Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) may persist after treatment ends and may lead to functional decline and falls. This study compared objective and self-report measures of physical function, gait patterns, and falls between women cancer survivors with and without symptoms of CIPN to identify targets for functional rehabilitation.
Methods
A secondary data analysis of 512 women cancer survivors (age, 62 ± 6 years; time since diagnosis, 5.8 ± 4.1 years) categorized and compared women self-reporting symptoms of CIPN (CIPN+) with asymptomatic women (CIPN−) on the following: maximal leg strength, timed chair stand, physical function battery, gait characteristics (speed; step number, rate, and length; base of support), self-report physical function and disability, and falls in the past year.
Results
After an average of 6 years after treatment, 47% of women still reported symptoms of CIPN. CIPN+ had significantly worse self-report and objectively measured function than did CIPN−, with the exception of maximal leg strength and base of support during a usual walk. Gait was slower among CIPN+, with those women taking significantly more, but slower and shorter, steps than did CIPN− (all P < .05). CIPN+ reported significantly more disability and 1.8 times the risk of falls compared with CIPN− (P < .0001). Increasing symptom severity was linearly associated with worsening function, increasing disability, and higher fall risk (all P < .05).
Conclusion
This work makes a significant contribution toward understanding the functional impact of CIPN symptoms on cancer survivors. Remarkably, 47% of women in our sample had CIPN symptoms many years after treatment, together with worse function, greater disability, and more falls. CIPN must be assessed earlier in the clinical pathway, and strategies to limit symptom progression and to improve function must be included in clinical and survivorship care plans.
INTRODUCTION
Aggressive treatments for cancer have improved survival but often cause serious long-term effects on daily life that last for many years. Cancer survivors report more functional limitations, including reduced mobility, compared with persons with no cancer history,1 and functional declines are linked to shorter survival.2 Falls share overlapping causes with functional declines and are another significant concern for cancer survivors. The risk of falls increases after a cancer diagnosis when compared with prediagnosis fall rates,3 and fall rate in cancer survivors can be twice that of cancer-free peers4 or community-dwelling older adults.5
A persistent cancer treatment–related symptom that may influence physical function, fall risk, and quality of life (QOL) is chemotherapy-induced peripheral neuropathy (CIPN). Neuropathies develop from nerve damage caused by cytotoxic chemotherapies and result in motor and sensory impairments and symptoms of sensory loss in hands and feet, burning, tingling, and pain.5 CIPN may occur in up to 90% of patients during chemotherapy4 and can persist in a proportion of survivors in the long term.6,7 CIPN is consistently associated with lower self-report physical function and QOL during or after chemotherapy.8 In older adults9 and in patients with diabetes,10,11 neuropathies are associated with poor mobility, balance impairments, and falls. Unfortunately, studies of physical function in people with CIPN have relied on self-report instruments that are subject to bias, do not identify the underlying causes of functional limitations, and are poor at detecting declines early on. Fall risk may be elevated in persons with CIPN, but studies are few and are limited by short recall periods or reliance on self-report measures of fall risk factors.12,13 A study of CIPN that includes objective measures of physical function and fall risk, together with patient-reported measures, would provide new information for oncology teams to potentially screen for referral to rehabilitation teams. Tailoring rehabilitation strategies to best prevent the disability and falls associated with CIPN could improve patient safety and enhance survivorship care plans for those receiving neurotoxic chemotherapies.
To address this need, we conducted a secondary data analysis of a pooled sample of women after cancer treatment. The purpose of this study was to (1) determine the prevalence of CIPN symptoms reported by women after chemotherapy; (2) compare objective and patient-reported measures of physical function, disability, and falls between women cancer survivors with and without CIPN symptoms; and (3) examine the relationship between CIPN symptom severity and outcomes.
METHODS
Sample and Setting
We analyzed the baseline data of women cancer survivors enrolled in four clinical exercise intervention trials (ClinicalTrials.gov identifiers: NCT01635413, NCT00662103, NCT00591747, and NCT00659906).14-16Common eligibility criteria included having a cancer diagnosis with no CNS involvement, being older than 50 years of age, being insufficiently active (< 60 minutes of moderate-intensity exercise per week), being free of metastatic disease and neurologic conditions, being ambulatory, and having medical clearance to exercise. Eligible women completed a baseline visit at Oregon Health & Science University that included self-report questionnaires and performance tests. The university’s institutional review board approved all study protocols, and the women consented to participate before testing.
Assessment of CIPN Symptoms
Symptoms associated with CIPN were determined by self-report of the presence or absence of noticeable dysthesia or parasthesia in the lower extremities. On the basis of the sensory neuropathy items in the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity,17 participants were asked if they experienced numbness, tingling, or discomfort in their feet in the past week and were then categorized into one of two groups: symptomatic (CIPN+) or asymptomatic (CIPN−). Women reporting symptoms were asked if the symptoms began during or after chemotherapy, and they were also asked to report the severity of symptoms on a four-point scale: 1 = a little bit, 2 = somewhat, 3 = quite a bit, 4 = very much. Because our study focused on mobility and falls, we assessed only symptoms in the lower extremities and only for sensory symptoms, which are the most common and distinguishable symptoms of CIPN compared with other symptoms (eg, weakness)18 that could be related to other treatment or age-related impairments (eg, sarcopenia). We could not verify a medical diagnosis of CIPN, nor could we confirm which women had received chemotherapy known to cause CIPN.
