Abstract
Source memory, or memory for the context in which a memory was formed, is a defining characteristic of human episodic memory and source memory errors are a debilitating symptom of memory dysfunction. Evidence for source memory in nonhuman primates is sparse despite considerable evidence for other types of sophisticated memory and the practical need for good models of episodic memory in nonhuman primates. A previous study showed that rhesus monkeys confused the identity of a monkey they saw with a monkey they heard, but only after an extended memory delay. This suggests that they initially remembered the source – visual or auditory - of the information but forgot the source as time passed. Here, we present a monkey model of source memory that is based on this previous study. In each trial, monkeys studied two images, one that they simply viewed and touched and the other that they classified as a bird, fish, flower, or person. In a subsequent memory test, they were required to select the image from one source but avoid the other. With training, monkeys learned to suppress responding to images from the to-be-avoided source. After longer memory intervals, monkeys continued to show reliable item memory, discriminating studied images from distractors, but made many source memory errors. Monkeys discriminated source based on study method, not study order, providing preliminary evidence that our manipulation of retention interval caused errors due to source forgetting instead of source confusion. Finally, some monkeys learned to select remembered images from either source on cue, showing that they did indeed remember both items and both sources. This paradigm potentially provides a new model to study a critical aspect of episodic memory in nonhuman primates.
Keywords: episodic memory, context, source monitoring, primate cognition
1. Introduction
During his 1980 presidential campaign, Ronald Reagan often earnestly repeated the story of a World War II bomber pilot who heroically went down with his damaged plane rather than abandon an injured crewman (Berger, 2004). Although Reagan correctly remembered the story, he had forgotten its source: the 1944 Hollywood film A Wing and a Prayer. This example demonstrates the importance of remembering the source of information, and that source memory can be dissociated from item memory.
Source memory is a defining characteristic of human episodic memory (Tulving, 1993). Practically, source memory errors are a debilitating symptom of age-, injury-, or drug-related memory impairment (e.g., Cansino, 2009; Janowsky et al., 1989; Mcintyre & Craik, 1987; Morgan et al., 2004). Therefore, tests of source memory and source memory errors in nonhuman animals are of great interest because they will inform our understanding of the evolution of memory, and provide models for neuroscientific investigations (Crystal, 2016; Templer & Hampton, 2013).
To assess source memory in nonhuman subjects, we adopt an operational definition of source memory that is grounded in studies of human memory. In many studies of human source memory, subjects study words, images, or abstract shapes presented with one of two secondary characteristics (e.g., presented in different colors, in different screen locations, in different sensory modalities). At test, subjects demonstrate item memory by discriminating studied items from non-studied items, and demonstrate source memory by additionally discriminating among studied items based on the secondary study characteristic. In one common variant, subjects are tested in an “exclusion” condition (Jacoby, 1991, 1999), in which they are instructed to accept only previously studied items from one source, avoid previously studied items from the other source, and also avoid unstudied items. For example, subjects might study line drawings in different colors (e.g., a green fork, a red key), and then see a mix of studied and unstudied black drawings at test and be instructed to only accept items if they had been studied in red (Cycowicz et al., 2001). In this example, item memory is operationalized as the ability to discriminate between studied and unstudied images (e.g., accept the key but reject a swan), and source memory is operationalized at the ability to discriminate between black test images based on the color they appeared in at study (e.g., accept the key and reject the fork).
Researchers studying source memory have used a wide variety of secondary study characteristics as the “source” of the item, including the item’s color (Cycowicz et al., 2001; Kensinger & Corkin, 2003), the color of a surrounding box (Mollison & Curran, 2012), whether the item was read or heard (Jacoby, 1999), the gender of the speaker during auditory study (Bornstein & Lecompte, 1995; Senkfor & Van Petten, 1998), item location on a computer screen (Mollison & Curran, 2012; Slotnick et al., 2003), or whether subjects were required to make a pleasant/unpleasant or a concrete/abstract judgement about the item during study (Dobbins et al., 2002). What is common across the rich variation in the nature of “source” in these studies is that the measure of source memory has been consistently operationalized as the ability to discriminate between items with different secondary study characteristics during a later memory test.
Despite the importance of studying source memory in nonhuman animals, evidence that nonhumans remember the source of learned information is scarce. One proposed example comes from studies of rats (Crystal & Alford, 2014; Crystal et al., 2013). In these tests, rats remembered not only where they previously found food, but also whether they learned the location of the food by navigating there themselves or by being placed there by the experimenter. If the rat found chocolate itself, it could find more chocolate in the same location later. But if the rat was placed at a chocolate location by the experimenter, there would be no chocolate in that location later. Rats demonstrated source memory by re-visiting the chocolate locations they found themselves more often than the chocolate locations at which they were placed by the experimenter. Their source memory was dependent on the integrity of the hippocampal CA3 subfield, and the rats could be retroactively cued after study as to which source predicted replenishing chocolate. However, the degree to which finding or being carried to a food site instantiates “source” in the same way this term is used with humans is not settled. In addition, evidence of source memory in rats would imply that it is a common feature of mammalian memory and that it should also be found in species that are more closely related to humans such as other primates; however, demonstrations of source memory in nonhuman primates are surprisingly lacking.
A recent study of auditory-visual memory integration (Adachi & Hampton, 2011) suggested the existence of source memory, source memory errors, and a delay-dependent dissociation of item and source memory in rhesus monkeys. During the study phase of each trial, monkeys watched videos of known conspecifics. After a retention interval, they were required to select the image of the studied individual from among four other known individuals. Some retention intervals included a vocalization from one of the four to-be-avoided distractor monkeys. This setup parallels human source memory methods in which source is defined by whether studied items had been seen or heard, and subjects must follow an exclusion rule of only selecting studied items from one modality (e.g., Jacoby, 1999). When subject monkeys erred, they chose the picture of the monkey they had heard during the retention interval more often than expected by chance. However, they chose the heard monkey in error only when the vocalization occurred at the beginning of the retention interval, not when it occurred at the end of the retention interval (unpublished data). One interpretation of this finding is that monkeys remembered the identities of both the seen monkey and the heard monkey, and generally selected the seen monkey at test, as was required. However, after a long delay they sometimes forgot which monkey was seen and which heard, and erroneously chose the heard monkey at test due to a source memory error. That this only happened when both pieces of information were presented at the beginning of the retention interval, and not when the heard monkey was presented at the end of the retention interval, may indicate that source memory is forgotten more quickly, or confused more easily, than item memory under these conditions.
