Abstract
Purpose
Docetaxel and cyclophosphamide (TC) was superior to doxorubicin and cyclophosphamide (AC) in a trial in early breast cancer. However, activity of TC relative to AC regimens with a taxane (TaxAC) is unknown.
Methods
In a series of three adjuvant trials, women were randomly assigned to TC for six cycles (TC6) or to a standard TaxAC regimen. US Oncology Research (USOR) 06-090 compared TC6 with docetaxel, doxorubicin, and cyclophosphamide (TAC6). National Surgical Adjuvant Breast and Bowel Project (NSABP) B-46-I/USOR 07132 compared TC6, TAC6, or TC6 plus bevacizumab. NSABP B-49 compared TC6 with several standard AC and taxane combination regimens. Before any analysis of individual trials, a joint efficacy analysis of TC versus the TaxAC regimens was planned, with invasive disease-free survival (IDFS) as the primary end point. Patients who received TC6 plus bevacizumab on NSABP B-46-I/USOR 07132 were not included. A hazard ratio (HR) from a stratified Cox model that exceeded 1.18 for TC6 versus TaxAC was predefined as inferiority for TC6. The prespecified interim monitoring plan was to report for futility if the HR was > 1.18 when 334 IDFS events were observed (50% of 668 events required for definitive analysis).
Results
A total of 2,125 patients were randomly assigned to receive TC6 regimens and 2,117 patients were randomly assigned to receive TaxAC regimens. The median follow-up time was 3.3 years. There were 334 IDFS events, and the HR for TC6 versus TaxAC was 1.202 (95% CI, 0.97 to 1.49), which triggered early reporting for futility. The 4-year IDFS was 88.2% for TC6 and was 90.7% for TaxAC (P = .04). Tests for treatment interaction by protocol, hormone receptor status, and nodal status were negative.
Conclusion
The TaxAC regimens improved IDFS in patients with high-risk human epidermal growth factor receptor 2–negative breast cancer compared with the TC6 regimen.
INTRODUCTION
Adjuvant chemotherapy substantially reduces the risk of breast cancer recurrences and death in early-stage breast cancer. A 15-year meta-analysis conducted by the Early Breast Cancer Trialists’ Collaborative Group of long-term outcomes among 100,000 women treated in 123 randomized trials to evaluate polychemotherapy demonstrated a 10-year breast cancer mortality reduction by one third with chemotherapy regimens that used an anthracycline, a taxane, and an alkylator compared with no chemotherapy.1 These triple-combination regimens were developed in two generations of trials, which improved the benefits noted in the landmark studies of alkylator-based chemotherapy.2-10 First-generation studies evaluated the addition of anthracyclines and demonstrated that, although 10-year mortality was not reduced with four cycles of anthracycline plus cyclophosphamide regimens relative to cyclophosphamide, methotrexate, and fluorouracil (relative risk [RR], 0.99; 95% CI, 0.90 to 1.08), combinations that used higher cumulative doses of anthracyclines were more effective (RR, 0.80; 95% CI, 0.72 to 0.93). Second-generation studies evaluated the incorporation of taxanes into anthracycline-based regimens, which further improved 8-year mortality (RR, 0.86; 95% CI, 0.79 to 0.93).1
Unfortunately, cardiac mortality was increased with anthracycline regimens (RR, 1.56; SE, 0.24; 2P = .02), which are also associated with increased risk for myelodysplastic syndromes and treatment-related leukemia.1,4 Because anthracyclines and taxanes were developed sequentially, it has been a challenge to evaluate de-escalation of the regimens doxorubicin and cyclophosphamide with a taxane (TaxAC). However, a study of 1,016 women conducted by US Oncology Research (USOR) compared four cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 administered every 3 weeks (AC) with four cycles of docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 administered every 3 weeks (TC). With a median follow-up time of 7 years, overall survival (OS) was superior for TC relative to AC (hazard ratio [HR], 0.69; 95% CI, 0.50 to 0.97; P = .032).11,12 These results established TC as an effective nonanthracycline regimen appropriate for comparison with TaxAC regimens.13
Subsequent collaborative efforts between USOR and the National Surgical Adjuvant Breast and Bowel Project (NSABP), in partnership with three separate sponsors, resulted in a series of three sequential trials, each of which randomly assigned women with early breast cancer to receive TC for six cycles (TC6) or one of several standard TaxAC regimens. As each of the first two trials closed to accrual and the subsequent trial was activated, agreements were reached with the sponsors to allow efficacy data from the preceding trial(s) to be combined prospectively with efficacy data from the subsequent trial(s). The statistical plans of the second and third trials were written to incorporate, monitor, and analyze the efficacy data from the preceding trial(s) in a joint analysis. Toxicity data continued to be collected and evaluated as specified in each original protocol. The efficacy data from the individual trials were never analyzed before they were combined and were only analyzed with the efficacy data from the other trials. The three trials are collectively referred to as the anthracyclines in early breast cancer (ABC) trials. The primary aim of the ABC trials was to determine if TC6 was noninferior to the TaxAC regimens. This report describes the efficacy analysis of these trials conducted after the prespecified futility boundary for demonstration of noninferiority of TC6 was crossed at the planned interim analysis.
