p21 and p27 revealed that expression of both proteins was decreased by CsA treatment in keratinocytes under growing conditions, and higher CsA concentrations were required to cause a similar suppression of p21 and p27 expression in keratinocytes induced to differentiate by high extracellular calcium (Fig. 2C). Levels of cyclin-dependent kinase 2 were little or not affected by the CsA treatment in both growing and differentiating cells (Fig. 2C).
Suppression of Calcineurin Activity by CsA Blocks Activation of the p21WAF1/CIP1 and Loricrin Promoters, Whereas Activity of These Promoters Is Induced by Calcineurin Overexpression.
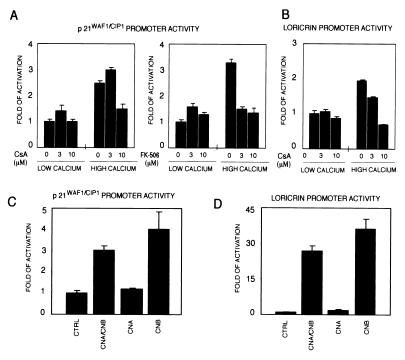
Induction of biochemical markers of differentiation and p21 expression occur at the transcriptional level (1). To test whether the effects of CsA on the expression of these genes also occur at the level of transcription, keratinocytes were transiently transfected with a luciferase reporter plasmid carrying either a 225-bp region of the p21 promoter (16) or the 2.4-kb region of the loricrin promoter (19), and promoter activity was measured in cells under basal conditions and after calcium treatment, in the presence or absence of CsA. As shown in Fig. 3 A and B, CsA treatment blocked the increase in p21 and loricrin promoter activity associated with calcium-induced differentiation in a dose-dependent fashion, whereas it had little or no effect on basal activity of these promoters. Similar inhibitory effects on p21 promoter activity also were observed after treatment of keratinocytes with FK506 (Fig. 3A Right).
Figure 3.
Suppression of calcineurin activity by CsA blocks activation of the p21WAF1/CIP1 and loricrin promoters, whereas activity of these promoters is induced by calcineurin overexpression. (A) Keratinocytes were transfected with a luciferase reporter plasmid carrying a 225-bp region of the p21 promoter, and maintained in low-calcium conditions or treated with calcium for the last 24 h of the experiment (72 h after transfection). CsA (Left) or FK506 (Right) was added at increasing concentrations 2 h before calcium. (B) Keratinocytes were transfected with a luciferase reporter plasmid carrying the 2.4-kb region of the loricrin promoter and treated as in A, except that high calcium exposure was for 48 h. (C and D) Keratinocytes were transfected with reporters for the 225-bp p21 promoter (C) or loricrin promoter (D), plus/minus CNA and CNB expression plasmids (2 μg), either alone or together. Each condition was tested in triplicate wells, and results are representative of three independent experiments.
To test whether increased calcineurin activity may induce activity of these promoters by itself, keratinocytes were transiently transfected with the p21 and loricrin promoters plus/minus expression vectors for the constitutive active CNA and CNB (17, 18). Expression of CNA and CNB was sufficient to transactivate both loricrin and p21 promoters to levels similar or higher than those induced by increased extracellular calcium (Fig. 3 C and D). Expression of the CNA subunit alone was not able to transactivate the p21 and loricrin promoters, whereas the regulatory CNB subunit was sufficient to elicit an induction similar to that observed after coexpression of the A and B subunits (Fig. 3 C and D).
Calcineurin Transactivates the p21 Promoter through Sp1/Sp3-Dependent Transcription.