In this study, we evaluated the source memory interpretation of the pattern of findings from the previous study of cross-modal integration. Monkeys studied two color images that were learned in two different ways, by touching one and by classifying the other. Our paradigm is similar to previous studies of source memory in humans, in that subjects must remember items that were studied in two different ways. Our approach most closely parallels studies of source memory in humans in which source is defined by the judgment subjects were required to make about items at study (e.g., Dobbins et al., 2002). In Experiment 1a, monkeys earned food at test by selecting the touched image and avoiding both the classified image and two unstudied distractor images. Thus, this paradigm also employs an exclusion rule (e.g., Jacoby, 1991, 1999) in which subjects must select items from one source but not the other. In accord with our operational definition, item memory was evaluated as the ability, at test, to discriminate studied items from unstudied items, and source memory was evaluated as the ability to discriminate between studied items based on how they were studied.
As in the previous monkey study, the item from the to-be-avoided source occurred at the end of the retention interval. In Experiment 1b, we randomly intermixed probe trials on which both images were studied at the start of the retention interval, reproducing the conditions from the previous study under which monkeys made apparent source errors. The study by Adachi and Hampton (2011; and unpublished data) suggests a source memory hypothesis whereby monkeys initially encode both item and source information but source information decays more rapidly than item information. This hypothesis makes three predictions for this experiment: 1) in Experiment 1a, when memory for the to-be-avoided source is still strong during test, monkeys should be able to learn to avoid the classified image; 2) in Experiment 1b, when memory for both sources is weaker during test, they will increase choices of the classified image; 3) because item memory is still relatively strong, errors will not be to unstudied distractors but will be selective to items from the to-be-avoided source.
2. Experiment 1a: Acquisition of source memory discrimination
2.1 Methods
2.1.1 Subjects
We tested twelve adult male rhesus monkeys (mean age at start: 8.5 years) in their home cages. Whenever possible, monkeys were pair-housed when not testing. Pair-housed monkeys were separated during testing by a protected-contact divider (a plastic dividing wall with small holes) that allowed them limited visual, auditory, and tactile access to their partner but not their partner’s computer screen. Monkeys received full food rations after each day’s testing, and water was available ad lib. All monkeys had prior experience with touchscreen-based cognitive tasks including perceptual classifications and delayed matching of images (Basile & Hampton, 2013a, 2013b, 2013c). All testing complied with US law and the National Institutes of Health guide for the care and use of laboratory animals.
2.1.2 Apparatus
We tested subjects six days a week using portable testing rigs equipped with a 15″ color LCD touch screen (3M, St. Paul, MN; and ELO, Milpitas, CA) running at a resolution of 1024 × 768 pixels, stereo speakers, and two automatic food dispensers (Med Associates Inc., St. Albans, VT) which dispensed nutritionally-complete food pellets into cups below the screen. Testing equipment was available to the monkeys seven hours a day. Stimuli were 40 color photographs of birds, fish, flowers, or people, similar to those used previously (Basile & Hampton, 2013a, 2013b; Diamond et al., 2016; Gazes et al., 2012).
2.1.3 Procedure
In the main test, monkeys studied two different images on each normal trial, one by touching it and one by classifying it as a bird, fish, flower, or person. Trials concluded with a memory test in which the touched image was the correct response, and the classified image appeared as one of three distractors (Figure 1a). Monkeys initiated each trial by touching a green start square (100 × 100 pixels). A color photograph (400 × 300 pixels) appeared in the center of the screen and the monkey touched it to advance the trial. An unfilled memory delay followed. At the start of training, this delay was 10 seconds for half the monkeys and 20 seconds for the other half because the latter six monkeys participated in another experiment between the start and end of training and that experiment used a longer delay (Basile & Hampton, 2013a). Because the different delay lengths did not produce significantly different performance (see Results), we treated all monkeys as one group for the remainder of the study. Regardless, the memory delay was 10 seconds for all monkeys at the end of training and in the probe tests. After the memory interval, a color photograph appeared in the center of the screen surrounded by four colored symbols that corresponded to the four possible image categories – bird, fish, flower, or person. Monkeys classified the photograph by touching the appropriate symbol, but did not have to touch the photograph itself. Classified samples were never the same photograph or category as the touched samples. These monkeys knew the classification task well (Basile & Hampton, 2013a, 2013b). Incorrect classification aborted the trial, produced an unfilled timeout of two seconds, and a negative audio cue (“d’oh!”). The aborted trial was then repeated as the subsequent trial following the inter-trial interval. Correct classifications produced an unfilled 200msec delay followed by the memory test.
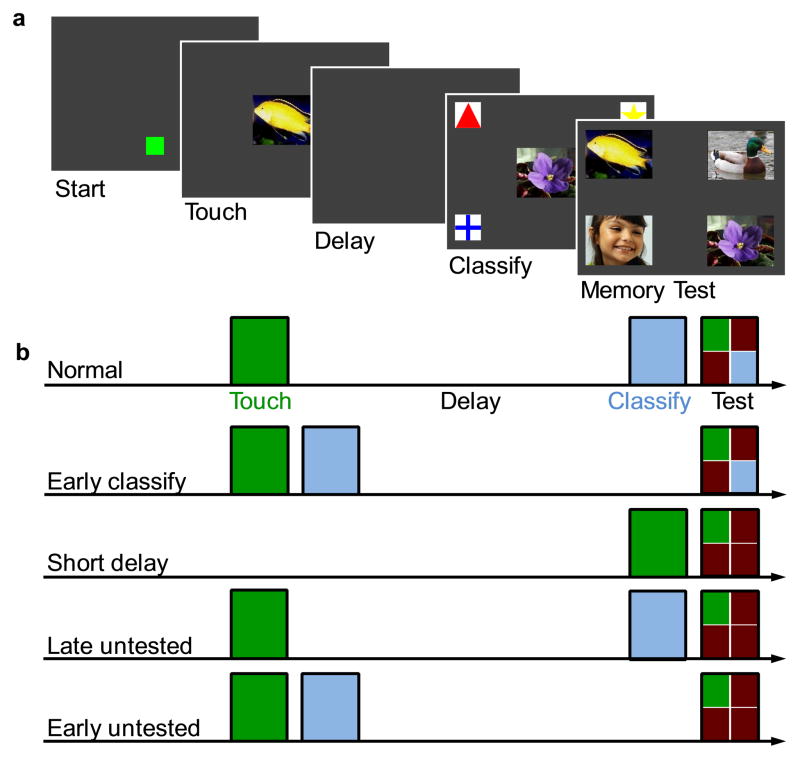
Fig. 1. Monkeys studied two images, one by touching it and one by classifying it, and were then tested for their memory of the touched image.
