Abstract
Purpose
Cognitive behavioral therapy for insomnia (CBT-I) and Tai Chi Chih (TCC), a movement meditation, improve insomnia symptoms. Here, we evaluated whether TCC is noninferior to CBT-I for the treatment of insomnia in survivors of breast cancer.
Patients and Methods
This was a randomized, partially blinded, noninferiority trial that involved survivors of breast cancer with insomnia who were recruited from the Los Angeles community from April 2008 to July 2012. After a 2-month phase-in period with repeated baseline assessment, participants were randomly assigned to 3 months of CBT-I or TCC and evaluated at months 2, 3 (post-treatment), 6, and 15 (follow-up). Primary outcome was insomnia treatment response—that is, marked clinical improvement of symptoms by the Pittsburgh Sleep Quality Index—at 15 months. Secondary outcomes were clinician-assessed remission of insomnia; sleep quality; total sleep time, sleep onset latency, sleep efficiency, and awake after sleep onset, derived from sleep diaries; polysomnography; and symptoms of fatigue, sleepiness, and depression.
Results
Of 145 participants who were screened, 90 were randomly assigned (CBT-I: n = 45; TCC: n = 45). The proportion of participants who showed insomnia treatment response at 15 months was 43.7% and 46.7% in CBT-I and TCC, respectively. Tests of noninferiority showed that TCC was noninferior to CBT-I at 15 months (P = .02) and at months 3 (P = .02) and 6 (P < .01). For secondary outcomes, insomnia remission was 46.2% and 37.9% in CBT-I and TCC, respectively. CBT-I and TCC groups showed robust improvements in sleep quality, sleep diary measures, and related symptoms (all P < .01), but not polysomnography, with similar improvements in both groups.
Conclusion
CBT-I and TCC produce clinically meaningful improvements in insomnia. TCC, a mindful movement meditation, was found to be statistically noninferior to CBT-I, the gold standard for behavioral treatment of insomnia.
INTRODUCTON
Insomnia is diagnosed by difficulty initiating sleep, frequent awakenings, and an inability to return to sleep, all of which lead to daytime impairments.1 For survivors of cancer, insomnia poses a significant public health concern; nearly 30% of survivors of breast cancer meet diagnostic criteria for insomnia, a prevalence rate almost twice that of the general population.2-4 Distress associated with cancer diagnosis and treatment is thought to precipitate and perpetuate insomnia symptoms5,6; hence, treatments that target distress might improve insomnia.7
Cognitive behavioral therapy for insomnia (CBT-I), considered the treatment of choice by the American Academy of Sleep Medicine,8-11 combines cognitive therapy, stimulus control, sleep restriction, sleep hygiene, and relaxation to improve sleep outcomes, with demonstrated efficacy in survivors of cancer.12,13 With no serious contraindications, CBT-I is more effective than pharmacotherapy at improving sleep for both short-term and long-term periods14-16; however, some question the feasibility of implementing CBT-I in routine clinical practice and oncology care.14
Tai Chi Chih (TCC), a manualized form of Tai Chi, is a mind–body intervention (MBI) that combines slow physical activity with relaxation to serve as a movement meditation.17 TCC has been found to improve insomnia symptoms,18-20 with one study demonstrating efficacy in the short-term that was comparable to CBT-I therapy in older adults with insomnia.21 It is not known whether TCC is an effective treatment of insomnia in survivors of breast cancer, even though up to 50% of survivors of breast cancer report annual use of MBIs, such as TCC, in an effort to promote health22; this practice can be readily implemented in the community. TCC has broad benefits with effects on multiple other health outcomes,21,23-28 and TCC improves depression29 and fatigue,30 which are frequently comorbid in survivors of breast cancer, especially those with insomnia.31,32
The primary objective of the current study was to establish whether TCC produces durable effects and effects that are similar to CBT-I in reducing insomnia symptoms in survivors of breast cancer. We hypothesized that TCC would be statistically noninferior to CBT-I for reducing insomnia severity at 15 months, 1 year after intervention or treatment exposure. Secondary outcomes were insomnia remission, subjective and objective sleep outcomes, and severity of insomnia-related daytime impairments of fatigue, sleepiness, and depressive symptoms.
PATIENTS AND METHODS
Trial Design
Trial design was registered and ethical approval was obtained from the UCLA Institutional Review Board. The study is a single-masked (rater), single-site, parallel-group, noninferiority trial of TCC versus CBT-I on sleep and related behavioral outcomes, which follows CONSORT guidelines for noninferiority randomized trials.33
After recruitment by advertisement, telephone screening, informed consent, completion of questionnaires and interviews, laboratory blood tests, and polysomnographic (PSG) exclusion of those with sleep apnea or nocturnal myoclonus,21 participants entered a 2-month phase-in period to establish stability of insomnia severity before intervention and to maximize participant retention and compliance for the subsequent random assignment portion. After a second baseline in which all questionnaires and interviews were readministered, participants were randomly assigned to CBT-I or TCC for 3 months. Assessments were at 2, 3, 6, and 15 months. Methods were not changed after trial commencement.
Study Participants
Recruitment was conducted from April 2008 to July 2012. Participants were survivors of breast cancer (age range, 42 to 83 years) who fulfilled criteria for insomnia in the Diagnostic and Statistical Manual (Fourth Edition, Text Revision [DSM-IV-TR])34 and for general insomnia in International Classification of Sleep Disorders (Second Edition),35 reported sleep difficulties ≥ 3 times per week for > 3 months consistent with DSM-5 criteria,1 had completed treatment with surgery, radiation, and/or chemotherapy at least 6 months before the study, who and showed no evidence of cancer recurrence or new primary tumor. Exclusion criteria were previously described.25 The Charlson comorbidity scale was administered by interview to evaluate medical comorbidity.36
Interventions
The CBT-I program was delivered to groups of 7 to 10 participants in weekly 120-minute sessions. CBT-I followed the format of previously published trials in adults, older adults, and patients with cancer,9,12,15,37,38 and contained the following five validated components: cognitive therapy, stimulus control, sleep restriction, sleep hygiene, and relaxation, which together target sleep-related physiologic and cognitive arousal to re-establish restorative sleep function.14 CBT-I teaching components were administered in 2 months, which was consistent with the duration of the majority of CBT-I treatment trials.8,10,12,37,39 Intervention exposure was extended to 3 months with 1 month of skill consolidation and adherence to specifically address issues related to adherence that might impact implementation of CBT-I during follow-up.