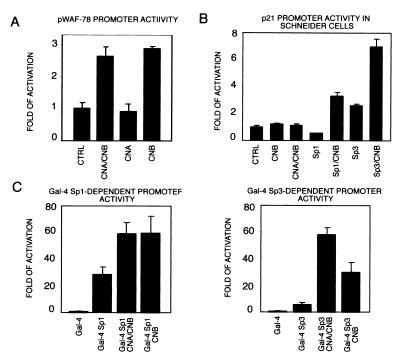
To gain mechanistic insights into the role of calcineurin in keratinocyte growth/differentiation control, we focused on calcineurin-dependent induction of the p21 promoter. The minimal region of the p21 promoter that retains full responsiveness to calcium-induced differentiation consists of a stretch of 78 nucleotides next to the TATA box, which serve as binding sites for Sp1/Sp3 transcription factors (16). Expression of the CNA and CNB subunits or CNB alone transactivated this minimal p21 promoter to an extent similar to larger promoter regions (Fig. 4A). To test whether this effect is mediated by Sp1/Sp3-dependent transcription, Drosophila Schneider cells that are devoid of endogenous Sp1/Sp3 (35) were transfected with the p21 promoter plus/minus expression vectors for Sp1/Sp3 and calcineurin. Unlike in keratinocytes, in Drosophila cells calcineurin transactivated the p21 promoter only when Sp1 or Sp3 were also coexpressed (Fig. 4B).
Figure 4.
Calcineurin activates the p21 promoter through Sp1/Sp3-dependent transcription. (A) Keratinocytes were transfected with the 78-bp minimal region of the p21 promoter next to the TATA box (pWAF-78) (16) plus/minus 2 μg CNA and CNB expression plasmids either alone or together. (B) Drosophila Schneider cells were transfected with the 225-bp p21 promoter (1 μg) plus/minus expression vectors for Sp1/Sp3 (2 μg) and calcineurin subunits (2 μg) in various combinations. Each condition is the result of two independent experiments. (C) Keratinocytes were transfected with 1 μg of a luciferase reporter plasmid carrying five consensus Gal4 DNA-binding sites (pGL5-Gal4) and 2 μg of expression vectors for Sp1 or Sp3 fused to Gal4 DNA-binding domain, plus/minus CNA and CNB (2 μg) in various combinations. Each condition was tested in triplicate wells, and results are representative of three independent experiments.
To test whether calcineurin also induces Sp1- and Sp3-dependent transcription in keratinocytes, these cells were cotransfected with a luciferase reporter plasmid carrying five consensus Gal4 DNA-binding sites (pGL5-Gal4), and expression vectors for Sp1 or Sp3 fused to a Gal4 DNA-binding domain (22). Activity of the Gal4-Sp1 and -Sp3 transcription factors was significantly increased by coexpression of the CNA and CNB subunits and, as with the p21 promoter, these enhancing effects also were observed after expression of CNB alone (Fig. 4C).
Sp1/Sp3 and NFAT1/2 Transcription Factors Functionally Interact in a Calcineurin-Dependent Manner.
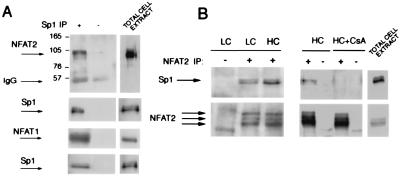
The phosphorylation state, relative abundance, and DNA-binding activity of endogenous Sp1/Sp3 are not modulated in keratinocytes in response to calcium (ref. 16; unpublished observations). An attractive possibility is that the observed enhancement of Sp1/Sp3 activity by calcineurin is mediated by calcineurin-responsive NFATs. As an initial test of whether Sp1 can associate with NFAT1/2 factors, 293 cells were cotransfected with the corresponding cDNAs. Immunoprecipitation with Sp1 followed by immunoblotting with antibodies against either NFAT1 or NFAT2 showed that these factors can be recovered in the same immunocomplexes (Fig. 5A). An important question was whether association of the endogenous proteins also exists in keratinocytes and whether this association is under calcineurin control. Accordingly, primary keratinocytes under low- versus high-calcium conditions and plus/minus CsA treatment were immunoprecipitated with antibodies against NFAT2 followed by immunoblotting with antibodies against Sp1. Association between NFAT2 and Sp1 was already detected in keratinocytes under growing conditions and was further increased in the differentiating cells (Fig. 5B Left). There was no coimmunoprecipitation of the two transcription factors in CsA-treated keratinocytes, implicating a calcineurin-dependent mechanism for their association (Fig. 5B Right).