METHODS
The ABC trials are a prospectively planned, joint efficacy analysis of three sequentially conducted, open-label, randomized, phase III trials in which 4,242 women with human epidermal growth factor receptor 2 (HER2)–negative breast cancer were enrolled after excision of the primary cancer and surgical evaluation of the axillary nodes. Patients were randomly assigned to receive TC for six cycles (TC6) or one of several triple-drug regimens that consisted of cyclophosphamide, doxorubicin, and a taxane, most often docetaxel (TaxAC). The initial trial conducted by USOR and supported by Sanofi (USOR 06-090) opened May 29, 2007, and included women with node-positive and high risk node-negative disease who were randomly assigned to receive docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 21 days for six cycles (TC6) or docetaxel, doxorubicin, and cyclophosphamide (75 mg/m2, 50 mg/m2, and 500 mg/m2, respectively) every 21 days for six cycles (TAC6). Designed as a superiority trial for TAC6, the projected sample size was 2,000 patients. The study was closed to accrual on June 1, 2009, after 1,295 patients were enrolled (TC6 [n = 648] and TAC6 [n = 647]) with a prespecified plan to combine the efficacy data with the common arms from NSABP B-46-I/USOR 07132, which had just been activated.
This second study developed by NSABP and USOR was supported by Genentech and opened to accrual on May 8, 2009, as a three-arm, open-label study, in essentially the same population as described for USOR 06-090. Patients were randomly assigned to receive one of three chemotherapy regimens for six cycles: TC6, TAC6, or TC6 plus bevacizumab (TCB). Bevacizumab 15 mg/kg was administered every 21 days until 1 year after the first cycle of TC. Planned enrollment was 3,600 patients. Efficacy data from both cohorts of the USOR 06-090 study and the TC6 and TAC6 groups of B-46-I/07132 were to be combined prospectively and analyzed to compare the TC6 and TAC6 regimens. The analysis of the end points for TC6 versus TAC6 was set to occur after the definitive analysis of TC6 versus TC6B on B-46-I/07132 and would exclude the patients in the TCB arm. Subsequent to well-publicized discussions by a US Food and Drug Administration (FDA) Oncologic Drug Advisory Committee and the FDA about withdrawal of approval for bevacizumab in metastatic breast cancer, declining enrollment occurred, which made it apparent that the study would not meet accrual goals. Therefore, B-46-I/07132 was closed to accrual on January 11, 2012, with a total accrual of 1,613 patients (TC [n = 539], TAC [n = 538], and TCB [n = 536]).
In aggregate, the initial two trials had enrolled 2,372 women into the TC6 and TAC6 arms (excluding the TC6B cohort from B-46-I/07132), which may have been sufficient to address the superiority question of TAC6 originally planned for USOR 06-0690 but was insufficient to formally address noninferiority of TC6. Therefore, the NSABP proposed a third trial of TC6 versus TAC6 to the Breast Cancer Steering Committee of the National Cancer Institute Cancer Therapy Evaluation Program to provide a sufficient number of additional women with high-risk breast cancer to allow for a noninferiority efficacy analysis by combining the efficacy data from a third trial with the efficacy data from the TC6 and TAC6 cohorts from the preceding two studies. The proposal was approved, but the Breast Cancer Steering Committee directed that investigators and patients should have a choice among the standard TaxAC regimens (TAC6; AC every 3 weeks for four cycles followed by paclitaxel 80 mg/m2 weekly for 12 doses; AC every 2 weeks for four cycles [DD AC] followed by paclitaxel 80 mg/m2 weekly for 12 doses; or DD AC followed by paclitaxel 175 mg/m2 every 2 weeks for four cycles). NSABP B-49 opened to accrual on April 4, 2012, 3 months after the closure of accrual to B-46-I/0732, and accrued 1,870 patients (TC6 [n = 938] and TaxAC [n = 932]) with HER2-negative, high-risk early breast cancer. Accrual closed on November 21, 2013.
Eligibility
Enrollment required a pathologic diagnosis of invasive breast cancer. Women with bilateral synchronous breast cancers were allowed in USOR 06-090 but were excluded from B-46-I/07132 and B-49. Patients with hormone receptor–positive or –negative disease were eligible. B-46-I/07132 and B-49 required HER2-negative status, as defined by the 2007 American Society of Clinical Oncology/College of American Pathologists guidelines. USOR 06-090 defined HER2-negative as immunohistochemistry of 0 to 1+ or a fluorescent in situ hybridization ratio ≤ 2.2 . Staging criteria for enrollment included pT1-3 if nodes were positive (including pN1Mi). For pN0, one of the following criteria was required: estrogen receptor (ER) and progesterone receptor (PgR) negative status, tumor size > 2.0 cm, or if T1c and ER or PgR positive, the tumor must also be grade 3 histology or have an Oncotype DX recurrence score ≥ 25 for B-46-I/07132 and B-49 enrollment or≥ 31 for USOR 06-090 enrollment. Other criteria included adequate hematopoietic, hepatic, and renal functions and a left ventricular ejection fraction of at least 50% regardless of the lower limit of normal at the facility. Surgical margins were required to be free of invasive disease and ductal carcinoma in situ (DCIS). Axillary evaluation was by axillary dissection or sentinel lymphadenectomy. Patients were excluded with definitive evidence of metastatic disease, T4 tumors, involvement of supraclavicular nodes, history of ipsilateral invasive cancer, ipsilateral DCIS, or nonbreast malignancies within 5 years before random assignment (except for curatively treated carcinoma in situ or nonmelanoma skin cancers).