a. Screens from an example trial. Monkeys initiated trials by touching a start square. They saw an image in the center of the screen and had to touch it to progress. After an unfilled delay, they then saw another image in the center of the screen and had to classify it as a bird, fish, flower, or person by touching one of the four associated symbols in the corners. Incorrect classification caused trials to abort. At test, monkeys saw the touched image, the classified image, and two unstudied distractors. Selecting the touched image earned food, whereas touching any other test image earned a time-out. b. Schematic of different probe and control trial types. Relative timing of touched (medium green) and classified (light blue) images is indicated by placement on the arrow. Quadrants at test indicate tested image types (dark red = distractor).
At test, monkeys saw the touched image, the classified image, and two unstudied distractors. On every trial, one image came from each of the four categories, with each category represented equally often as touched or classified samples, and each appearing equally often in the four test locations. Selecting the touched image produced one food pellet and a positive audio reinforcer (“excellent!”), whereas selecting any other image produced an unfilled timeout of 2 seconds and a negative audio cue (“d’oh!”). All trials were followed by an unfilled 10-second inter-trial interval, in addition to any timeout. To prevent registration of spurious screen contacts, all responses required two consecutive touches to the same image.
Training sessions also contained two types of control trials, which are depicted in Figure 1b. On short delay trials, we removed the classified image and used a short 200msec delay. On late untested trials, the classified image was presented at the end of the retention interval and we replaced the classified image at test with an unstudied image from the same category.
Training and testing progressed as follows. We first made an initial assessment of the degree to which monkeys chose both studied stimuli at test in a single session. We next trained monkeys to avoid the classified sample at test using two-choice training sessions, which contained only the touched and classified samples at test, followed by normal four-choice training sessions, as described above. Monkeys progressed through each training phase after their choice of the touched sample on normal trials and on late untested trials were within five percentage points of each other. Near equivalence of selection of the touched image in these conditions indicated that the monkeys were successfully rejecting the classified image at test on normal trials. Training sessions contained 100 normal trials, 100 short delay trials, and 100 late untested trials, pseudorandomly intermixed.
2.2 Results and discussion
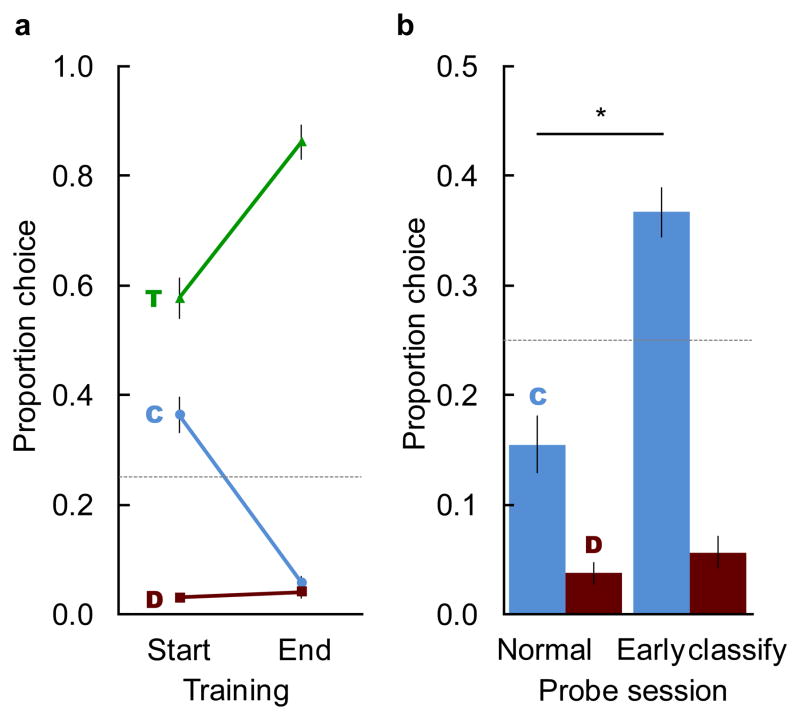
Selection of the touched or classified image in the initial session did not differ as a function of initial delay (touched mean(SEM): short delay = 0.58(0.03), long delay = 0.58(0.07); t10 = 0.08, p = .94; classified mean(SEM): short delay = 0.34 (0.02), long delay = 0.38(0.06); t10 = 0.48, p = .64), thus we treated all 12 monkeys as one group for the remainder of the study. During training, monkeys learned to avoid selecting the classified image. At the start of training, monkeys selected the classified image significantly more often than expected by chance (Figure 2a; mean = 0.36, SEM = 0.03, chance = 0.25; t11 = 3.33, p = .007, d = 0.96). Monkeys received an average of 13,910 trials (SD= 4118) before meeting the criterion for successfully rejecting the classified image at test. At the end of training, they chose the classified image significantly less often than at the start (Figure 2a; mean = 0.06, SEM = 0.01; t11 = 12.77, p < .001, d = 3.69), and at levels significantly below chance (Figure 2a; t11 = −10.41, p < .001, d = 3.14). Thus, monkeys demonstrated memory for both images and could discriminate between these memories at test based on their source (touched vs classified).
Fig. 2. Monkeys learned to avoid choosing the classified image, but then erroneously chose it when the classification task occurred at the start of the delay.
a. Mean proportion choice of the touched image (T; green), classified image (C; blue), and distractor images (D; red) on the first and last training session in Experiment 1a. b. Mean proportion choice of the classified image (C; blue), or of a distractor (D; red), in the first probe session of Experiment 1b, in which the classified image appeared at the end of the delay interval as during training (Normal) or at the beginning of the memory delay (Early classify). Proportion choice of distractors is the average of both distractors. Error bars represent ±SEM, dashed lines represent chance, * = p < .05.