Figure 5.
Physical association between the Sp1 and NFAT1/2 transcription factors. (A) 293 cells were cotransfected with 10 μg of mammalian expression vectors for Sp1 and either NFAT1 or NFAT2. Cell lysates were immunoprecipitated with mouse monoclonal antibodies against Sp1 or affinity-purified mouse IgG1. Immune complexes were analyzed by SDS/6% polyacrylamide gel and sequential immunoblotting with the indicated proteins. Similar results were obtained in three independent experiments. (B) Primary keratinocytes were either left under low- or high-calcium conditions for 48 h (Left), or were treated with calcium for 48 h plus/minus CsA (10 μM). Two milligrams of total cell lysates were immunoprecipitated with mouse monoclonal antibodies against NFAT2 or affinity-purified mouse IgG1. Immune complexes were analyzed by SDS/6% polyacrylamide gel and immunoblotting with the goat polyclonal antibodies against Sp1 or mouse monoclonals against NFAT2. Similar results were obtained in a third independent experiment.
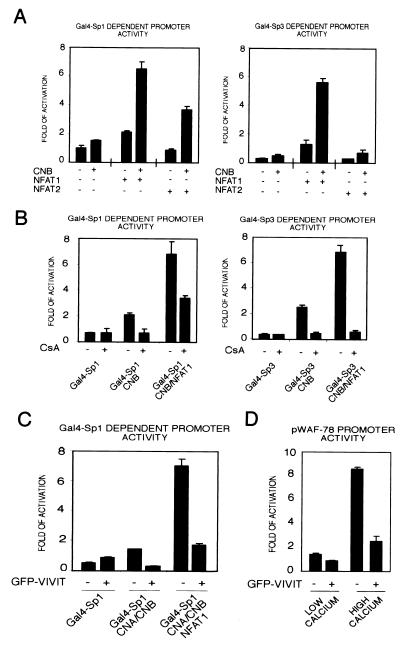
To test whether the interaction between Sp1/Sp3 and NFATs is of functional significance, keratinocytes were transfected with the Gal4 reporter plasmid and expression vectors for Gal4-Sp1 and -Sp3 plus/minus NFAT1, NFAT2, and calcineurin, in various combinations. Sp1-dependent transcription was increased by overexpression of NFAT1 alone, and a much stronger induction was observed when calcineurin was coexpressed (Fig. 6A). Unlike NFAT1, NFAT2 expression did not increase Sp1-dependent transcription by itself, but did so in combination with calcineurin. Analogous results were observed in the analysis of Sp3-dependent transcription (Fig. 6A). Similar transient transfection experiments were performed plus/minus CsA treatment (Fig. 6B). CsA had no effect on basal Sp1- or Sp3-dependent transcription, whereas it suppressed the enhancing effects of calcineurin alone or in combination with NFAT1 (Fig. 6B). As an alternative to CsA treatment, cells were transfected with the above-mentioned combination of plasmids plus an expression vector for a GFP-fusion peptide (GFP-VIVIT), which functions as a specific inhibitor of calcineurin-dependent NFAT activation (25). Like CsA treatment, expression of GFP-VIVIT did not decrease basal Sp1-dependent transcription, but suppressed induction of Sp1 activity by calcineurin expressed alone or in combination with NFAT (Fig. 6C). In parallel with the responsiveness of the minimal p21 promoter region (pWAF78) to Sp1/Sp3- and calcineurin-dependent transcription, activity of this promoter was also suppressed by GFP-VIVIT expression in keratinocytes in high-calcium medium and, to a lesser extent, under low-calcium conditions (Fig. 6D).