Demographics, Health, and Clinical History
Self-report prevalence of chronic medical conditions was obtained by the Charlson comorbidity index,19 physical activity levels with the Community Healthy Activities Model Program for Seniors questionnaire,3 and cancer history and demographics with an in-house questionnaire.
Objectively Measured Outcomes
Lower-extremity maximal strength.
Lower-extremity maximal strength was evaluated by a one-repetition maximal leg press test (kilograms) using standard protocols.20 This test determines the maximal amount of weight a woman can push a single time with her legs from a seated upright position.
Objective physical function.
Objective physical function was assessed by the short physical performance battery (sPPB) consisting of the summed score (0 to 12) from three timed tests: five-time chair stand, standing balance, and 4-meter usual walk speed21; low scores predict activities of daily living disability, hospitalization, nursing home admission, and mortality.21-24 Chair stand time and walk speed were also evaluated separately because these tests independently predict poor outcomes in older adults. Minimal clinically important differences are 0.5 for PPB and 0.1 meters per second for gait speed25,26, whereas chair stand time ≥ 12 seconds predicts a 2.4-fold increased risk of falls in older adults.27
Characteristic gait speed and patterns.
In addition to habitual walking speed, abnormalities in gait (stepping) patterns can predict poor function and fall risk.28 We measured characteristic gait speed and gait patterns by having participants walk on an electronic walkway (GAITRite; CIR System, Sparta, NJ). Gait patterns included step number, rate, and length; stride length; base of support; and percentage of time in single support and double support phases of the gait cycle.
Patient-Reported Outcomes
Physical function and mobility disability.
Perceived physical function and mobility disability were measured with the valid and reliable Late-Life Function and Disability Instrument, which contains subscales of function including basic and advanced lower-extremity function and disability.29,30 Scales on all three instruments range from 0 to 100, with higher scores indicating better function or less disability.
Falls.
A fall was defined as unintentionally coming to rest on the ground or at some other lower level, not as a result of a major intrinsic event (eg, stroke or syncope) or an overwhelming hazard.31 Participants indicating a fall were asked about any resultant injury (ie, fractures, head injuries, sprains, bruises, scrapes, or joint injuries) or need to seek medical care.31 Recall periods for falls assessment varied slightly across studies; women were asked if they experienced a fall in the past 6 months15 or in the past year.14,16 We collapsed data into a single assessment of falls in the past year.
Analysis