Study Oversight
Each trial was designed and conducted separately and approved by institutional review boards at each participating institution or by a central institutional review board. Efficacy and toxicity data were collected and monitored by the individual groups and safety monitoring committees. The joint analysis was performed by the NSABP Biostatistical Center. The joint writing committee members vouch for the accuracy and completeness of the data and analyses.
Adverse Events
Adverse events were graded by the National Cancer Institute Common Toxicity Criteria (CTCAE) version 3.0 (USOR 06-090 and B-46-I/07132) and version 4.0 (B-49) and were collected after each cycle of chemotherapy and 3 to 4 weeks after the last chemotherapy treatment. Because different toxicity criteria were used in B-49 and because the toxicity profile of the regimens evaluated in these three trials are well established, only toxicity data for the B-49 trial will be reported here. However, toxicity data from the USOR 06-090 and B-46-I/07132 trials are provided in the Data Supplement.
Stratification and Random Assignment
Patients were randomly assigned to TC6 or TAC6 in USOR 06-090; to TC6, TAC6, or TC6B in B-46-I/07132; and to TC6 or TaxAC regimens in B-49, and patients were stratified by pathologic nodal status (0, 1-3, 4-9, or ≥ 10) and hormone receptor status (ER and PgR negative or ER or PgR positive). Minimization algorithms that were based on the method described by White and Friedman14 and that incorporated the biased-coin approach proposed by Ephron15 were used for these trials. Random assignment was done independently in the three studies. The combined analyses included a study identifier treated as an additional stratification factor.
End Point Definitions
The primary end point was invasive disease-free survival (IDFS), defined as time from random assignment to local recurrence after mastectomy, invasive local recurrence in the ipsilateral breast after lumpectomy, regional recurrence, distant recurrence, invasive contralateral breast cancer, second primary cancer (other than squamous or basal cell carcinoma of the skin, melanoma in situ, or carcinoma in situ), or death as a result of any cause before recurrence or second primary cancer.
Secondary end points included recurrence-free interval, defined as time from random assignment until first local, regional, or distant recurrence or death as a result of breast cancer reported without prior documentation of recurrence; disease-free survival–DCIS, which adds DCIS of the ipsilateral or contralateral breast as a primary end point events; overall survival (OS), defined as time from random assignment to death as a result of any cause; and toxicity.
Primary Aim, Noninferiority
The primary aim of the joint efficacy analysis was to determine if the nonanthracycline regimen (TC) was noninferior to standard doxorubicin-containing regimens (TaxAC). A HR for TC versus TaxAC from a Cox model stratified for parent trial (USOR 06-090, NSABP B-46-I/USOR 07132, NSABP B-49), positive nodes (0, 1-3, 4-9, or ≥ 10), and hormonal status (ER and PgR negative, or ER or PgR positive) of > 1.18 was predefined as a demonstration of inferiority, which would correspond to an absolute difference of ≥ 2% in the 5-year IDFS rate of TC6 relative to TaxAC.
Interim Monitoring Plan
A single interim analysis was planned when 334 IDFS events (50% of the planned 668 events) for the definitive analysis were observed. The futility rule for the interim analysis was based on the Wieand Rule16, which would recommend consideration of reporting the study for futility to demonstrate noninferiority if the observed HR was > 1.18 when 334 events had been reported. The futility analysis was also to be conducted on the basis of treatment received rather than treatment randomly assigned.
Statistical Analysis
All analyses subsequent to the interim futility analysis included all patients with follow-up data analyzed according to randomly assigned treatment (intent to treat [ITT]) and were evaluated for significance at an α level of .05; there was no control for multiple comparisons. Time to event was measured from random assignment, and time-to-event plots were estimated by the Kaplan-Meier method. HRs were calculated from stratified Cox models, and P values for the time to event were from stratified log-rank tests. Strata used for analyses were parent trial (USOR 06-090, B-46-I/07132, B-49), positive nodes (0, 1-3, 4-9, or ≥ 10), and hormone-receptor status (ER and PgR negative or ER or PgR positive) or the appropriate subset of factors for subset analyses. Fisher’s exact test or Cochran-Mantel-Haenszel tests were used to test for differences in proportions.