The TCC program was delivered to groups of 7 to 10 in weekly 120 sessions. TCC emphasized control over physical function and arousal-related responsiveness, which are thought to contribute to insomnia,40 through the mindful performance of repetitious, nonstrenuous, slow-paced movement, that is, movement meditation, which made this MBI highly accessible. A week-by-week description of the program has been previously published.17 TCC teaching components were administered over 2 months, followed by 1 month of skill consolidation and adherence, for 3 months of treatment exposure.
Treatment Fidelity
Therapists were experienced and trained in one modality but not in the other. Another therapist who had extensive experience (> 10 years) in either CBT-I or TCC provided weekly supervision, evaluated treatment integrity, and attended three or more sessions with rating of treatment elements, which contained > 95% of required components.
Treatment Credibility
After the second session, participants rated treatment acceptance, credibility, and expectation for change.41 At the end of treatment exposure, therapists and participants rated insomnia treatment credibility.
Primary Outcome
The primary outcome was insomnia treatment response at month 15, which was defined by a decrease (≥ 5 points) on the Pittsburgh Sleep Quality Index (PSQI) or marked clinical improvement of symptoms.42 A decrease of ≥ 5 points on the PSQI also corresponds to treatment response of > 7 points15 or > 8 points43 derived from the Insomnia Severity Index, which also indicates marked improvement or nearly complete or complete remission of symptoms.44 Furthermore, this response criterion is consistent with clinically meaningful improvements in insomnia severity in published clinical trials of survivors of cancer.7,12,45-48
Secondary Outcomes
Insomnia remission was a secondary outcome. An assessor who was blind to treatment allocation administered a structured interview, and DSM-IV-TR criteria of insomnia remission were ascertained in a consensus meeting of at least two board-certified psychiatrists or psychologists.
Other secondary outcomes were sleep quality (PSQI and Athens Insomnia Severity Index [AISI]) and daily diaries of sleep parameters for 2 weeks (ie, Pittsburgh Sleep Diary).49 After a night of adaptation, PSG was obtained at baseline and month 3, as previously described.50,51 Behavioral outcomes included insomnia-related daytime symptoms of fatigue (Multidimensional Fatigue Symptom Inventory),52 sleepiness (Epworth Sleepiness Scale),53 and clinician-rated depressive symptoms (ie, Inventory of Depressive Symptoms).54 Body mass index and physical activity (Yale Physical Activity Survey55,56) were evaluated.
Sample Size
An a priori power analysis was conducted in G*Power (http://www.gpower.hhu.de/en.html). On the basis of prior meta-analytic findings and mean treatment effect (d = 0.4, a medium effect size),39 in which there is, at minimum, complete or nearly complete remission of symptoms,48 25 participants per treatment group provided statistical power of 80% (α = .05) to detect insomnia treatment response (ie, decrease of ≥ 5 points on the PSQI) at month 15 relative to baseline. The noninferiority margin was set at a minimum value of 50% of insomnia treatment response, which was consistent with two prior noninferiority trials on insomnia,7 or a 2.5-point decrease on the PSQI. With an estimated 20% attrition, 45 participants in each group provided adequate power (80% with α = .05) to reject the null hypothesis that the insomnia treatment response induced by TCC is inferior to that achieved by CBT-I.
Random Assignment
Random assignment sequence was generated via a computerized random number generator in blocks of 7 to 10 participants in TCC and CBT-I (1:1) by R.O., who did not view participant data before allocation. To maintain concealment, no research staff had access to allocation sequence, which was recorded on sequentially numbered, opaque, and sealed envelopes.
Blinding
The study was advertised as a research study to evaluate whether one or another insomnia treatment would improve insomnia, and participants remained blind to hypotheses and the content of the other treatment group through study duration. Use of a modified blind-to-treatment protocol—that is, partial blinding—is thought to reduce selection bias that is frequently associated with trials of behavioral interventions. Investigators and outcome assessors were blinded to allocation.
Statistical Methods
Noninferiority of the primary outcome was assessed by using an F statistic from linear mixed models and the appropriate CI side; significant P values indicate noninferiority, in which the observed difference between the two treatment means is significantly less than the noninferiority margin F-statistic (2.5).58 Intervention effects on secondary outcomes of proportion of insomnia remission and treatment response were tested by using Fisher’s exact test.
Intervention effects on secondary, continuous outcomes were tested on an intention-to-treat basis using a mixed model approach. Data from all randomly assigned participants were included. For each of the models, the random effect was participant and the fixed effects were group (TCC v CBT-I), time, and group × time interaction. We tested whether two baselines differed for any secondary outcome and found no differences. Subsequent analyses covaried for baseline immediately before treatment, that is, baseline 2. The restricted maximum likelihood estimate method estimated model parameters and standard errors with a compound symmetry covariance structure to account for the correlation between measurements. We used type III fixed effects (F and t) and set the statistical significance at P < .05. The mixed model approach generated unbiased estimates under the assumption that data are missing completely at random and the missing completely at random assumption was tested. Additional comparisons were made by using either t test or Fisher’s exact test as appropriate.
A priori linear contrasts tested group differences from baseline to 15-month follow-up, controlling for multiple comparisons. Data were available on > 95% of retained participants at all time points. Analyses were performed with SPSS for Windows version 21 (SPSS, Chicago, IL).