Figure 6.
Functional interaction between the Sp1/Sp3 and NFAT1/2 transcription factors and NFAT-dependent control of the p21 promoter. (A) Keratinocytes under low-calcium conditions were transfected with the Gal4 reporter plasmid and expression vectors for Gal4-Sp1 or Gal4-Sp3, NFAT1 or NFAT2, and CNB in various combinations as indicated; promoter activity was determined 72 h after transfection. Results are representative of three independent experiments. (B) Keratinocytes were transfected with the Gal-4 reporter and various expression plasmids as in A. Keratinocytes were untreated or treated with 10 μM CsA for the last 24 h of the experiment. Results are representative of two independent experiments. (C) Keratinocytes were transiently transfected with the same plasmids as in A and B, plus/minus the mammalian expression vector for GFP-VIVIT (2 μg) (25). As control for GFP-VIVIT, cells were transfected with the same vector expressing GFP alone. (D) Keratinocytes were transiently transfected with 1 μg of the minimal region of the p21 promoter (pWAF-78) plus/minus 2 μg of the NFAT inhibitor GFP-VIVIT. Keratinocytes were either kept under low-calcium conditions or exposed to high-calcium concentrations (2 mM) for the last 48 h before termination of the experiment (72 h). Similar results were obtained in a second independent experiment.
Discussion
Like other complex biological processes, the switch between epithelial cell growth and differentiation is controlled by several signaling pathways functioning in both sequential and parallel fashion. Calcium-induced differentiation of primary mouse keratinocytes provides a well defined system to dissect these pathways (1). In this context, involvement of calcium/calmodulin-dependent kinases or phosphatases has not been explored in any detail. We have shown here that at least one of these enzymes, calcineurin, is likely to play an important role in keratinocyte differentiation control, acting downstream of cell/cell adhesion formation and affecting Sp1/Sp3-dependent transcription in synergism with NFAT activation.
One of the major known targets of calcineurin is the NFAT family of transcription factors (5) and, consistent with an enhancement of calcineurin activity in differentiating keratinocytes, NFAT-dependent transcription increases in these cells, and this increase can be blocked by calcineurin inhibition through CsA treatment. Calcineurin inhibition produced no apparent effect on the structural changes associated with differentiation including cadherin-mediated cell/cell adhesion, but blocked expression of specific differentiation markers and expression of p21WAF1/CIP1 and p27KIP1, two cyclin-dependent kinase inhibitors, which are usually induced with differentiation (15, 34). Inhibition of endogenous p21 and loricrin expression was paralleled by decreased promoter activities of their corresponding genes, whereas calcineurin overexpression was sufficient to cause transactivation of these promoters. p21 and loricrin promoter activity were not induced by a constitutively active form of CNA expressed by itself, whereas they were strongly induced after overexpression of CNB either in combination with the A subunit or alone. This finding suggests that CNB plays a preferential regulatory function in this context and/or its concentration is rate-limiting.