RESULTS
Patient Characteristics
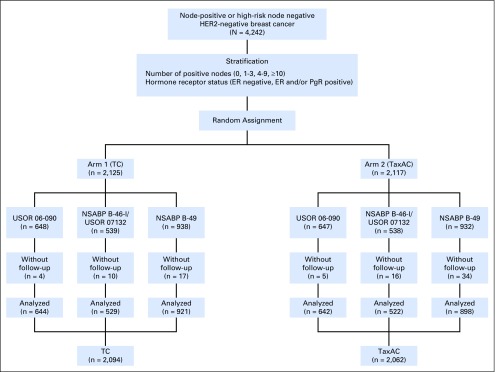
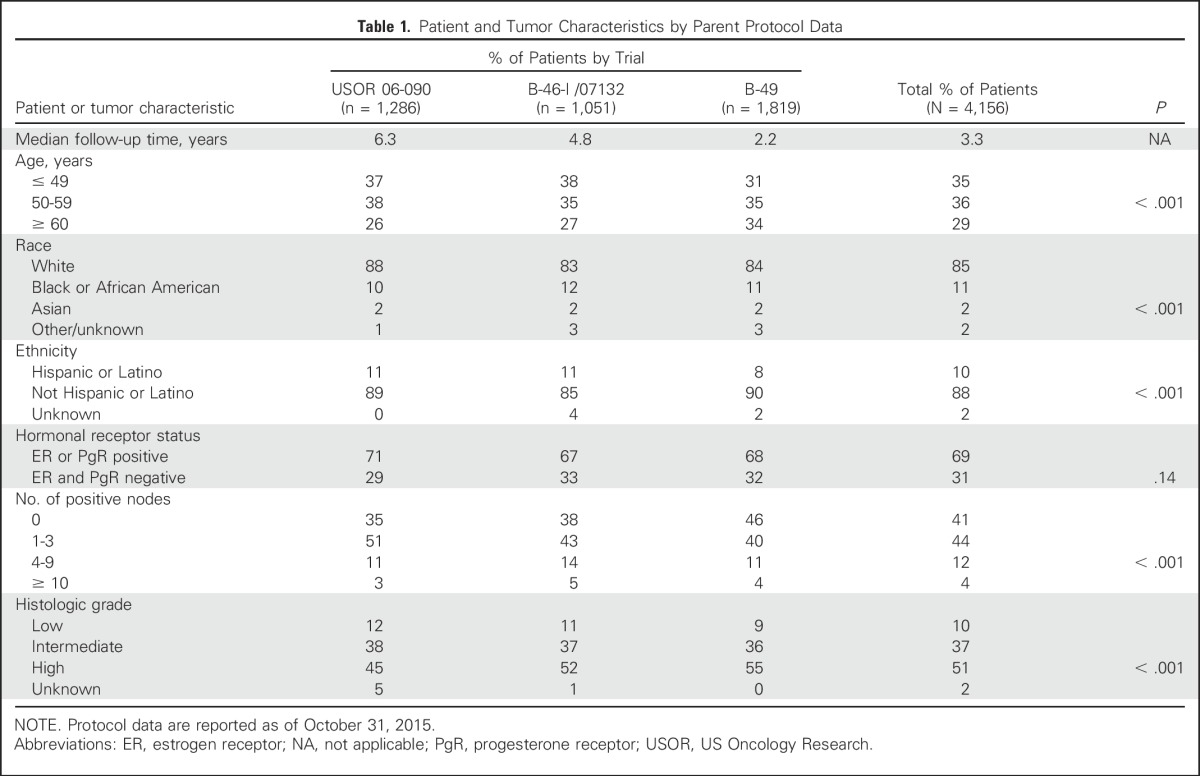
Between May 2007 and November 2013, 4,242 patients enrolled in the USOR 06-090 trial, in the TC6 and TAC6 arms of B-46-I/07132, and in B-49. Of these, the data of 4,156 patients were analyzed with 2,094 women assigned to TC6, and 2,062 women assigned to TaxAC regimens. Figure 1 demonstrates the random assignment and data analysis in each of the three trials. Table 1 lists the patient characteristics. Approximately 40% had node-negative disease, and nearly one third of patients were hormone receptor negative. The median follow-up times were 6.3 years for USOR 06-090, 4.8 years for B-46-I/07132, 2.2 years for B-49, and 3.3 years for the combined studies. Although the distribution of characteristics differs statistically by protocol, the absolute differences were minimal and not clinically relevant.
Fig 1.
Consort diagram. Anthracyclines in early breast cancer (ABC) trials. ER, estrogen receptor; HER2, human epidermal growth factor receptor; NSABP, National Surgical Adjuvant Breast and Bowel Project; PgR, progesterone receptor; TaxAC, doxorubicin and cyclophosphamide regimens with a taxane; TC, docetaxel and cyclophosphamide; USOR, US Oncology Research.
Table 1.
Patient and Tumor Characteristics by Parent Protocol Data
Interim Analysis
An October 31, 2015, data cutoff was used for the interim analysis. By that time, 399 IDFS events (59.7% of the required 668 events for the definitive analysis) had been reported. For the formal interim analysis, an administrative censoring date (prior to the cutoff) was used to alter the time and censoring variable so that the data contained exactly the prespecified 334 events required by using the as-treated cohorts. With the restriction, the observed HR was 1.202, which exceeded the 1.18 prespecified threshold for futility. Because all patients had completed chemotherapy, the data monitoring committee recommended a report of the observation in accordance with the prespecified rules in the B-49 statistical plan. After the decision to report, a full analysis of the joint efficacy data received by the data cutoff on the basis of random assignment (ITT) was performed. All analyses subsequent to the planned interim futility analysis used all available information up to the October 31, 2015, cutoff without administrator censoring.