3. Experiment 1b – Retention interval dissociated source and item memory
The evidence for source memory in rats suggested that source memory and location memory were dissociable on the basis of retention interval (Crystal et al., 2013). In humans, source memory is usually worse than item memory (e.g., Bornstein & Lecompte, 1995; Brown & Halliday, 1991; Glisky et al., 1995). The previous cross-modal integration study in monkeys suggested a delay-based dissociation, with source memory impaired at longer delays that left identity memory intact (Adachi & Hampton, 2011). If source memory is forgotten more rapidly than item memory, then moving the item from the to-be-avoided source (classified) to the beginning of the retention interval should produce source memory errors as evidenced by increased choice of the classified image.
3.1 Methods
2.1.1 Subjects and apparatus
Subjects and apparatus were the same as in Experiment 1a.
3.1.2 Procedure
In probe sessions, we sometimes presented both images at the start of the retention interval. On these early classify trials, the classified image occurred 200msec after the touched image and before the 10-second memory delay (Figure 1b). All other aspects of the trial were as described in normal trials in Experiment 1a. Similar to the late untested control trials in the training sessions, probe sessions contained early untested trials, during which the classified image was presented at the start of the retention interval and we replaced the classified image at test with an unstudied image from the same category. Probe sessions contained 50 normal trials, 50 early classify trials, 100 short delay trials, 50 late untested trials, and 50 early untested trials, pseudorandomly intermixed. We tested monkeys on ten probe sessions, analyzing the first probe session as our critical data and the remaining sessions to determine the effect’s robustness.
3.2 Results and discussion
Presenting the classified image at the start of the delay selectively increased erroneous choices of the classified image. In the first probe session, monkeys chose the classified image significantly more often on the early classify trials than on the normal trials (Figure 2b; early: mean = 0.37, SEM = 0.02; normal: mean = 0.16, SEM = 0.03; t11 = 9.95, p < .001, d = 2.87), and at levels significantly above chance (Figure 2b; t11 = 5.32, p < .001, d = 1.54). These choices were specific to the classified image and were not due to a general decline in accuracy, as choice of the unstudied distractor images did not change significantly (Figure 2b; early: mean = 0.06, SEM = 0.01; normal: mean = 0.04, SEM = 0.01; t11 = 1.37, p = .198). The selective increase in choice of the studied image from the incorrect source, but not increased errors to the unstudied distractors indicates a source memory error. Monkeys likely remembered both images, but had forgotten or confused which image came from which source.
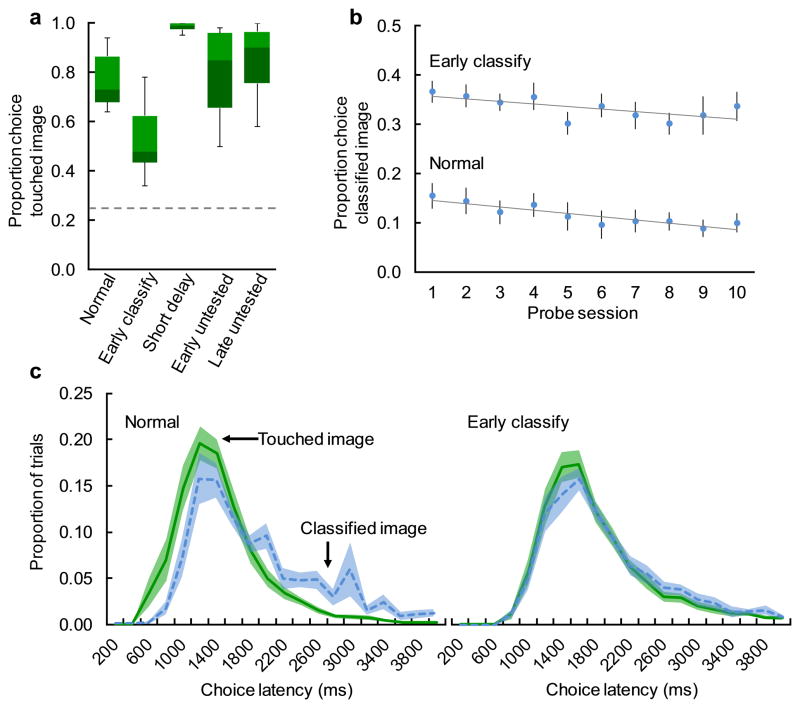
The three types of control trials demonstrated that increased choice of the classified image was not an epiphenomenon due to difficulty selecting images after a short delay, or to decreased choice of the touched image per se. First, the short 200 ms delay between the classified image and the test could have somehow inhibited selecting that image on normal trials. This was not the case. When the short-delay image was the touched image (Figure 1b), monkeys correctly selected it significantly above chance (Figure 3a; mean = 0.98, SEM = 0.01, chance = 0.25; t11 = 38.48, p < .001, d = 11.11) and were even significantly more accurate than on normal trials as would be expected given the shorter retention interval (mean = 0.77, SEM = 0.03; t11 = 10.99, p < .001, d = 3.17). Second, it is possible that the increase in choices of the classified image when classification was moved to the beginning of the delay was actually due to monkeys avoiding the touched image at test. This was also not the case. When the classified image was present during the delay, but not present at test (Figure 1b), accuracy was either not significantly different from that on normal trials (Figure 3a; late untested trials; mean = 0.80, SEM = 0.05; Figure 3a; t11 = 1.40, p = .189), or superior to that on normal trials (Figure 3a; early untested trials; mean = 0.85, SEM = 0.04; t11 = 3.21, p = .008, d = 0.93). For all three of these control trial types, accuracy was significantly higher than in the early classify trials (Figure 3a; mean = 0.52, SEM = 0.04; short delay: t11 = 18.09, p < .001, d = 5.22; late untested: t11 = 7.66, p < .001, d = 2.21; early untested: t11 = 10.44, p < .001, d = 3.01). That the source memory errors go away when the item from the to-be-avoided source is absent at test indicates that these errors are indeed due to competition between memories of the two studied images at test.