Standard t tests and χ2 tests were used to compare clinical and demographic variables between groups, and variables that differed significantly were controlled for in subsequent analyses. Logistic and linear regression models were used to examine the association between neuropathy symptoms and outcomes. Linear regression coefficients and odds ratios (with 95% CIs) were used to quantify effect size for the linear and logistic regression models, respectively. We also applied the Hochberg adjustment to fully adjusted models to account for potential false discovery rates.32 Because gait characteristic variables are highly interdependent, we included only walk speed as an outcome when adjusting P values.
RESULTS
Sample
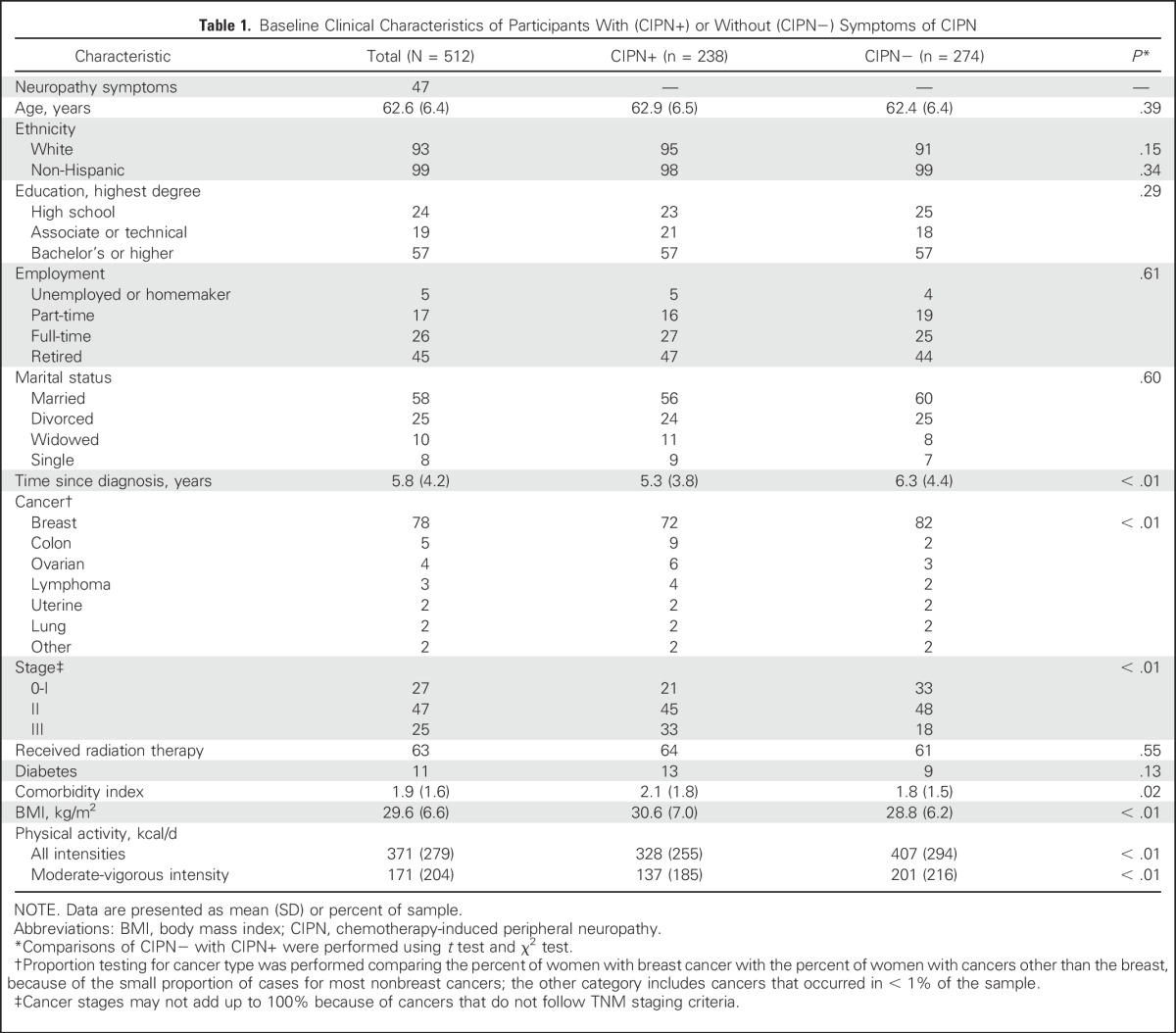
Women were older (63 ± 6 years), obese (body mass index [BMI], 29.6 ± 6.6 kg/m2), had low comorbidity scores (1.9 ± 1.6) and a low prevalence of diabetes (11%), and were diagnosed 3 months to 33 years before enrollment (, 6 years; Table 1). Forty-seven percent of the sample reported that they currently experienced sensory loss in their lower extremities. Women with neuropathy symptoms (CIPN+) were significantly more likely than were asymptomatic women (CIPN−) to be closer to their cancer diagnosis, to have been diagnosed with stage II or III cancer, to have been treated for a cancer other than breast cancer, to be obese, to be less physically active, and to have worse comorbidities. Groups were similar in terms of age, ethnicity, employment, education, marital status, diabetes prevalence, and past radiation therapy.
Table 1.
Baseline Clinical Characteristics of Participants With (CIPN+) or Without (CIPN−) Symptoms of CIPN
Objectively Measured Outcomes
Linear regression models were used to determine associations between CIPN symptoms and outcomes, serially adjusting for the group differences identified in Table 1. Model 1 was completely unadjusted, with models 2, 3, and 4 accounting for the additional influence of clinical characteristics, comorbidities, and lifestyle factors (BMI plus physical activity), respectively. In unadjusted models, symptomatic women had significantly worse performance on functional tests compared with asymptomatic women (sPPB, chair stand, and characteristic gait, all P < .01) but had similar maximal leg strength. For characteristic gait, symptomatic women took significantly more, but shorter and slower, steps, had shorter strides, and spent more time in stance and double support phases of the gait cycle than did asymptomatic women (all P < .05). The abnormal gait pattern observed in symptomatic women is known as a conservative gait pattern and is also seen in persons with diabetic peripheral neuropathy.33,34 Significant differences remained in fully adjusted models, with the exception of gait characteristics, where significance was lost with final adjustment for BMI and physical activity.
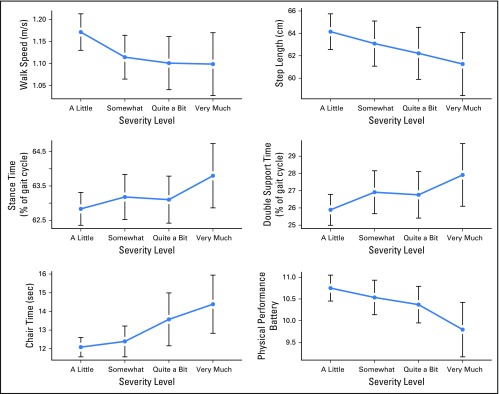
Increasing CIPN symptom severity was linearly associated with slower chair stand times, slower walking speeds, and worse sPPB scores (P < .05; Appendix Table A1, online-only). Walking speed slowed more when symptoms progressed from mild to moderate, whereas chair stand and PPB scores were lowest when symptoms were moderate to severe (Fig 1). Increasing symptom severity resulted in increasingly worsening gait patterns (P < .05), with changes apparent when severity progressed from mild to moderate (Fig 1).