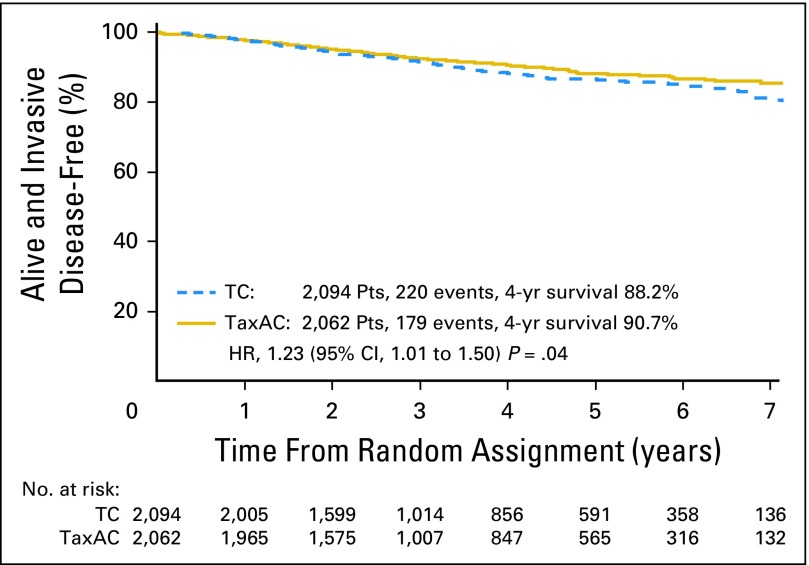
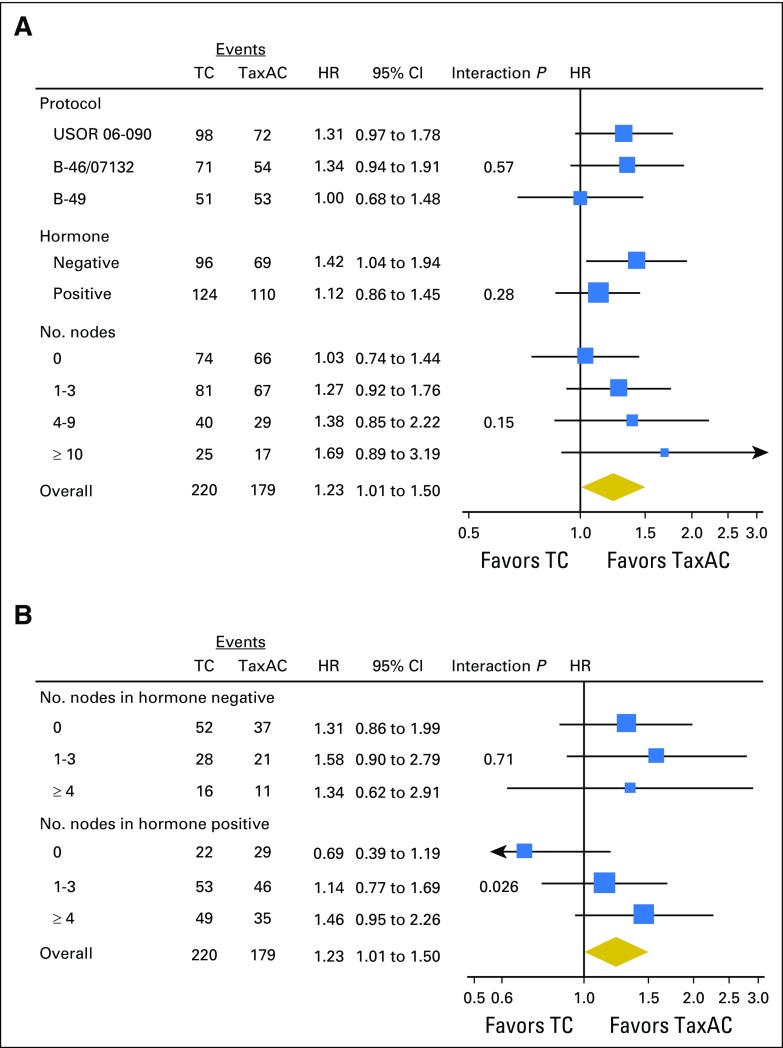
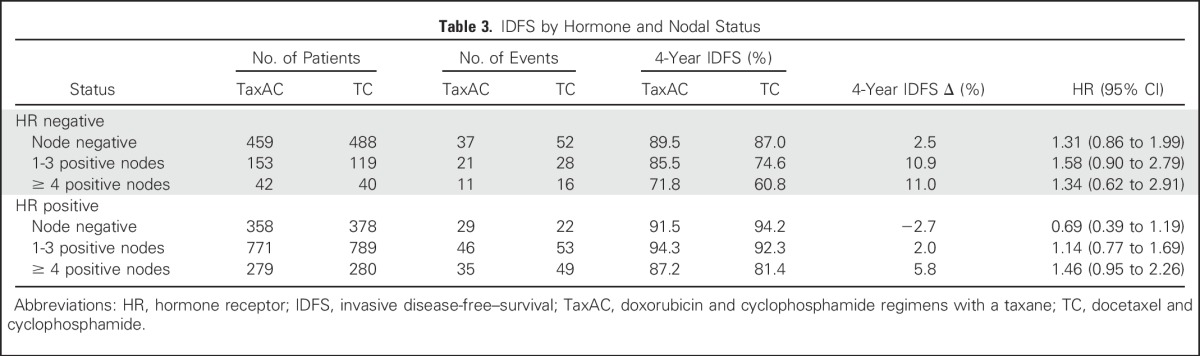
The observed HR for IDFS on the basis of the ITT analysis with all available information through October 31, 2015, for TC6 versus TaxAC, was 1.23 (95% CI, 1.01 to 1.50; P = .04), which also exceeded the 1.18 noninferiority threshold. Figure 2 shows Kaplan-Meier plots of IDFS. Table 2 lists the numbers and types of first IDFS events by treatment. The 4-year IDFS rates were 88.2% for TC6 and 90.7% for TaxAC. Figure 3A shows a forest plot of the IDFS HR according to stratification variables. Planned exploratory tests for treatment interaction by protocol, hormone receptor status, and nodal status were negative. However, unplanned tests for interaction by combined hormone receptor status and nodal status demonstrated a significant interaction for positive hormone receptor status with nodal status (P = .026) but no interaction in the patients with hormone receptor–negative status (P = .71), as shown in Fig 3B. Table 3 lists IDFS data by treatment, hormone receptor status, and nodal status.
Fig 2.