RESULTS
Baseline Characteristics of Participants
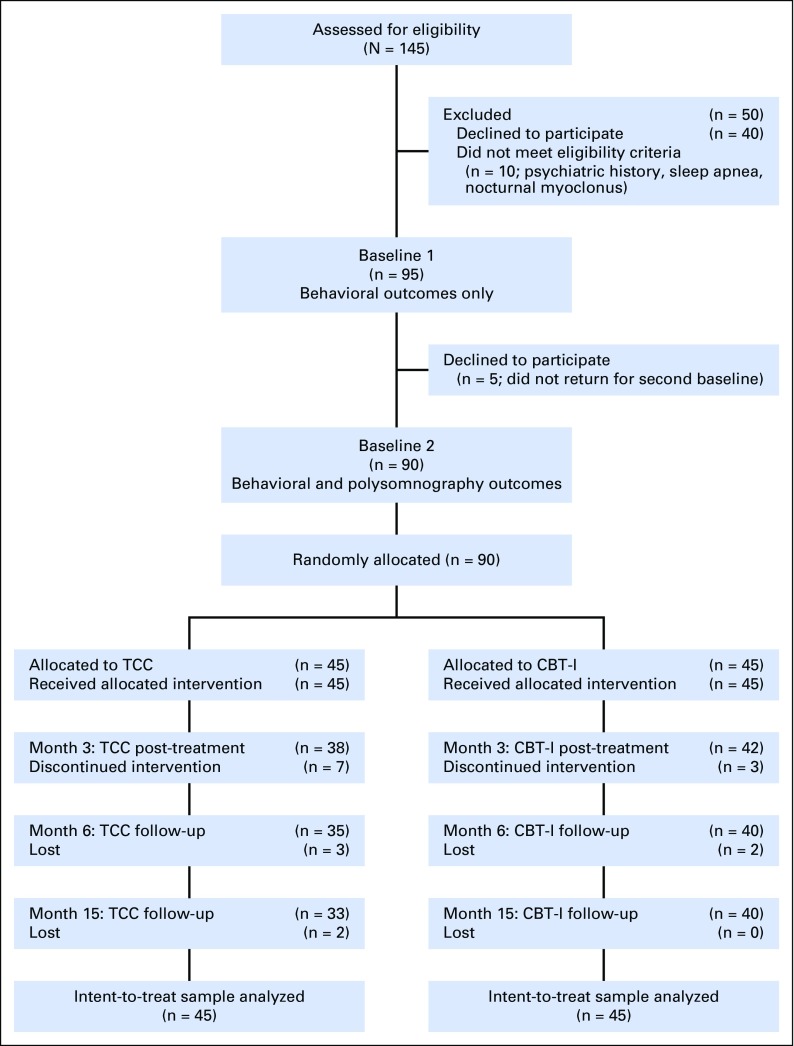
Table 1 lists the baseline characteristics of the two groups. Figure 1 shows participant flow and reasons for ineligibility. Those who completed and did not complete intervention and follow-up did not differ on any baseline characteristic. CBT-I and TCC showed similar rates of session attendance (80.6% v 75.8%; t[65] = 1.12; P = .27) and drop-out at month 3 (Fisher’s P = .32), but an increased rate of drop-out for TCC at month 15 (Fisher’s P = .06). CBT-I and TCC were perceived by participants as equally acceptable to improve insomnia after session 2 (100% v 94.7%; P = .49) and at month 3 (100% v 100%), with similar results rated by therapists. In TCC, 79.2% continued to practice > 30 min per day during follow-up, although days per week decreased from month 3 (5.3 ± 1.2) to month 15 (2.1 ± 1.6; T23 = 3.5; P < .05). No significant between-group change from baseline to month 15 was found for sedative-hypnotic medication use (F1,77.0 = 0.99; P = .40), body mass index (F1,49.0 = 0.01; P = .94), or physical activity (ie, metabolic equivalents per week59; F1,77.0 = 0.38; P = .55).
Table 1.
Baseline Characteristics of Participants by Treatment Group
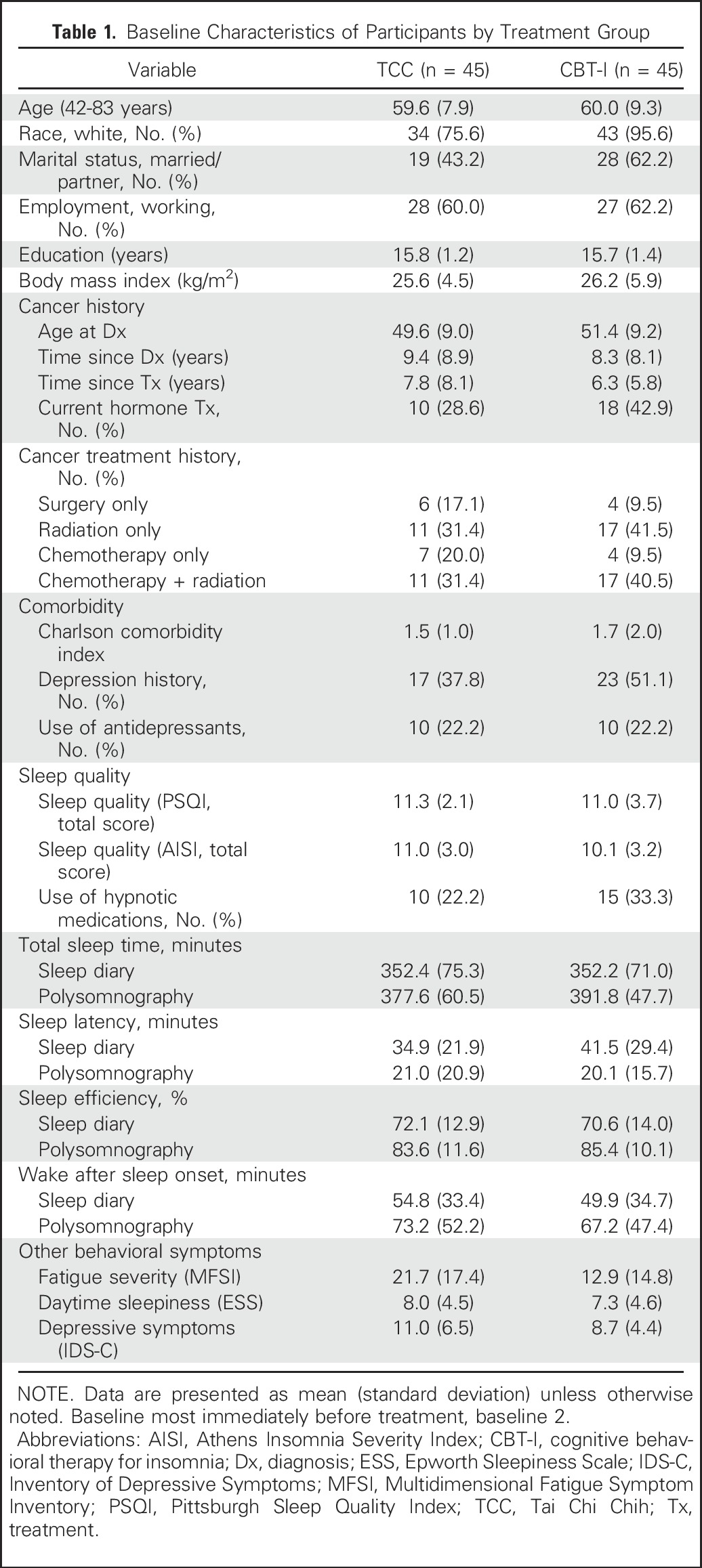
Fig 1.