Fig 1.
Effect of increasing chemotherapy-induced peripheral neuropathy symptom severity on objectively measured outcomes. m/s; meters per second.
Patient-Reported Outcomes
In both unadjusted and adjusted models, symptomatic women reported significantly lower levels of basic and advanced lower-extremity function and overall physical function and less ability to perform activities of daily living independently, indicating greater mobility disability than asymptomatic women (P < .01). Increasing symptom severity was also linearly associated with declining physical function and increasing mobility disability (P < .01; Appendix Table A1). Physical function declined proportionately with worsening symptoms, whereas disability worsened linearly with moderate severity, but then reached a nadir (Fig 2).
Fig 2.
Effect of increasing chemotherapy-induced peripheral neuropathy symptom severity on patient-reported outcomes.
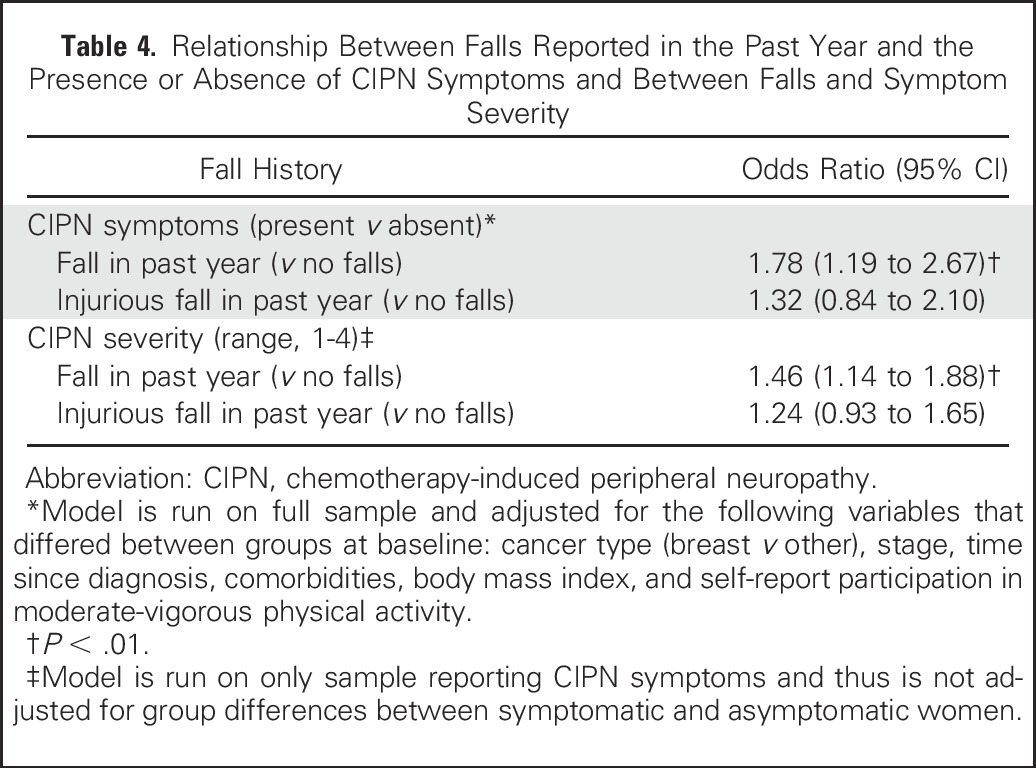
A greater proportion of symptomatic women reported falling in the past year (57%; mean number of falls per year, 0.7; median, 0; range, 0 to 20 falls) compared with asymptomatic women (43%; mean number of falls per year, 0.3; median, 0; range, 0 to 10 falls; Table 2). Having CIPN symptoms significantly increased the odds of falling in the past year compared with being symptom free, even after adjusting for all covariates (P < .01; Table 3). Symptomatic women were 1.8 times more likely to report a fall than were asymptomatic women. Increasing symptom severity increased the odds of falling by 1.5 times with each unit increase in severity (P < .01; Table 4), but mostly when symptoms were moderate or severe (Fig 2). The odds of experiencing an injurious fall were elevated in symptomatic women and with increasing symptom severity, although neither significantly so (Table 4).
Table 2.