Kaplan-Meier plots of invasive disease-free survival. HR, hazard ratio; TaxAC, doxorubicin and cyclophosphamide regimens with a taxane; TC, docetaxel and cyclophosphamide.
Table 2.
First Invasive Disease-Free–Survival Event by Treatment
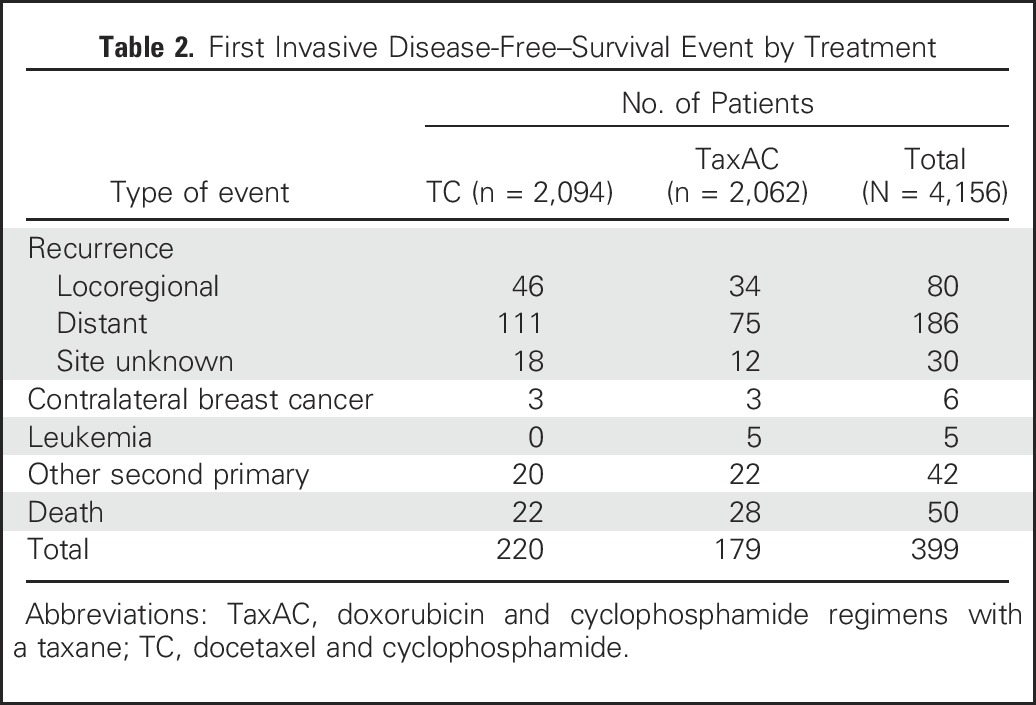
Fig 3.
Forest plots of (A) invasive disease-free survival hazard ratio (HR) according to stratification variables and (B) with test for interaction by combined hormone receptor and nodal status. TaxAC, doxorubicin and cyclophosphamide regimens with a taxane; TC, docetaxel and cyclophosphamide.
Table 3.
IDFS by Hormone and Nodal Status
Secondary End Points
There was a statistically significant difference in recurrence-free interval in favor of TaxAC—179 events occurred in the TC6 group and 121 events occurred in the TaxAC group (HR, 1.51; 95% CI, 1.20 to 1.90; P < .001). DFS-DCIS results were similar to the primary end point of IDFS, because only seven DCIS events were observed in the combined studies. At this time, there is no significant difference in OS—111 deaths occurred in the TC6 groups and 102 deaths occurred in the TaxAC groups (HR, 1.08; 95% CI, 0.82 to 1.41; P = .60).