Screening, random assignment, and completion of postintervention. CBT-I, cognitive behavioral therapy for insomnia; TCC, Tai Chi Chih.
Primary Outcome
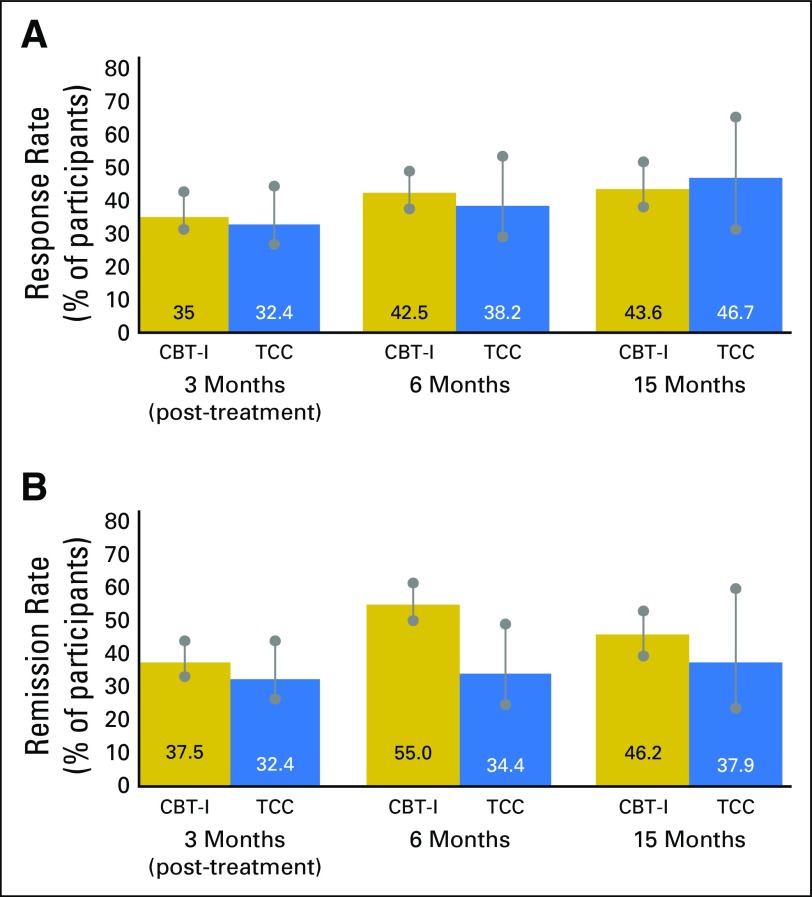
CBT-I and TCC resulted in a similar rate of treatment response at the primary outcome end point, month 15 (43.6% and 46.7%, respectively; P = .82; d = −0.07), with a similar proportion of CBT-I and TCC participants showing treatment response at months 3 (35% and 32.4%, respectively; P = 1.0; d = 0.06) and 6 (42.5% and 38.2%, respectively; P = .82; d = 0.10; Fig 2). Reported effect sizes use the d metric to reflect differences between groups; positive values indicate CBT-I > TCC, negative values TCC > CBT-I. CBT-I and TCC were not substantively different if those lost were considered responders at months 3 (P = 1.0; d = −0.05), 6 (P = .84; d = −0.10), and 15 (P = .29; d = −0.30), or were considered nonresponders at months 3 (P = .82; d = 0.12), 6 (P = .51; d = 0.22), and 15 (P = .66; d = 0.16).
Fig 2.
(A) Treatment response of patients with insomnia (change in Pittsburgh Sleep Quality Index score of ≥ 5 points) at 3 months (post-treatment), 6 months, and 15 months in the cognitive behavioral therapy for insomnia (CBT-I) and Tai Chi Chih (TCC) groups. Rate of response is indicated as a percentage of observed cases. Vertical bars indicate range of response with the assumption that missing cases are all responders or nonresponders. Rate of response was similar between the two groups at month 3 (35% and 32.4%, respectively; P = 1.0; d = 0.06) and month 6 (42.5% and 38.2%, respectively; P = .82; d = 0.10), and at the primary outcome end point, month 15 (43.6% and 46.7%, respectively; P = .82; d = −0.07). Effect sizes are reported using the d metric to reflect differences between groups. Positive values indicate CBT-I > TCC, negative values TCC > CBT-I. (B) Percentage of participants with remission of Diagnostic and Statistical Manual (Fourth Edition, Text Revision; DSM-IV-TR) insomnia at 3 months (postintervention), 6 months, and 15 months in the CBT-I and TCC groups. Insomnia diagnosis was made by clinician interview using DSM-IV-TR criteria. Rate of remission is indicated as a percentage of observed cases. Vertical bars indicate range of remission with the assumption that missing cases are all remitters or nonremitters. Rate of remission was similar between the two groups at 3 months (CBT-I, 37.5%; TCC, 32.47%; P = .81), 6 months (CBT-I, 55%; TCC, 34.4%; P = .10), and 15 months (CBT-I, 46.2%; TCC, 37.9%; P = .62).
With the noninferiority margin equal to 50% of the treatment response, the observed difference between CBT-I and TCC supported noninferiority at the primary outcome end point, month15 (mean 0.52; CI, 2.36; P = .02) and at months 3 (mean 0.84; upper 95% CI, 2.42; P = .02) and 6 (mean 0.42; CI, 1.97; P < .01). A sensitivity analysis was performed that assumed that all patients in the TCC arm who were lost showed no change and were nonresponders, and that all those in the CBT arm who were lost were responders. Estimated difference between CBT-I and TCC supported noninferiority at the primary outcome end point, month15 (mean 1.21; CI, 2.77; P = .05) and at months 3 (mean 0.95; CI, 2.37; P = .01) and 6 (mean 0.86; CI, 2.25; P < .01).
Secondary Outcomes of Sleep
CBT and TCC resulted in a similar rate of insomnia remission at the primary end point, month 15 (46.2% and 37.9%, respectively; P = .62; d = 0.18) and at months 3 (37.5% and 32.4%, respectively; P = .81; d = 0.12) and 6 (55.0% and 34.4%, respectively; P = .10; d = 0.46; Fig 2). The number needed to treat at month 3 was 3.0 (95% CI, 1.9 to 4.2) for CBT-I and 3.0 (95% CI, 2.0 to 4.6) for TCC. CBT-I and TCC were not substantively different if those lost were considered remitters at months 3 (P = 1.0; d = 0.00), 6 (P = .68; d = 0.13), and 15 (P = .68, d = −0.15), or were considered nonremitters at months 3 (P = .65; d = 0.18), 6 (P = .028; d = 0.60), and 15 (P = .18; d = 0.40).