Comparison of Means for Women With (CIPN+) or Without (CIPN−) Symptoms of CIPN on Outcome Measures
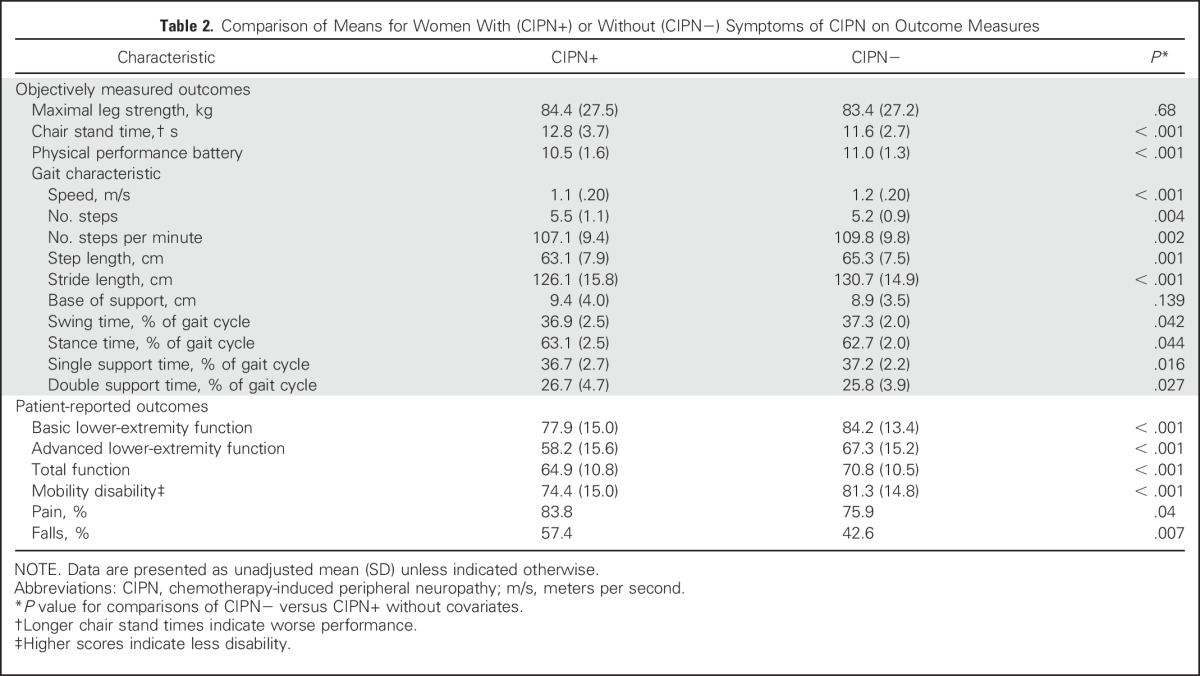
Table 3.
Regression Coefficients and 95% CIs Comparing Women With CIPN Symptoms With Asymptomatic Women (N = 512)

Table 4.
Relationship Between Falls Reported in the Past Year and the Presence or Absence of CIPN Symptoms and Between Falls and Symptom Severity
Significance remained with Hochberg adjustment for all outcomes, with the exception of group differences for chair stand, where significance weakened (P = .08).
DISCUSSION
Among our sizeable sample of women cancer survivors, we observed that nearly one half still experienced sensory symptoms associated with CIPN many years after completing chemotherapy. Women with persistent CIPN symptoms performed worse on several objective tests of physical function and reported poorer functioning, more disability, and nearly twice the rate of falls compared with asymptomatic women. Outcomes worsened with increasing symptom severity. To our knowledge, this is the first study to compare fall rates and both objectively measured and patient-reported physical function among women with and without persistent symptoms of CIPN. Our findings suggest that CIPN symptoms remain a significant and potentially life-threatening problem for cancer survivors well beyond completion of their chemotherapy and close to the time that many will transition out of oncologic care.
Women with CIPN symptoms were 1.8 times more likely to experience a recent fall than were asymptomatic women, even after adjustment for covariates. Nearly one third of persons older than 65 years of age experience one or more falls every year.35 Despite their younger age, the rate of falls in symptomatic women in our study exceeded the rate in the general older adult population by 24%. Our findings corroborate a recent study in which post-treatment breast cancer survivors who reported moderate to severe CIPN symptoms had a 2.3 times greater fall risk than did asymptomatic women,7 and a short prospective study that detected an increase in fall risk with increasing CIPN symptoms over 2 months of neurotoxic chemotherapy.36 Our data extend these findings to the broader population of women cancer survivors whose CIPN symptoms persist years beyond treatment completion and elevate fall risk.
Women with persistent symptoms reported significantly worse physical function and more mobility disability than did asymptomatic women. Differences remained after adjusting for clinical characteristics and comorbidities, suggesting that CIPN symptoms were not merely a proxy for worse disease and health status. CIPN is linked to poorer health-related QOL8 and worse self-report physical functioning using subscales of QOL instruments.12,37 Our study used patient-reported instruments predictive of poor outcomes in older adults38 and found meaningful group differences—symptomatic women had more difficulty in tasks ranging from recreational activities to tasks necessary for independent functioning than did asymptomatic women. To our knowledge, we are also the first to study falls and CIPN symptoms using objective measures that are sensitive and specific indicators of functional mobility problems and fall risk and are predictive of morbidity and mortality in older adults.39-42 Symptomatic women had significantly lower scores for PPB and gait speed than did asymptomatic women, and group differences were clinically meaningful25; however, absolute scores for both groups were above that predictive of poor outcomes in older adults,22,39,40 which may reflect the younger age of our sample. In contrast, a measure of functional strength that requires patients to stand repeatedly from a chair was abnormal in symptomatic women, with their average score indicating increased fall risk.27 The inability to stand quickly during this performance test may be attributable to poor sensorimotor feedback from the feet rather than to muscle weakness, consistent with observations that sensory impairment is more common than motor impairment in CIPN18 and our findings of similar maximal leg strength in symptomatic and asymptomatic women. Current exercise guidelines for cancer survivors recommend general muscle-strengthening exercises for overall conditioning,43 but our findings suggest this approach may be insufficient to prevent functional declines, disability, and falls in survivors with persistent CIPN symptoms, who would benefit from a more task-specific and functionally oriented approach to strength and mobility training.