Adverse Events
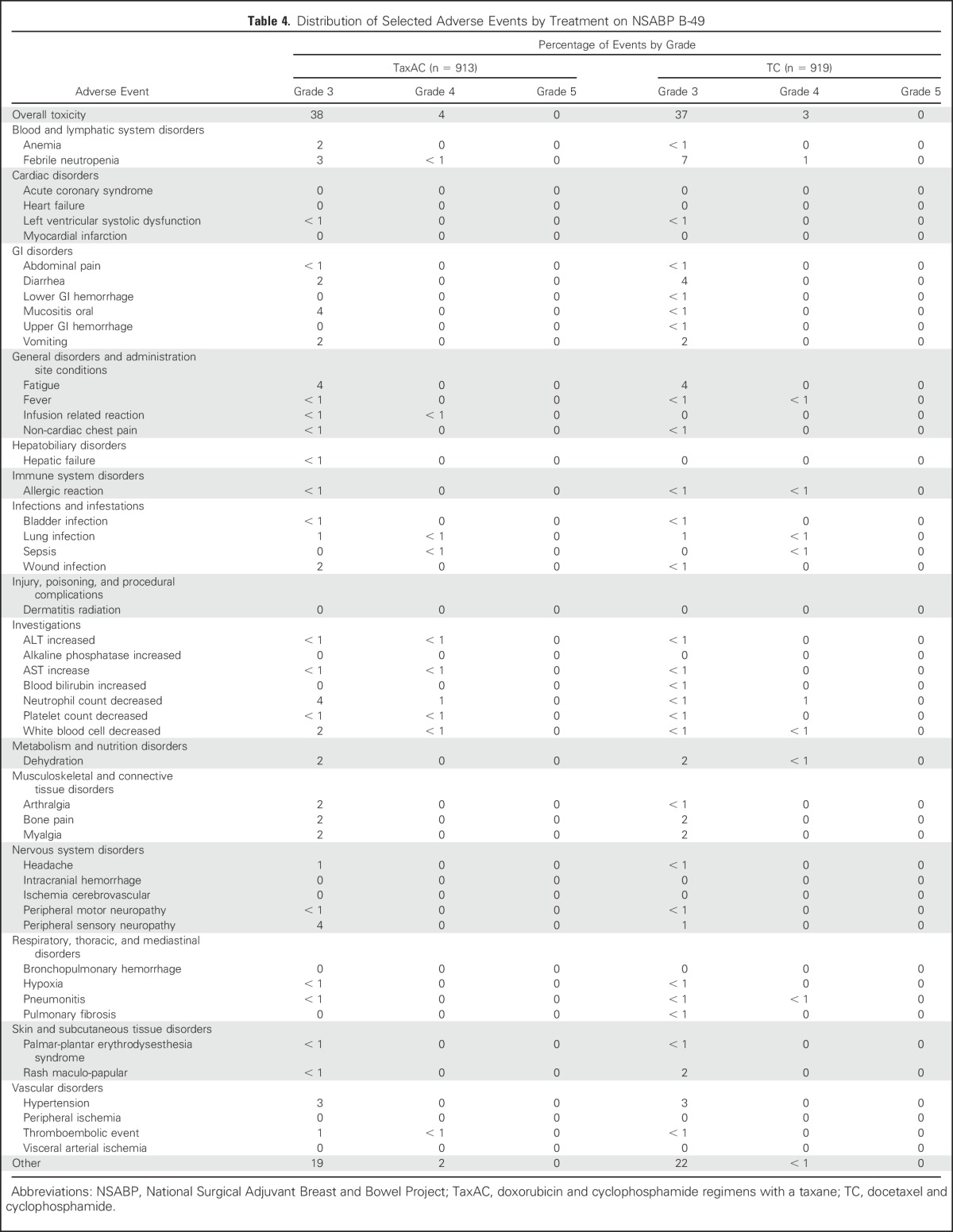
Table 4 lists the toxicity summary from B-49. The reported toxicities were consistent with the well-established toxicities associated with these regimens. Summary toxicity tables for USOR 06-090 and B-46-I/07132 are provided in the Data Supplement.
Table 4.
Distribution of Selected Adverse Events by Treatment on NSABP B-49
DISCUSSION
The interim futility analysis of the ABC trials demonstrated futility for demonstration of noninferiority of TC6 relative to TaxAC for the primary end point of IDFS in women with HER2-negative, operable breast cancer. In fact, statistical inferiority for IDFS of TC6 relative to TaxAC was evident with a HR of 1.23 (95% CI, 1.01 to 1.50; P = .04) with 59% of the events originally required for the definitive analysis. Exploratory analyses by stratification variables suggested that the benefit of TaxAC was more evident in the studies that had longer follow-up times, for the patients with hormone receptor–negative disease, and for the women who had the highest number of positive axillary lymph nodes, although individual tests for treatment interaction with these factors were negative. Of interest, unplanned tests for treatment interaction with nodal status demonstrated a significant interaction in the hormone receptor–positive subset but no apparent benefit in patients with node-negative disease, in contrast to the benefit observed in patients with positive axillary nodes.