The minimal region of the p21 promoter under calcineurin control is the same as that required for induction by increased extracellular calcium (16). This region consists of a short stretch of 80 nt around the TATA box, characterized by GC-rich repeats and bound by Sp1 and Sp3 transcription factors (16). By using Schneider cells, which lack endogenous Sp1 and Sp3 (35), we have shown that induction of the p21 promoter by calcineurin is mediated by Sp1/Sp3-dependent transcription. Additionally, Sp1/Sp3-dependent transcription in keratinocytes, as assessed by the activity of Sp1- and Sp3-Gal4 fusion proteins, was enhanced by calcineurin overexpression. Because the phosphorylation state and the relative abundance of endogenous Sp1/Sp3 do not seem to be affected in response to calcium (ref. 16; unpublished observations), calcineurin might control specific transcription factors that bind directly or indirectly to Sp1/Sp3, and/or affect the interactions of Sp1/Sp3 with the basal transcription apparatus. In fact, Sp1 family members have been reported to regulate transcription through direct association with components of the basal transcription machinery (36, 37), and other transcription factors such as NF-κB, c-jun, E2F1, pRb, and YY1 (20, 38–41). We have provided here the first evidence that members of the NFAT family can also associate with Sp1 and enhance Sp1- and Sp3-dependent transcription in a calcineurin-dependent manner. NFAT family members have been shown to regulate transcription through the cooperative binding to a specific DNA recognition sequence in concert with other transcription factors, such as AP1, GATA, and EGR-1 (5). The present findings suggest that the direct binding of NFAT to the DNA is dispensable and can be replaced by the interaction of these factors with Sp1/Sp3. The notion that NFAT family members can function as coactivators of other DNA-binding transcription factors also is supported by recent evidence in T cells, where the Nur77 promoter is transactivated by NFAT in synergism with MEF2D (42). At the level of the p21 promoter, the same minimal region responsive to calcium in mouse primary keratinocytes coincides with that responsive to transforming growth factor-β in the HaCaT keratinocyte cell line (16, 43). Our finding of a functional cross talk among calcineurin, Sp1/Sp3, and NFAT have revealed a unique mode of regulation of the p21 gene with its critical role in cell growth and differentiation.
Acknowledgments
We thank Drs. A. Rao, F. McKeon, G. R. Crabtree, T. Sakai, and D. Roop for their generous gifts of plasmids and/or antibodies. We thank Dr. Cathrin Brisken for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AR39190, CA16038, and CA73796 (to G.P.D.), and, in part, by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd.
Abbreviations
- CsA
cyclosporin A
- CNA/B
calcineurin A/B
- GFP
green fluorescent protein
References
- 1.Dotto G P. Crit Rev Oral Biol Med. 1999;10:442–457. doi: 10.1177/10454411990100040201. [DOI] [PubMed] [Google Scholar]
- 2.Reiss M, Lipsey L R, Zhou Z L. J Cell Physiol. 1991;147:281–291. doi: 10.1002/jcp.1041470213. [DOI] [PubMed] [Google Scholar]
- 3.Yokokura H, Terada O, Naito Y, Sugita R, Hidaka H. Adv Second Messenger Phosphoprot Res. 1997;31:151–157. doi: 10.1016/s1040-7952(97)80016-x. [DOI] [PubMed] [Google Scholar]
- 4.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 5.Rao A, Luo C, Hogan P G. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, McKeon F. Nature (London) 1999;398:256–260. doi: 10.1038/18473. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber S L. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 8.Siekierka J J, Sigal N H. Curr Opin Immunol. 1992;4:548–552. doi: 10.1016/0952-7915(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 9.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree G R. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 10.Friday B B, Horsley V, Pavlath G K. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magocsi M, Apati A, Gati R, Kolonics A. Immunol Lett. 1999;68:187–195. doi: 10.1016/s0165-2478(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Peter R U, Ruzicka T. Hautarzt. 1992;43:687–694. [PubMed] [Google Scholar]