Results from the gait analysis indicate that even after adjustment for clinical characteristics and comorbidities, women with CIPN symptoms displayed an abnormal gait pattern in which they walked more slowly, took shorter steps, and spent more time in the standing phases of gait to maintain stability while walking. This conservative gait pattern, which is different from that of normal walking,44 is associated with fall risk in aging and diabetic peripheral neuropathy40-42 and stems from decreased proprioceptive sensory feedback to the lower extremities, ankle weakness, and foot pain.33 The significance between groups in terms of gait patterns disappeared after final adjustment for BMI and physical activity, indicating a potential interplay between these factors and CIPN symptoms on gait that is worth additional study.
In our sample, increasing symptom severity was strongly and linearly associated with worsening objective and subjective function and increasing patient-reported disability and falls. Gait instability may worsen early when symptoms progress from mild to moderate, whereas functional strength declines as symptoms become more severe. This pattern may reflect the additional motor impairment that compounds sensory impairment with more severe CIPN.5 Although falls did not seem to be elevated above age-expected rates in women with mild CIPN symptoms, fall risk increased sharply as symptoms progressed from mild to moderate and moderate to severe. Our findings point to the importance of early detection and management of CIPN to limit the progression of symptoms before they affect function and also concomitantly to timely referral to rehabilitation services to learn compensatory strategies that preserve function and prevent disability and falls, even if symptoms progress.
Our study had limitations, including the cross-sectional design that prevents establishing cause and effect between CIPN symptoms and study outcomes and the use of self-report measures for clinical history. Our assessment of CIPN was limited to self-report of sensory symptoms, rather than being based on an objective neurologic examination. However, Huang et al17 reported that a reduced four-item scale of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity that assesses only sensory neuropathy is sufficient to separate groups relative to clinically relevant CIPN, so it is likely that we accurately classified our sample on the basis of self-report symptoms.17 In our community-based sample, we could not verify that participants received a chemotherapy agent known to cause CIPN, so symptoms could be related to a different condition; however, women confirmed that symptoms began after chemotherapy and were thus less likely to be related to other causes (ie, diabetes). Our data may or may not generalize to male cancer survivors, although sex is not a known risk factor for CIPN.6
Our findings suggest that a high proportion of women treated with chemotherapy for a variety of cancers may experience persistent symptoms of CIPN many years after their treatment ends and that these women have abnormal gait patterns and more functional deficits, patient-reported disability, and falls compared with women with no symptoms. Falls are disabling and life threatening and lead to health care costs of approximately $17,000 per event,35 and poor self-report physical function has been linked to shorter survival times in cancer survivors.2 CIPN contributes to excess health care costs, including those associated with hospitalizations, neurology specialist visits, and outpatient visits, and may influence return to work, adding to the economic and societal burden of cancer.45 Currently, clinical practice guidelines focus on pharmacologic management of CIPN,46 which remains suboptimal, and on the use of patient report to identify dose-limiting toxicities during therapy, which miss detecting long-term functional deficits and associated disability. Using standardized clinical tests and patient-reported outcomes validated in older adults, we determined that persistent CIPN symptoms may have serious functional consequences. Thresholds for early detection of functional declines related to CIPN must be established and functional screening subsequently integrated into the clinical pathway of patients receiving neurotoxic treatment. Rehabilitation focused on strength and balance training is known to prevent disability and falls in older adults and in persons with neurologic conditions47-49 and may be a reasonable initial recommendation for persons with CIPN.50 However, our data suggest that the etiology of disability and falls associated with CIPN symptoms may be unique; thus, efforts to define, implement, and evaluate the efficacy and cost effectiveness of specific prevention strategies for persons with CIPN are urgently needed.