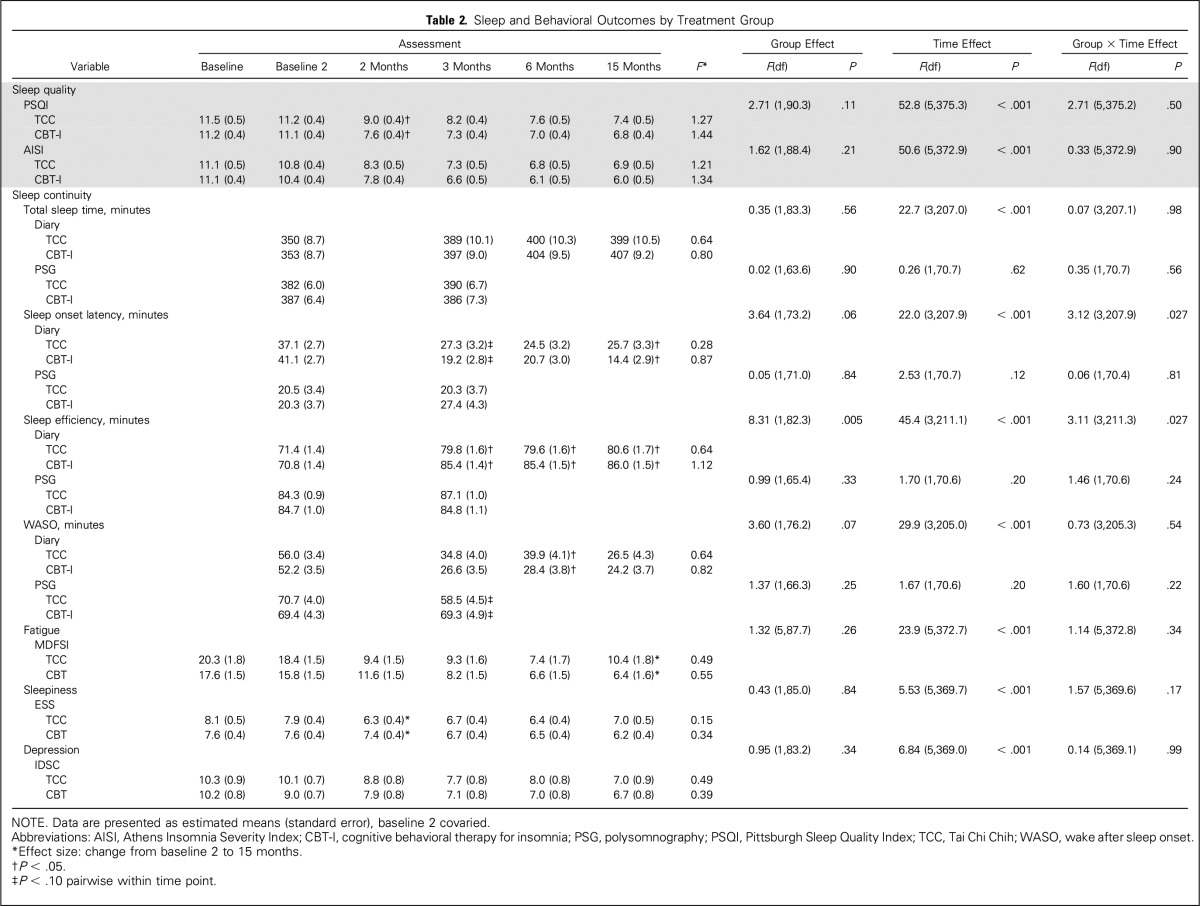
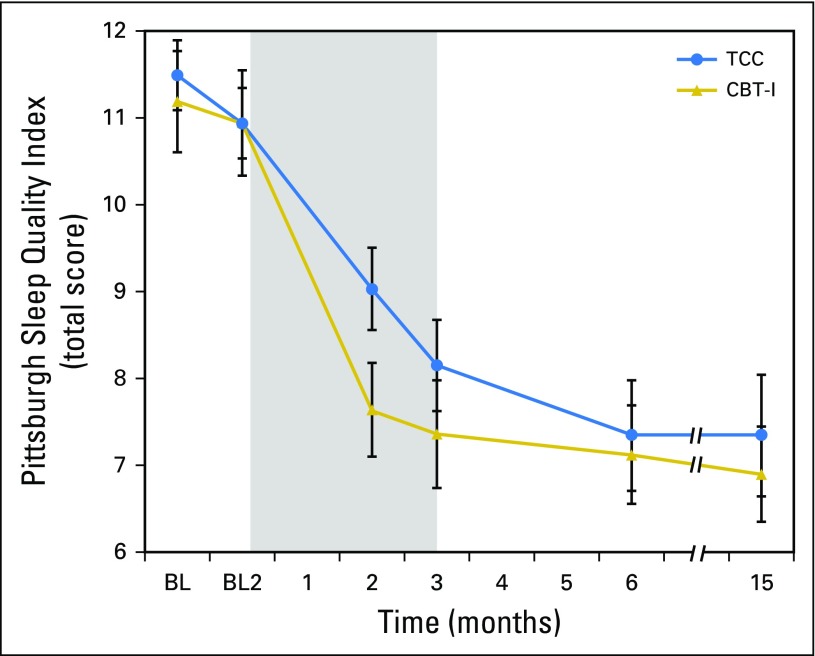
PSQI showed overall treatment effects (P < .001), with large treatment effect sizes for CBT-I and TCC (d = 1.34 and 1.21, respectively; Table 2 and Fig 3) in which mean PSQI scores at month 15 were less than the threshold of clinical sleep disturbance (ie, PSQI < 8 in survivors of cancer).60 AISI showed similar results. Between-group differences in change of PSQI or AISI from baseline to months 3, 6, and 15 were not significant (all P > .3; Table 3). TCC practice time was not correlated with PSQI or AISI at months 3, 6, or 15 (all P < .3).
Table 2.
Sleep and Behavioral Outcomes by Treatment Group
Fig 3.