- 13.Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Lab Invest. 1987;56:684–686. [PubMed] [Google Scholar]
- 14.Yamamoto S, Jiang H, Kato R. J Invest Dermatol. 1994;102:160–164. doi: 10.1111/1523-1747.ep12371755. [DOI] [PubMed] [Google Scholar]
- 15.Missero C, Calautti E, Eckner R, Chin J, Tsai L H, Livingston D M, Dotto G P. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prowse D M, Bolgan L, Molnar A, Dotto G P. J Biol Chem. 1997;272:1308–1314. doi: 10.1074/jbc.272.2.1308. [DOI] [PubMed] [Google Scholar]
- 17.O'Keefe S J, Tamura J, Kincaid R L, Tocci M J, O'Neill E A. Nature (London) 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 18.Milan D, Griffith J, Su M, Price E R, McKeon F. Cell. 1994;79:437–447. doi: 10.1016/0092-8674(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 19.DiSepio D, Jones A, Longley M A, Bundman D, Rothnagel J A, Roop D R. J Biol Chem. 1995;270:10792–10799. doi: 10.1074/jbc.270.18.10792. [DOI] [PubMed] [Google Scholar]
- 20.Udvadia A J, Templeton D J, Horowitz J M. Proc Natl Acad Sci USA. 1995;92:3953–3957. doi: 10.1073/pnas.92.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizushima S, Nagata S. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowa Y, Orita T, Minamikawa-Hiranabe S, Mizuno T, Nomura H, Sakai T. Cancer Res. 1999;59:4266–4270. [PubMed] [Google Scholar]
- 23.Ho S N, Thomas D J, Timmerman L A, Li X, Francke U, Crabtree G R. J Biol Chem. 1995;270:19898–19907. doi: 10.1074/jbc.270.34.19898. [DOI] [PubMed] [Google Scholar]
- 24.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. Nature (London) 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 25.Aramburu J, Yaffe M B, Lopez-Rodriguez C, Cantley L C, Hogan P G, Rao A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 26.Roop D R, Cheng C K, Titterington L, Meyers C A, Stanley J R, Steinert P M, Yuspa S H. J Biol Chem. 1984;259:8037–8040. [PubMed] [Google Scholar]
- 27.Rothnagel J A, Mehrel T, Idler W W, Roop D R, Steinert P M. J Biol Chem. 1987;262:15643–15648. [PubMed] [Google Scholar]
- 28.Mehrel T, Hohl D, Rothnagel J A, Longley M A, Bundman D, Cheng C, Lichti U, Bisher M E, Steven A C, Steinert P M, et al. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 29.Calautti E, Missero C, Stein P L, Ezzell R M, Dotto G P. Genes Dev. 1995;9:2279–2291. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- 30.Musaro A, McCullagh K J, Naya F J, Olson E N, Rosenthal N. Nature (London) 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 31.Calautti E, Cabodi S, Stein P L, Hatzfeld M, Kedersha N, Dotto G P. J Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Keefe E J, Briggaman R A, Herman B. J Cell Biol. 1987;105:807–817. doi: 10.1083/jcb.105.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Tennenbaum T, Yuspa S H. J Invest Dermatol. 1996;106:254–260. doi: 10.1111/1523-1747.ep12340654. [DOI] [PubMed] [Google Scholar]
- 34.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto G P. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 35.Courey A J, Tjian R. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 36.Gill G, Pascal E, Tseng Z H, Tjian R. Proc Natl Acad Sci USA. 1994;91:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saluja D, Vassallo M F, Tanese N. Mol Cell Biol. 1998;18:5734–5743. doi: 10.1128/mcb.18.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano F, Tanaka H, Hirano Y, Hiramoto M, Handa H, Makino I, Scheidereit C. Mol Cell Biol. 1998;18:1266–1274. doi: 10.1128/mcb.18.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kardassis D, Papakosta P, Pardali K, Moustakas A. J Biol Chem. 1999;274:29572–29581. doi: 10.1074/jbc.274.41.29572. [DOI] [PubMed] [Google Scholar]
- 40.Lin S Y, Black A R, Kostic D, Pajovic S, Hoover C N, Azizkhan J C. Mol Cell Biol. 1996;16:1668–1675. doi: 10.1128/mcb.16.4.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J S, Galvin K M, Shi Y. Proc Natl Acad Sci USA. 1993;90:6145–6149. doi: 10.1073/pnas.90.13.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youn H D, Chatila T A, Liu J O. EMBO J. 2000;19:4323–4331. doi: 10.1093/emboj/19.16.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datto M B, Li Y, Panus J F, Howe D J, Xiong Y, Wang X F. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]