Fig. 3. Source memory errors were specific to early classify trials, were stable across sessions, and were as quick as correct choices.
a. Proportion choice of the touched image on normal trials, early classify trials, and control trials in the first probe session. Bars represent medians and quartiles, whiskers represent minimum and maximum values, and the dashed horizontal line represents chance. b. Mean proportion choice (±SEM) of the classified image on normal trials and early classify trials across ten consecutive probe sessions. c. Distributions of latency to choose the touched (solid green) or classified (dashed blue) image at test. Lines represent the proportion of each trial type (±SEM) that fell within each 200ms bin.
The increase in erroneous choices of the classified image was stable and robust across ten probe sessions. Although monkeys got significantly better at avoiding the classified image (Figure 3b; main effect of session: F(9,99) = 2.63, p = .009, partial η2 = .19), they always selected it significantly more on early classify trials than on normal trials (main effect of trial type: F(9,99) = 186.97, p < .001, partial η2 = .94). The size of this increase remained constant, as there was no significant interaction between trial type and session (F(9,99) = 0.51, p = .866).
Latency analysis of the early classify trials suggested that monkeys were equally confident when choosing the correct touched image and the incorrect classified image. In typical memory tests, monkeys choose relatively slowly when about to make an incorrect choice and relatively quickly when about to make a correct choice, and this latency difference correlates with confidence measures in metamemory tests (Basile et al., 2015). In the current study, visual inspection of choice latency distributions indeed showed relatively slower reaction times when choosing the classified image on normal trials, but overlapping latency distributions when choosing it on early classify trials (Figure 3c). On normal trials, mean latency to choose the classified image was slower than for the touched image but not different than for the un-studied distractors (means(SEM) seconds: touched = 1.38(0.08), classified = 1.88(0.14), distractor = 2.33(0.36); touched vs classified: t11 = 9.49, p < .001, d = 2.74; classified vs distractor: t11 = 2.14, p = .056; Bonferroni corrected α = .025), suggesting that the monkeys treated images from the to-be-avoided source as more similar to the distractors. In contrast, on early classify trials, mean latency to choose the classified image was not different than for the touched image and was quicker than for the un-studied distractors (means(SEM) seconds: touched = 2.71(0.73), classified = 2.40(0.21), distractor = 4.62(0.81); touched vs classified: t11 = 0.21, p = .838; classified vs distractor: t11 = 3.74, p = .003, d = 1.08; Bonferroni corrected α = .025), suggesting that they were now treating images from the to-be-avoided source as more similar to images from the to-be-selected source. Note that the absolute test latencies were longer on early classify trials than on normal trials because those were the trials on which monkeys had touched the screen less recently. Equally quick choice of the classified image and the touched image suggests that monkeys were equally confident about the two studied items and more confident about those items than the distractor items. Together, this is consistent with the idea that they remembered both items but could no longer discriminate those items’ sources.
4. Experiment 2 – Monkeys flexibly selected images from either source on cue
Experiment 1 suggested that monkeys remembered both images and both sources on normal trials. If this is true, they should be able to flexibly select images from either source on cue. In Experiment 2, monkeys again studied images from two sources (touched or classified), but we did not cue them until test, via the background color of the screen, as to which was the correct image on that trial. This parallels the manipulation used in the rat studies of source memory, in which rats did not know until just before test which source predicted replenished food (Crystal & Alford, 2014). As in Experiment 1, we looked for source memory errors during a probe session, in which we sometimes presented the classified image at the end of the retention interval, and sometimes presented both images at the beginning of the retention interval. If monkeys do remember both images and both sources, and source memory is more affected by delay than item memory, then monkeys should be able to learn to select images from either source on cue, and presenting both images at the beginning of the retention interval should increase erroneous selection of the image from the un-cued source.
4.1 Methods
4.1.1 Subjects
For all subsequent experiments, data come from six of the original twelve monkeys. All twelve monkeys were previously scheduled to participate in a different experiment, and only six completed these experiments before all had to start the new experiment.
4.1.2 Procedure
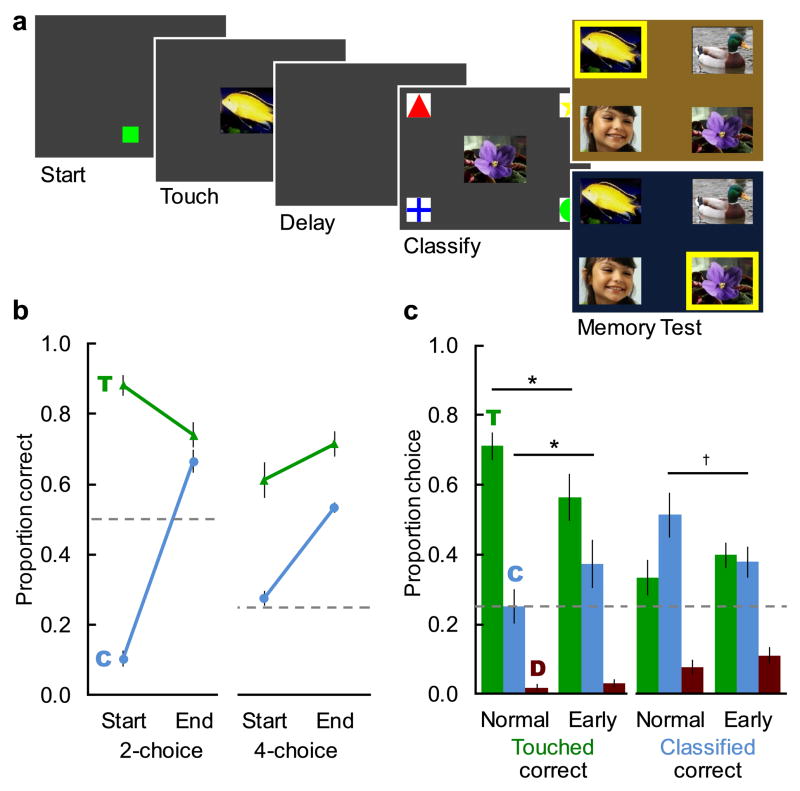
Trials progressed as in Experiment 1, with the exception that the color of the screen background at test signaled whether selecting the touched sample or the classified sample would be rewarded (Figure 4a). The background was either blue or brown, with the assignment of color to test requirement counterbalanced across monkeys. Training proceeded as in Experiment 1. Monkeys first met criterion with tests that omitted the two un-studied distractors, and then met criterion with tests that contained both studied images and two un-studied distractors. Monkeys progressed if they correctly selected the cued sample on more than 60% of trials for each type for the two-choice task (chance = 50%), and on more than 50% of trials for each type on the four-choice task (chance = 25%). For this experiment, we used only normal trials, in which both samples were present at test. Training sessions consisted of 500 trials in which the touched image was correct and 500 trials in which the classified image was correct, pseudorandomly intermixed.