Change in global sleep quality from baseline to month 15 follow-up in the cognitive behavioral therapy for insomnia (CBT-I) and Tai Chi Chih (TCC) treatment groups. Total scores on the Pittsburgh Sleep Quality Index range from 0 to 21, with higher scores indicating worse sleep quality. Values are means and bars indicate standard error of measurement. Measurements were obtained at baseline 1 (BL1; 2 months before intervention) and baseline 2 (BL2; immediately before intervention), and months 2 (midintervention), 3 (postintervention), and 6 and 15 (follow-up). The numbers of participants evaluated at each time point for each group are as follows: BL1: TCC, n = 45; CBT-I, n = 45; BL2: TCC, n = 45; CBT-I, n = 45; month 2: TCC, n = 38; CBT-I, n = 44; month 3: TCC, n = 38; CBT-I, n = 42; month 6: TCC, n = 35; CBT-I, n = 40; and month 15: TCC, n = 33; CBT-I, n = 40. Shaded area indicates period of exposure to treatment after baseline assessment. Comparisons between BL1 and BL2 were not significant (t377.7 = 0.2; P = .60). Significant pairwise comparisons were found between BL2 and months 2, 3, 6, and 15 (all P < .001). No significant differences were found between CBT-I and TCC at BL1, BL2, months 3, 6, and 15 (all P > .10), but CBT-I and TCC differed at month 2 (P < .02).
Table 3.
Changes by Treatment Group
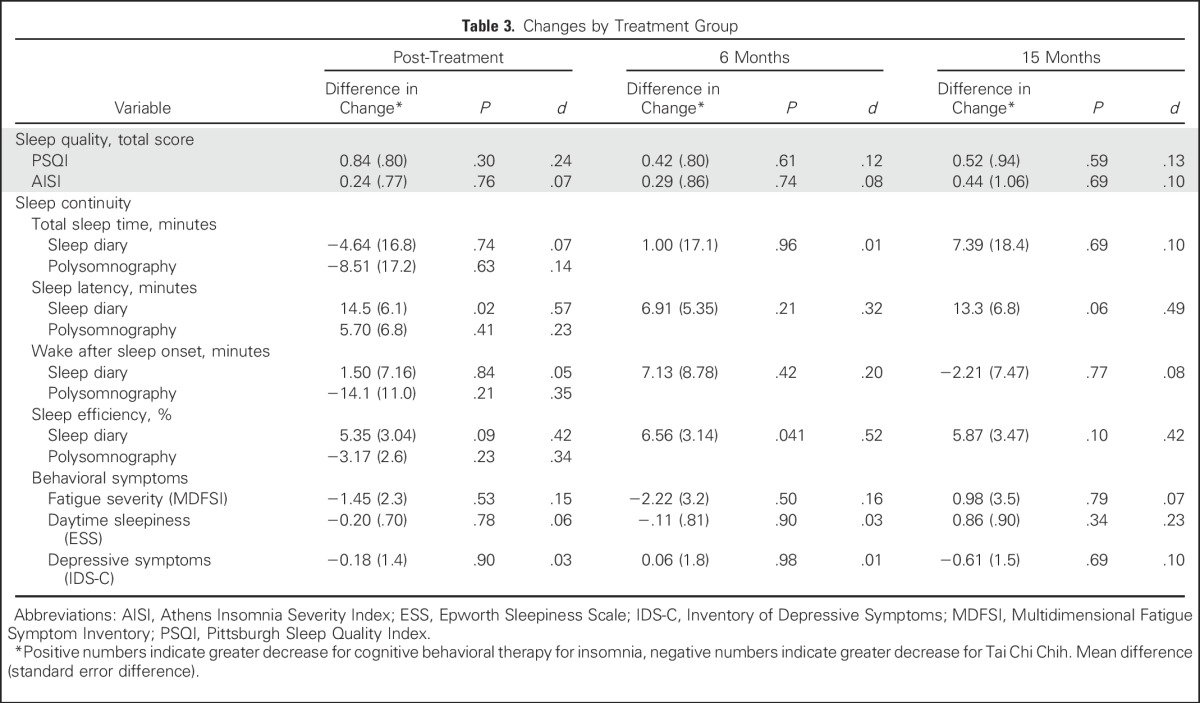
For sleep diaries, there were overall treatment effects for total sleep time, sleep onset latency, sleep efficiency, and wake after sleep onset (all P < .001; Table 2). Between-group differences in change of total sleep time and wake after sleep onset from baseline to months 3, 6, and 15 were not significant (all P > .4), although CBT-I was more likely to reduce sleep onset latency at month 3 (P < .05) and improve sleep efficiency at month 6 (P < .05) compared with TCC (Table 3).
For PSG, no treatment effects were found (all P > .10; Table 2), and there were no between-group differences for any measure (all P > .10; Table 3)
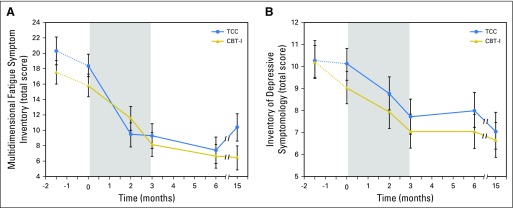
Secondary Outcomes of Fatigue, Daytime Sleepiness, and Depression
For behavioral outcomes of fatigue severity (Fig 4A), daytime sleepiness, and depression (Fig 4B), CBT-I and TCC produced robust improvements (overall treatment effects; all P < .001; Table 2). Between-group differences in change of fatigue, sleepiness, or depression from baseline to months 3, 6, and 15 were not significant (all P > .5; Table 3)
Fig 4.