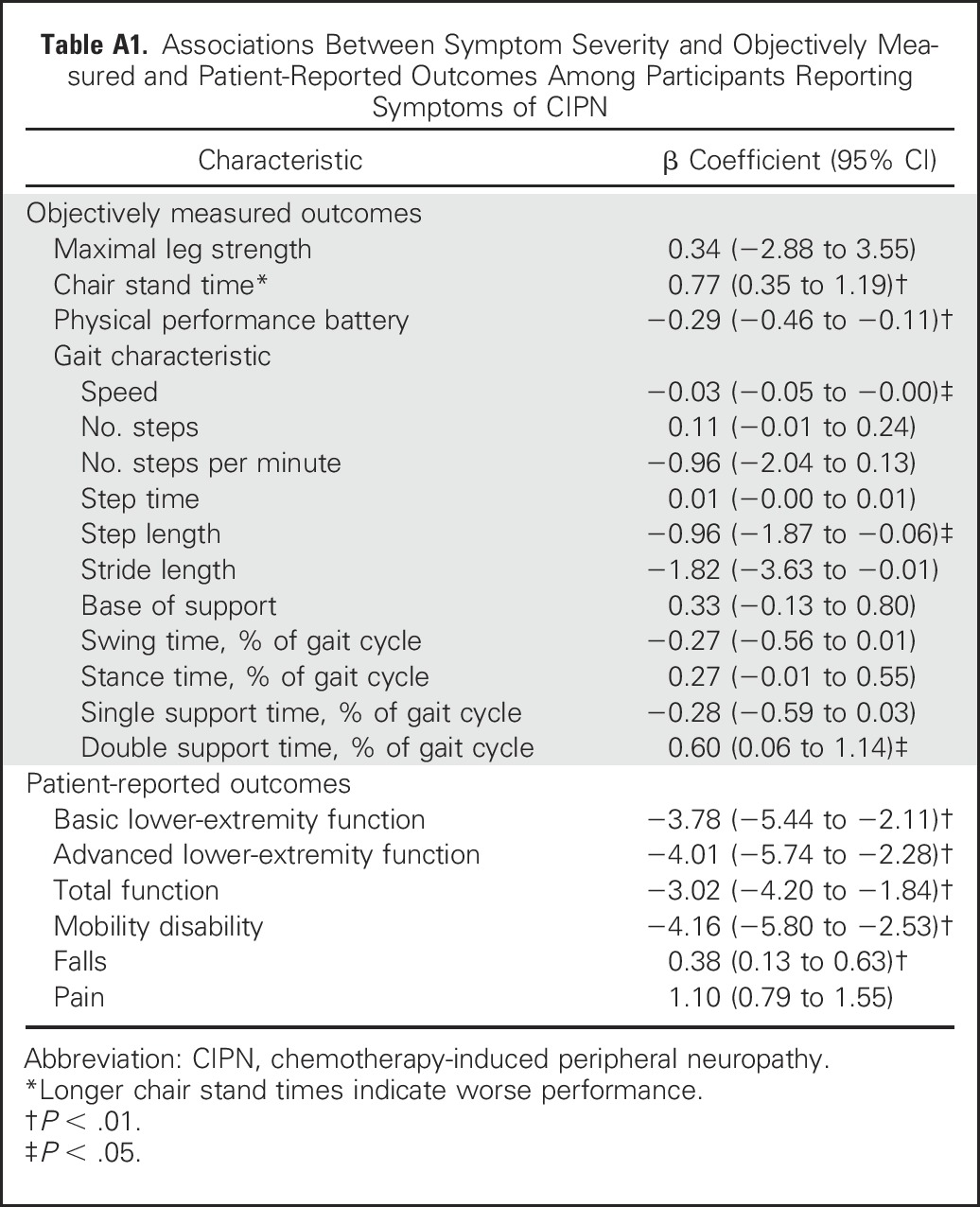
Appendix
Table A1.
Associations Between Symptom Severity and Objectively Measured and Patient-Reported Outcomes Among Participants Reporting Symptoms of CIPN
Footnotes
Supported in part by National Institutes of Health Grants P30 CA069533 and R01 CA163474.