Fig. 4. Monkeys learned to choose either image on cue, and asymmetrically increased source errors when both samples occurred before the delay.
a. Screens from an example 4-choice normal trial. At test, the color of the background cued the monkey as to whether selecting the touched image or the classified image would be rewarded. A yellow border indicates the correct choice here, but it was not present during actual testing. b. Training data. Mean proportion correct (±SEM) on the two-choice task (left) and the four-choice task (right), on trials for which the monkey was supposed to report the touched image (T; green) or the classified image (C; blue). c. Mean proportion (±SEM) choice of each image type on the first probe session. Proportion choice of distractors (D; red) is the average of both distractors. The dashed lines represent chance. * = p < .05, † = p < .1.
After learning the two cues, monkeys completed a single 200-trial probe session, in which the classified sample appeared at the beginning of the delay for half of trials of each type. Trials were pseudorandomly intermixed such that no trial type (touched correct or classified correct) or study timing (normal or early classify) appeared more than four times in a row, and assignment of image category to samples was done as in Experiment 1.
4.2 Results and discussion
Monkeys learned to select either the touched or classified image on cue. At the start of training with only two choices, monkeys selected the touched image significantly above chance, and the classified image significantly below chance (Figure 4b; chance = 0.50, touched: mean = 0.88, SEM = 0.02; t5 = 9.77, p < .001, d = 3.99; classified: mean = 0.10, SEM = 0.02; t5 = −13.48, p < .001, d = 5.50). Monkeys received an average of 43,746 (SD= 13,645) trials during training. At the end of training, they chose both the touched and classified images significantly above chance (Figure 4b; chance = 0.25, touched: mean = 0.72, SEM = 0.04; t5 = 11.86, p < .001, d = 4.84; classified: mean = 0.53, SEM = 0.02; t5 = 19.00, p < .001, d = 7.75), but remained significantly more accurate at choosing the touched image than the classified image (t5 = 4.47, p = .007, d = 1.83). This is likely due to their long training history of selecting the touched image.
Because we were not able to complete training with all 12 monkeys, we cannot know how many of the remaining six monkeys would have learned to select images from the two sources on cue. However, all 12 did begin training on Experiment 2, and 5 of the 6 monkeys who did not complete Experiment 2 were performing more accurately than expected by chance for both types of cued trials at the time they left this study.
Placing the classified image at the start of the delay asymmetrically increased erroneous choices of the non-cued image. When cued to select the touched image, monkeys erroneously chose the classified image significantly more often on the early classify trials than on the normal trials (Figure 4c, left; early classify: mean = 0.37, SEM = 0.07; normal: mean = 0.25, SEM = 0.05; t5 = 3.29, p = .022, d = 1.34). These choices were specific to the classified image and were not due to a general decline in accuracy, as choice of the unstudied distractor images did not change significantly (early classify: mean = 0.03, SEM = 0.01; normal: 0.02, SEM = 0.01; t5 = 1.51, p = .191). This replicated the initial effect in Experiment 1b. In contrast, when cued to select the classified image, erroneous choices of the touched image increased numerically in five of the six monkeys, but the group effect did not reach statistical significance (Figure 4c, right; early classify: mean = 0.40, SEM = 0.04; normal: mean = 0.33, SEM = 0.05; t5 = 1.30, p = .249). This asymmetry is most likely due to the monkeys having much more training, and higher baseline accuracy, for selecting the touched image than selecting the classified image. Thus, we replicated the delay-based dissociation of source and item memory, but we provide only preliminary evidence for its generality. Overall, Experiment 2 demonstrated that monkeys did remember both sources and could be cued to flexibly select images from either source.
5. Experiment 3 – Study method, not temporal order, as the source of information
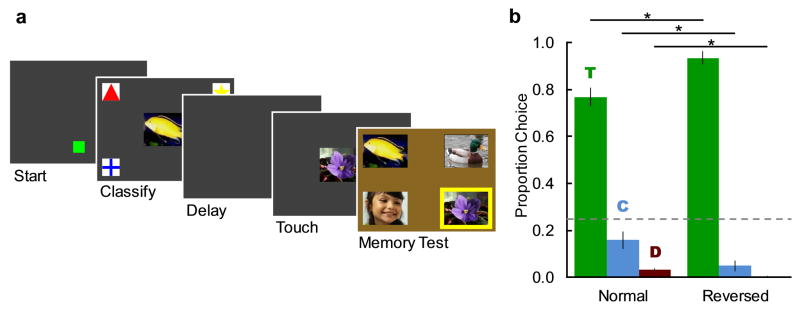
Monkeys may have selected one of the studied items at test based on memory for whether they touched it or classified it. However, it is also possible that monkeys instead encoded which sample came first in the trial, and used temporal order to discriminate between test items. These two types of source information are consistent with different accounts of the source memory errors we observed. If monkeys discriminated the two samples on the basis of when they occurred in the trial, then making the temporal placement more similar makes that temporal discrimination difficult, and thus source errors may be due to source confusion. In contrast, if the monkeys remembered whether they touched or classified the image, then making the temporal placement more similar is less likely to cause confusion, and the increase in errors is more likely due to source forgetting. To evaluate whether study method or temporal order defined the source of memories, and thus provide preliminary evidence as to whether source errors were due to confusion or forgetting, we pitted study method against temporal order by reversing the temporal sequence of the two samples and then cueing them to report the touched image (Figure 5a). Thus, if source is defined by temporal order, then reversing the sample order should reverse the monkeys’ choices; whereas if source if defined by study method, then reversing the sample order should either not affect choices or should improve accuracy due to the shorter delay between touched sample and test.