(A) Change in severity of fatigue symptoms from baseline to month 15 follow-up in the cognitive behavioral therapy for insomnia (CBT-I) and Tai Chi Chih (TCC) treatment groups. Higher scores on the Multidimensional Fatigue Symptom Inventory indicate more fatigue severity. Values are means, and bars indicate standard error of measurement. Measurements were obtained at baseline 1 (BL1), baseline 2 (BL2), and months 2, 3, 6, and 15. The numbers of participants evaluated at each time point for each group are as follows: BL1: TCC, n = 45; CBT-I, n = 45; BL2: TCC, n = 45; CBT-I, n = 45; month 2: TCC, n = 38; CBT-I, n = 44; month 3: TCC, n = 38; CBT-I, n = 42; month 6: TCC, n = 35; CBT-I, n = 40; and month 15: TCC, n = 33; CBT-I, n = 40. Shaded area indicates period of exposure intervention after baseline assessment. Comparisons between BL1 and BL2 were not significant (t374.9 = 1.8; P = .20). Significant pairwise comparisons were found between BL2 and months 2, 3, 6, and 15 (all P < .001). No significant differences were found between CBT-I and TCC at BL1, BL2, months 2, 3, 6, and 15 (all P > .10). (B) Change in clinician-rated severity of depressive symptoms from baseline to month 15 follow-up in the CBT-I and TCC intervention groups. Higher scores on the Inventory of Depressive Symptomology indicate worse depressive symptoms. Values are means, and bars indicate standard error or measurement. Measurements were obtained at BL1, BL2, and months 2, 3, 6, and 15. The numbers of participants evaluated at each time point for each group are as follows: BL1: TCC, n = 45; CBT-I, n = 45; BL2: TCC, n = 45; CBT-I, n = 45; month 2: TCC, n = 38; CBT-I, n = 44; month 3: TCC, n = 38; CBT-I, n = 42; month 6: TCC, n = 35; CBT-I, n = 40; and month 15: TCC, n = 33; CBT-I, n = 40. Shaded area indicates period of administration of intervention after baseline assessment. Comparisons between BL1 and B2 were not significant (t371.5 = 0.7; P = .34). Significant pairwise comparisons were found between BL2 and months 2, 3, 6, and 15 (all P < .01). No significant differences were found between CBT-I and TCC at BL1, BL2, months 2, 3, 6 and 15 (all P > .10).
None of the background variables (Table 1) moderated treatment effects, except nonwhite participants, who showed more improvement for sleep quality with CBT-I than with TCC (P < .04). No adverse events, as monitored by project personnel and reported to the principal investigator and data safety monitoring board, occurred.
DISCUSSION
This randomized, partially blinded, noninferiority trial examined the effects of TCC versus CBT-I on insomnia treatment response and secondary outcomes of insomnia remission and sleep and behavior in survivors of breast cancer with comorbid insomnia. Both interventions yielded robust rates of insomnia treatment response, as indicated by marked clinical improvement, or complete or nearly complete remission of insomnia symptoms that were comparable to reported treatment response for CBT-I in survivors of cancer,7,12,43-46 adults, and older adults.15,21,39,47,61-64 Furthermore, TCC, a mindful movement meditation, was found to be noninferior to CBT-I. Indeed, TCC and CBT-I yielded equivalent rates of insomnia treatment response that were durably maintained over 1 year of follow-up. The noninferiority of TCC relative to CBT-I contrasts with mindfulness-based stress reduction in which noninferiority was found only at 5 months of follow-up.7
Effect sizes of both TCC and CBT-I for improvement in sleep quality and sleep diary measures were large and exceeded mean efficacy of CBT-I for these outcomes in patients diagnosed with cancer, as reported in a recent meta-analysis.12 Furthermore, CBT-I and TCC yielded improvements in sleep outcomes that were at the level of a minimally important difference for insomnia severity,48 with effects comparable to all types of behavioral interventions, including CBT-I and pharmacotherapy.7,12,39,43-46,64-69 Both CBT-I and TCC showed similar rates of insomnia remission and similar improvements in sleep quality, sleep diary measures, and daytime impairments of fatigue, sleepiness, and depressive symptoms. The mechanisms that contribute to the benefit of TCC on insomnia are not known, although this practice has been found to reduce sympathetic arousal70 and inflammation,25-27,71 both of which influence sleep.72-74
This study is characterized by several strengths, including defined eligibility criteria, use of a modified blinded-to-treatment protocol that was intended to reduce selection bias, random assignment, manualized interventions, matching of treatment exposure, and use of a no-treatment lead-in phase to establish stability of insomnia severity before intervention. TCC and CBT-I were equally credible with similar rates of session attendance, which is clinically significant because self-reported motivation to change sleep behaviors and adherence to treatment is one of the best predictors of treatment outcome. Compared with older adults,21 survivors of breast cancer report more severe insomnia complaints and impairments in daytime functioning, which might contribute to motivation and sustained practice of TCC.
This study had several limitations. Women were primarily white and well educated. There was a lack of treatment effect on PSG, which is consistent with prior treatment studies.16,21,44,61,68 A lack of correlation between subjective and objective sleep measures is common and is often interpreted as a consequence of the weak ecologic validity of PSG—that is, only one night in the laboratory—rather than a lack of validity of subjective measures.74 Finally, there was evidence of a differential rate of drop-out in TCC at month 15 as a result, in part, of reported difficulties adhering to TCC practice outside of sessions, although tests of noninferiority remained robust when taking this into account.
This investigation demonstrates that a mindful movement meditation, TCC, was noninferior to CBT-I for long-term treatment of insomnia in survivors of breast cancer. Given that standardized TCC is both scalable and community accessible compared with the limited availability of CBT in most medical centers, immediate access to TCC would address the need to reduce the morbidity associated with insomnia in survivors of breast and other cancers with treatment benefits commensurate with the status quo of clinical treatment approaches to insomnia.
Footnotes
Supported by National Institutes of Health Grants No. R01-AG034588, R01-AG026364, R01 CA160245-01, CA195637-01, and R01-CA119159 (to M.R.I.), and the Cousins Center for Psychoneuroimmunology.
Clinical trial information: NCT00690196.