See accompanying Editorial on page 2593
AUTHOR CONTRIBUTIONS
Conception and design: Kerri M. Winters-Stone, Nathan F. Dieckmann
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kerri M. Winters-Stone
No relationship to disclose
Fay Horak
Employment: APDM
Stock or Other Ownership: APDM
Peter G. Jacobs
Stock or Other Ownership: MotioSens
Patents, Royalties, Other Intellectual Property: 2003 US Patent No. 6558321: Systems and methods for remote monitoring and modulation of medical devices; 2012 US Patent No. 8317700: Method and device for non-invasive analyte measurement; 2011 US Patent No. 7976466: Use of multiple data points and filtering in an analyte sensor; 2011 US Patent No. 8810388: Position tracking and mobility assessment system; 2013 US Patent No. 9480418: Systems and methods for hearing loss screening and monitoring
Phoebe Trubowitz
No relationship to disclose
Nathan F. Dieckmann
No relationship to disclose
Sydnee Stoyles
No relationship to disclose
Sara Faithfull
Other Relationship: Prostate Cancer Charity UK
REFERENCES
- 1.Sweeney C, Schmitz KH, Lazovich D, et al. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98:521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 2.Brown JC, Harhay MO, Harhay MN. Patient-reported versus objectively-measured physical function and mortality risk among cancer survivors. J Geriatr Oncol. 2016;7:108–115. doi: 10.1016/j.jgo.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad M, Tafani C, Psimaras D, et al. Chemotherapy-induced peripheral neuropathy in the adult. Curr Opin Oncol. 2014;26:634–641. doi: 10.1097/CCO.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 4.Beijers A, Mols F, Dercksen W, et al. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12:401–406. doi: 10.12788/jcso.0086. [DOI] [PubMed] [Google Scholar]
- 5.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159:327–333. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mols F, Beijers T, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer. 2014;22:2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor AB. Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Macgilchrist C, Paul L, Ellis BM, et al. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010;27:162–168. doi: 10.1111/j.1464-5491.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 10.Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318. doi: 10.3122/jabfm.17.5.309. [DOI] [PubMed] [Google Scholar]
- 11.Gewandter JS, Fan L, Magnuson A, et al. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): A University of Rochester CCOP study. Support Care Cancer. 2013;21:2059–2066. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters-Stone KM, Dobek J, Nail L, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: A randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winters-Stone KM, Li F, Horak F, et al. Comparison of tai chi vs. strength training for fall prevention among female cancer survivors: Study protocol for the GET FIT trial. BMC Cancer. 2012;12:577. doi: 10.1186/1471-2407-12-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loprinzi PD, Cardinal BJ, Si Q, et al. Theory-based predictors of follow-up exercise behavior after a supervised exercise intervention in older breast cancer survivors. Support Care Cancer. 2012;20:2511–2521. doi: 10.1007/s00520-011-1360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HQ, Brady MF, Cella D, et al. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: A gynecologic oncology group study. Int J Gynecol Cancer. 2007;17:387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 17.Argyriou AA, Kyritsis AP, Makatsoris T, et al. Chemotherapy-induced peripheral neuropathy in adults: A comprehensive update of the literature. Cancer Manag Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 20. American College of Sports Medicine: ACSM’s Guidelines for Exercise Testing and Prescription (ed 7). Philadelphia, PA, Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 25.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J Eval Clin Pract. 2014;20:295–300. doi: 10.1111/jep.12158. [DOI] [PubMed] [Google Scholar]
- 27.Tiedemann A, Shimada H, Sherrington C, et al. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 28.den Ouden MEM, Schuurmans MJ, Arts IEMA, et al. Physical performance characteristics related to disability in older persons: A systematic review. Maturitas. 2011;69:208–219. doi: 10.1016/j.maturitas.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 30.Haley SM, Jette AM, Coster WJ, et al. Late life function and disability instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 31.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 33. doi: 10.1155/2016/9305025. Mustapa A, Justine M, Mohd Mustafah N, et al: Postural control and gait performance in the diabetic peripheral neuropathy: A systematic review. BioMed Res Int 10.1155/2016/9305025 [epub ahead of print on July 20, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrobel JS, Crews RT, Connolly JE.Clinical factors associated with a conservative gait pattern in older male veterans with diabetes J Foot Ankle Res 211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Costs of falls among older adults. http://www.cdc.gov/homeandrecreationalsafety/falls/fallcost.html
- 36.Kolb NA, Smith AG, Singleton JR, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73:860–866. doi: 10.1001/jamaneurol.2016.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 38.Beauchamp MK, Jette AM, Ward RE, et al. Predictive validity and responsiveness of patient-reported and performance-based measures of function in the Boston RISE study. J Gerontol A Biol Sci Med Sci. 2015;70:616–622. doi: 10.1093/gerona/glu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wennie Huang WN, Perera S, VanSwearingen J, et al. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58:844–852. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pamoukdjian F, Paillaud E, Zelek L, et al. : Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review Journal of Geriatric Oncology. doi:10.1016/j.jgo.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 41.Thaler-Kall K, Peters A, Thorand B, et al. Description of spatio-temporal gait parameters in elderly people and their association with history of falls: Results of the population-based cross-sectional KORA-Age study BMC Geriatr 1532, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor ME, Delbaere K, Mikolaizak AS, et al. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture. 2013;37:126–130. doi: 10.1016/j.gaitpost.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 44.Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age Ageing. 2003;32:137–142. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- 45. doi: 10.1155/2012/913848. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl, et al: Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract 10.1155/2012/913848 [epub ahead of print on March 14, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Harmer P, Stock R, et al. Implementing an evidence-based fall prevention program in an outpatient clinical setting. J Am Geriatr Soc. 2013;61:2142–2149. doi: 10.1111/jgs.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. doi: 10.1002/mds.25787. Li F, Harmer P, Liu Y, et al: A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson's disease. Mov Disord 29:539-545,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. doi: 10.1002/14651858.CD007146.pub2. Gillespie LD, Robertson MC, Gillespie WJ, et al: Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 15:CD007146, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Tofthagen C, Visovsky C, Berry DL. Strength and balance training for adults with peripheral neuropathy and high risk of fall: Current evidence and implications for future research. Oncol Nurs Forum. 2012;39:E416–E424. doi: 10.1188/12.ONF.E416-E424. [DOI] [PMC free article] [PubMed] [Google Scholar]