Fig. 5. Source discrimination was controlled by whether the image was touched or classified, not whether it came first or second.
a. Example screens from a reverse order probe trial, in which the first image was classified, the second was touched, and the test screen cued the monkey to report the touched image. A yellow border indicates the correct choice here, but it was not present during actual testing. Compare to 1a and 4a. b. Mean proportion choice (±SEM) on the single probe session for touched images (T; green), classified images (C; blue), and distractors (D; red). Proportion choice of distractors is the average of both distractors. The dashed line represents chance. * = p < .05.
5.1 Methods
5.1.1 Procedure
Monkeys completed a single 200-trial probe session. Half of trials were normal, as described previously. In the other half of trials, the monkeys classified the first image and touched the second image (Figure 5a). For all trials, the background color at test cued the monkey to report the touched sample. Trial type was pseudorandomly intermixed such that no trial type occurred more often than four times in a row.
5.2 Results and discussion
Study method and not temporal order controlled source discrimination. When the temporal order was reversed so that the touched image came second, but the test background still instructed monkeys to select the touched image, monkeys continued to select the touched image rather than switching to select the classified image (Figure 5b). Indeed, accuracy in choosing the touched image significantly improved when the temporal order was reversed, most likely because the retention interval between the touched image and the memory test was now shorter (Figure 5b; normal: mean = 0.77, SEM = 0.04; reversed: mean = 0.94, SEM = 0.03; t5 = 3.11, p = .027, d = 1.27). As expected from an increase in accuracy due to a shorter retention interval, this was a general improvement in accuracy, as choice of the un-studied distractors also decreased significantly (normal: mean = 0.03, SEM = 0.01; reversed: mean = 0.01, SEM < 0.01; t5 = 5.10, p = .004, d = 2.08).
6. General Discussion
Monkeys remembered images and the method in which they were studied. Source memory was dissociated from item memory on the basis of retention interval. Some monkeys flexibly learned to select information from either source on cue. Finally, monkeys discriminated between sources based on study method, not temporal order.
These findings bolster the source memory forgetting interpretation of the study of cross-modal integration in monkeys (Adachi & Hampton, 2011). In that experiment, monkeys erroneously chose a previously-heard monkey when they were supposed to choose a previously-seen monkey. Monkeys initially learned the visual memory test in their home room, which contained other monkeys that vocalized throughout the day. Thus, learning to select the seen monkey in the test may have required inhibiting choice based on memory of any heard monkeys. When they were in a sound-attenuated testing room for cross-modal sessions, they continued to avoid selecting the monkey heard during the test, but only when they could remember which monkey was heard and which was seen. Moving information from both sources to the beginning of a long retention interval likely caused forgetting of the source, which in turn produced selective errors to the heard monkey. Our current study reinforces this interpretation. Using neutral stimuli in a more controlled test, we verified the assumption that monkey initially remembered information from a to-be-avoided source and learned to avoid it. We reproduced the finding that monkeys erroneously chose to-be-avoided samples when they occur at the start of the delay and near information from the relevant source. Finally, we reinforce the case that monkeys remember the source of information with the finding that they could select information from either source on cue.
This monkey model of source memory and source memory errors parallels the rat model of source memory (Crystal & Alford, 2014; Crystal et al., 2013) in several ways. Both studies presented two pieces of information on each trial that were learned in different manners. Rats learned spatial locations by finding the locations or by being placed there, whereas monkeys learned image identities by touching the image or classifying it. In both paradigms, subjects were able to flexibly select the information from either source on cue. Both studies also resulted in delay-based dissociations of source memory and item or location memory. However, in the rat study it was location information that was more sensitive to delay, whereas here it was source memory that was more sensitive. This apparent discrepancy may be due to methodological differences. In the rat study, source memory was tested with more-preferred chocolate rewards and location memory was tested with less-preferred rat chow. In our study, all memory items were assessed with the same food rewards. Our current finding that source memory is more susceptible than item memory to delay is consistent both with the robust evidence from humans that source memory is generally less accurate than item memory (e.g., Bornstein & Lecompte, 1995; Brown & Halliday, 1991; Glisky et al., 1995), and with the day-to-day observation that most people know large collections of information that once were tied to a source but are now purely semantic. Regardless, both studies demonstrate that item memory and source memory have different functional properties.
The asymmetrical source memory errors reported in Experiment 2 would likely be symmetrical if not for monkeys’ training history. When the two sources occurred together at the start of the retention interval, monkeys increased choice of items from the un-cued source only when cued to select the touched item, but not when cued to select the classified item. However, when cued to select the classified item, five of the six monkeys did numerically increase source errors. All monkeys also had substantially more training in selecting the touched image than selecting the classified image, and were substantially better at doing so at the end of training prior to the probe session. It is likely that monkeys with equivalent training in both cues would show equivalent source error effects. A similar issue is that performance on touched trials might have been facilitated by the match in required action; that is, touching the item at test is more similar to touching it during study than to classifying it during study. The fact that the monkeys did learn to touch the classified image on cue suggests that this action match was not the primary driver of choice at test. Nonetheless, this provides another reason for future studies to include a group in which subjects initially learn to select the classified image, or to use a source manipulation that holds the action constant for all studied items (e.g., colored borders: Mollison & Curran, 2012; screen location: Slotnick et al., 2003).
Our operational definition of source memory as the ability to discriminate between studied items on the basis of secondary study characteristics is consistent with a wide variety of literature on source memory in both humans and nonhumans (e.g., Bornstein & Lecompte, 1995; Brown & Halliday, 1991; Crystal, 2016; Crystal & Alford, 2014; Crystal et al., 2013; Cycowicz et al., 2001; Dobbins et al., 2002; Glisky et al., 1995; Jacoby, 1999; Janowsky et al., 1989; Kensinger & Corkin, 2003; Mcintyre & Craik, 1987; Mollison & Curran, 2012; Senkfor & Van Petten, 1998; Slotnick et al., 2003). Nonetheless, we acknowledge that it may not fully capture some aspects of source memory of interest to particular researchers. Many cases of real-world human source memory are inherently social, or involve discriminating real events from imagined ones, and these specifics are better captured with other paradigms. Additionally, source memory is sometimes treated as a proxy for, or as equivalent to, episodic memory. Episodic memory is a broad construct with a definition that has changed multiple times over the past several decades and that is almost always tested using verbal report (see Tulving, 2002 for a review). Because nonhumans cannot give meaningful verbal reports, comparative researchers have made progress in the study of episodic memory by focusing on its most tractable sub-components (e.g., Clayton & Dickinson, 1998; Fortin et al., 2002; for similar arguments, see also Basile, 2015; Templer & Hampton, 2013). Our approach here has been to assess the ability to discriminate remembered items at test on the basis of how the items were originally studied. Thus, while we cannot know from these results whether monkeys have “full” episodic memory, laden with autobiographical self-awareness or other properties sometimes associated with episodic recall, we can conclude that they discriminate between study sources.