AUTHOR CONTRIBUTIONS
Conception and design: Michael R. Irwin, Richard Olmstead
Collection and assembly of data: Michael R. Irwin, Richard Olmstead, Carmen Carrillo, Nina Sadeghi, Perry Nicassio
Data analysis and interpretation: Michael R. Irwin, Richard Olmstead, Patricia A. Ganz, Julienne E. Bower
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tai Chi Chih Compared With Cognitive Behavioral Therapy for the Treatment of Insomnia in Survivors of Breast Cancer: A Randomized, Partially Blinded, Noninferiority Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Michael R. Irwin
No relationship to disclose
Richard Olmstead
Employment: Boston Scientific (I)
Stock or Other Ownership: Boston Scientific (I)
Carmen Carrillo
No relationship to disclose
Nina Sadeghi
No relationship to disclose
Perry Nicassio
No relationship to disclose
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock or Other Ownership: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Merganser Biotech (I), Teva Pharmaceuticals, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Honoraria: Biogen Idec (I)
Consulting or Advisory Role: Keryx (I), Merganser Biotech (I), Silarus Therapeutics (I), InformedDNA, Eli Lilly, Gilead Sciences (I), Akebia (I)
Research Funding: Keryx (I)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease (I), Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I), Keryx (I)
Julienne E. Bower
No relationship to disclose
REFERENCES
- 1.American Psychiatric Association . DSM-5 Task Force: Diagnostic and Statistical Manual of Mental Disorders. ed 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Savard J, Villa J, Ivers H, et al. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 3.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Irwin MR. Depression and insomnia in cancer: Prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15:404. doi: 10.1007/s11920-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. doi: 10.1002/pon.2004. Sharma N, Hansen CH, O’Connor M, et al: Sleep problems in cancer patients: Prevalence and association with distress and pain. Psychooncology 21:1003-1009, 2012 [Erratum: Psychooncology 22:1198, 2013] [DOI] [PubMed] [Google Scholar]
- 7.Garland SN, Carlson LE, Stephens AJ, et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 8.Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann Intern Med. 2015;163:191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 10.Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 12.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25:791–800. doi: 10.1093/annonc/mdt506. [DOI] [PubMed] [Google Scholar]
- 14.Morin CM. Cognitive behavioral therapy for chronic insomnia: State of the science versus current clinical practices. Ann Intern Med. 2015;163:236–237. doi: 10.7326/M15-1246. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 17.Stone JF. Tai Chi Chih, Joy Through Movement. Albuquerque, NM: Good Karma Publishing; 1996. [Google Scholar]
- 18.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Fisher KJ, Harmer P, et al. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: A randomized controlled trial. J Am Geriatr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 20.Raman G, Zhang Y, Minichiello VJ, et al. Tai Chi improves sleep quality in healthy adults and patients with chronic conditions: A systematic review and meta-analysis. J Sleep Disord Ther. 2013;141:2–6. doi: 10.4172/2167-0277.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGianni LM, Garber JE, Winer EP.Complementary and alternative medicine use among women with breast cancer J Clin Oncol 20: 34S–38S.2002suppl 18 [PubMed] [Google Scholar]
- 23.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: A systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Schmid CH, Rones R, et al. A randomized trial of Tai Chi for fibromyalgia. N Engl J Med. 2010;363:743–754. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MR, Olmstead R, Breen EC, et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. J Natl Cancer Inst Monogr. 2014;2014:295–301. doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: A randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20:764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin MR, Olmstead R, Breen EC, et al. Cognitive behavioral therapy and Tai Chi reverse cellular and genomic markers of inflammation in late-life insomnia: A randomized controlled trial. Biol Psychiatry. 2015;78:721–729. doi: 10.1016/j.biopsych.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: A randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–517. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 29.Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: A randomized controlled trial. Am J Geriatr Psychiatry. 2011;19:839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campo RA, Agarwal N, LaStayo PC, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8:60–69. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin MR, Olmstead RE, Ganz PA, et al. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;30(suppl):S58–S67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. ed 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 35.American Academy of Sleep Medicine . The International Classification of Sleep Disorders: Diagnostic and Coding Manual. ed 2. Darien, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 36.Beloosesky Y, Weiss A, Mansur N. Validity of the Medication-based Disease Burden Index compared with the Charlson Comorbidity Index and the Cumulative Illness Rating Scale for geriatrics: A cohort study. Drugs Aging. 2011;28:1007–1014. doi: 10.2165/11597040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65(suppl 16):33–40. [PubMed] [Google Scholar]
- 38.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 39.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Nicassio PM, Mendlowitz DR, Fussell JJ, et al. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behav Res Ther. 1985;23:263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 41.Borkovec T, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 42.Cole JC, Motivala SJ, Buysse DJ, et al. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 43.Harvey AG, Bélanger L, Talbot L, et al. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: A randomized controlled trial. J Consult Clin Psychol. 2014;82:670–683. doi: 10.1037/a0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiorentino L, McQuaid JR, Liu L, et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: A randomized controlled crossover pilot study. Nat Sci Sleep. 2010;2:1–8. doi: 10.2147/NSS.S8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savard J, Ivers H, Savard MH, et al. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37:1305–1314. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savard J, Simard S, Ivers H, et al. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23:6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 48.Matthews EE, Berger AM, Schmiege SJ, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: A randomized, controlled trial. Oncol Nurs Forum. 2014;41:241–253. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 49.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 50.Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 51.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 53.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 54.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert AL, Lee J, Ma M, et al. Comparison of subjective and objective measures of sedentary behavior using the Yale Physical Activity Survey and accelerometry in patients with rheumatoid arthritis. J Phys Act Health. 2016;13:371–376. doi: 10.1123/jpah.2015-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harada ND, Chiu V, King AC, et al. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Blom K, Tarkian Tillgren H, Wiklund T, et al. Internet-vs. group-delivered cognitive behavior therapy for insomnia: A randomized controlled non-inferiority trial. Behav Res Ther. 2015;70:47–55. doi: 10.1016/j.brat.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Mascha EJ, Sessler DI. Equivalence and noninferiority testing in regression models and repeated-measures designs. Anesth Analg. 2011;112:678–687. doi: 10.1213/ANE.0b013e318206f872. [DOI] [PubMed] [Google Scholar]
- 59.Tzeng JI, Fu YW, Lin CC. Validity and reliability of the Taiwanese version of the Pittsburgh Sleep Quality Index in cancer patients. Int J Nurs Stud. 2012;49:102–108. doi: 10.1016/j.ijnurstu.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carney CE, Segal ZV, Edinger JD, et al. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–260. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 62.Krystal AD, Erman M, Zammit GK, et al. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: A 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: A meta-analysis of treatment efficacy. JAMA. 1997;278:2170–2177. [PubMed] [Google Scholar]
- 64.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 65.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 66.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34:E51–E59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 67.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 68.Ritterband LM, Bailey ET, Thorndike FP, et al. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21:695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motivala S, Sollers J, Thayer DT, et al. Tai Chi Chih acutely decreases sympathetic outflow in older adults J Gerontol A Biol Sci Med Sci 611177–1180., 2005 [DOI] [PubMed] [Google Scholar]
- 70.Black DS, Irwin MR, Olmstead R, et al. Tai chi meditation effects on nuclear factor-κB signaling in lonely older adults: A randomized controlled trial. Psychother Psychosom. 2014;83:315–317. doi: 10.1159/000359956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Irwin MR, Opp MR. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42:129–155. doi: 10.1038/npp.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Ann Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19:478–486. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]