Future studies are needed to establish whether these source memory errors represent source forgetting or source confusion. The source forgetting hypothesis posits that monkeys remembered both items but forgot which had been touched and which classified. The source confusion hypothesis posits that sometimes monkeys erroneously thought that they had touched the classified image. Our finding from Experiment 1, that a delay manipulation caused source errors, suggests source forgetting. Our finding from Experiment 3, that making temporal order more confusable did not cause source errors, provides complementary evidence against source confusion as an explanation for our results. In contrast, our latency analysis from Experiment 1b can be interpreted as supporting the source confusion hypothesis. Similarly quick latency to choose images from either the to-be-selected or to-be-avoided source, but slower latency when choosing unstudied distractors, might indicate relatively high confidence in these source errors. Future studies might discriminate between source confusion and source forgetting by manipulating both the retention interval and the confusability of the sources.
In conclusion, this study provides evidence of source memory in nonhuman primates, elucidates some initial findings about the different functional properties of source and item memory in monkeys, and provides a nonhuman primate model of source memory that will allow for future psychological and neuroscientific investigations of source memory and source memory errors.
Highlights.
There is little evidence that nonhumans remember where or how they learned
We found that monkeys can remember which image was seen in which encoding context
Memory for images lasted longer than memory for source
Source memory may be a common feature of mammalian memory
Acknowledgments
We thank Steven L. Sherrin, Tara A. Dove-VanWormer, Emily K. Brown, and Jessica A. Joiner for help running subjects. This work was supported by the National Science Foundation (Grants 0745573; 1146316), the National Center for Research Resources P51RR000165, the Office of Research Infrastructure Programs/OD P51OD011132, the Center for Behavioral Neuroscience under the Science and Technology Center Program of the National Science Foundation (under agreement IBN-9876754), and the National Institute of Mental Health (Grant R01M H082819). These funding sources had no involvement in the design, execution, writing, or submission of this manuscript. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi I, Hampton RR. Rhesus Monkeys See Who They Hear: Spontaneous Cross-Modal Memory for Familiar Conspecifics. PLoS ONE. 2011;6(8):e23345. doi: 10.1371/journal.pone.0023345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM. Rats remind us what actually counts in episodic memory research. Frontiers in Psychology. 2015;6:75. doi: 10.3389/fpsyg.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition. 2013a;126(3):391–396. doi: 10.1016/j.cognition.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Monkeys show recognition without priming in a classification task. Behavioural Processes. 2013b;93:50–61. doi: 10.1016/j.beproc.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Recognition errors suggest fast familiarity and slow recollection in rhesus monkeys. Learning & Memory. 2013c;20(8):431–437. doi: 10.1101/lm.029223.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Schroeder GR, Brown EK, Templer VL, Hampton RR. Evaluation of seven hypotheses for metamemory performance in rhesus monkeys. Journal of Experimental Psychology: General. 2015;144(1):85–102. doi: 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. Ronald Reagan dies at 93; Fostered cold-war might and curbs on government. The New York Times 2004 Jun 6; [Google Scholar]
- Bornstein BH, Lecompte DC. A comparison of item and source forgetting. Psychonomic Bulletin & Review. 1995;2(2):254–259. doi: 10.3758/BF03210966. [DOI] [PubMed] [Google Scholar]
- Brown AS, Halliday HE. Crytomnesia and Source Memory Difficulties. The American Journal of Psychology. 1991;104(4):475–490. [Google Scholar]
- Cansino S. Episodic memory decay along the adult lifespan: A review of behavioral and neurophysiological evidence. International Journal of Psychophysiology. 2009;71(1):64–69. doi: 10.1016/j.ijpsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Animal models of source memory. Journal of the Experimental Analysis of Behavior. 2016;105(1):56–67. doi: 10.1002/jeab.173. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Alford WT. Validation of a rodent model of source memory. Biology Letters. 2014;10(3) doi: 10.1098/rsbl.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Alford WT, Zhou W, Hohmann AG. Source Memory in the Rat. Current Biology. 2013;23(5):387–391. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG. Remembering the Color of Objects: An ERP Investigation of Source Memory. Cerebral Cortex. 2001;11(4):322–334. doi: 10.1093/cercor/11.4.322. [DOI] [PubMed] [Google Scholar]
- Diamond RFL, Stoinski TS, Mickelberg JL, Basile BM, Gazes RP, Templer VL, Hampton RR. Similar stimulus features control visual classification in orangutans and rhesus monkeys. Journal of the Experimental Analysis of Behavior. 2016;105(1):100–110. doi: 10.1002/jeab.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive Control during Episodic Retrieval: Multiple Prefrontal Processes Subserve Source Memory. Neuron. 2002;35(5):989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazes RP, Brown EK, Basile BM, Hampton RR. Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory-based testing. Anim Cogn. 2012 doi: 10.1007/s10071-012-0585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9(2):229–235. [Google Scholar]
- Jacoby LL. A Process Dissociation Framework - Separating Automatic from Intentional Uses of Memory. Journal of Memory and Language. 1991;30(5):513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(1):3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27(8):1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & cognition. 2003;31(8):1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Mcintyre JS, Craik FI. Age differences in memory for item and source information. Canadian Journal of Psychology/Revue canadienne de psychologie. 1987;41(2):175–192. doi: 10.1037/h0084154. [DOI] [PubMed] [Google Scholar]
- Mollison MV, Curran T. Familiarity in source memory. Neuropsychologia. 2012;50(11):2546–2565. doi: 10.1016/j.neuropsychologia.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug and Alcohol Dependence. 2004;75(3):301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24(4):1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. Episodic Memory in Nonhuman Animals. Current Biology. 2013;23(17):R801–R806. doi: 10.1016/j.cub.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. What Is Episodic Memory? Current Directions in Psychological Science. 1993;2(3):67–70. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]