The secondary end point of RFI also demonstrated a significant HR of 1.51 (95% CI, 1.20 to 1.90; P < .001) in favor of TaxAC. Survival data are still immature, and longer follow-up times will be necessary to determine the impact on survival.
To our knowledge, this is the first study to specifically evaluate the role of doxorubicin in taxane-based adjuvant therapy of HER2-negative breast cancer. Interpretation of older studies, which accrued patients before routine testing for HER2 status, was confounded by the inclusion of patients with HER2-amplified breast cancer, because this important subset was found to derive clear benefit from the incorporation of anthracyclines into adjuvant therapies before the introduction of trastuzumab.17
Although results of the ABC trials demonstrated a statistically significant improvement in IDFS with the administration of anthracyclines in patients with HER2-negative disease, the absolute benefits were small, and the majority of patients who received TC6 have done well without an anthracycline. Exploratory tests for treatment interaction by nodal status and hormonal status suggest that the benefits appear to be meaningful in patients with hormone receptor–negative tumors or those with hormone receptor–positive tumors and positive axillary nodes.
Because the largest of the three trials has only 2.2 years of median follow-up time and is contributing only 25% of the reported events, additional follow-up time will be needed to fully interpret the data from these trials. Additional events also will be essential for critical correlative studies of predictive biomarkers or expression profiles, which may identify subsets of patients who derive substantial benefit from inclusion of the anthracyclines and thus help identify a larger subset who could be spared the risk of serious cardiac and long-term hematologic toxicities.
ACKNOWLEDGMENT
We thank the patients who made this study possible, the co-authors, and participating investigators at US Oncology Research (USOR), National Surgical Adjuvant Breast and Bowel (NSABP), The Alliance, and Eastern Cooperative Oncology Group–American College of Radiology Imaging Network, and their research staffs. We also thank USOR, NSABP, and NRG Oncology staff at the Operations and Biostatical Centers.
Footnotes
Supported by Public Health Service Grants No. U10CA-180868, -21115, -180822, -180820, -180821, -180791, and UG1-189867 from the from the National Cancer Institute, Department of Health and Human Services, and by grants from Susan G. Komen for the Cure; Sanofi, and Genentech.
Presented in part (related material) at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016.
Clinical trial information: USOR 06-090, NCT00493870; NSABP B-46-I/USOR 07132, NCT00887536; NSABP B-49, NCT01547741.
See accompanying Oncology Grand Rounds on page 2600
AUTHOR CONTRIBUTIONS
Conception and design: Joanne L. Blum, Patrick J. Flynn, Greg Yothers, Lina Asmar, Charles E. Geyer Jr, Samuel A. Jacobs, Nicholas J. Robert, Joyce A. O'Shaughnessy, Jong-Hyeon Jeong, Sandra M. Swain, Eleftherios P. Mamounas, Stephen E. Jones, Norman Wolmark
Administrative support: Greg Yothers, Norman Wolmark
Provision of study materials or patients: Joanne L. Blum, Patrick J. Flynn, Charles E. Geyer Jr, Nicholas J. Robert, Judith O. Hopkins, Henry Leonidas Gómez, Louis Fehrenbacher, Devchand Paul, Adam M. Brufsky
Collection and assembly of data: Joanne L. Blum, Patrick J. Flynn, Greg Yothers, Lina Asmar, Joyce A. O'Shaughnessy, Chau T. Dang, Henry Leonidas Gómez, Louis Fehrenbacher, Svetislava J. Vukelja, Devchand Paul, Adam M. Brufsky, Eleftherios P. Mamounas
Data analysis and interpretation: Joanne L. Blum, Patrick J. Flynn, Greg Yothers, Lina Asmar, Charles E. Geyer Jr, Nicholas J. Robert, Judith O. Hopkins, Joyce A. O'Shaughnessy, Chau T. Dang, Henry Leonidas Gómez, Louis Fehrenbacher, Alan P. Lyss, Devchand Paul, Linda H. Colangelo, Sandra M. Swain, Eleftherios P. Mamounas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Anthracyclines in Early Breast Cancer: the ABC Trials—USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Joanne L. Blum
Honoraria: Medivation, Biomarin, Pfizer, Myriad Genetics
Consulting or Advisory Role: Biomarin, Medivation, Pfizer, Myriad Genetics
Travel, Accommodations, Expenses: Biomarin, Medivation, Pfizer, Myriad Genetics
Patrick J. Flynn
Employment: ARIAD Pharmaceuticals (I), Sanofi/Genzyme (I)
Stock or Other Ownership: ARIAD Pharmaceuticals (I)
Speakers' Bureau: Lilly
Travel, Accommodations, Expenses: Lilly
Greg Yothers
Employment: Mountainview Pediatrics (I)
Consulting or Advisory Role: Pharmacyclics, Orbus Therapeutics
Lina Asmar
No relationship to disclose
Charles E. Geyer Jr
Consulting or Advisory Role: Stemnion, Myriad Genetics
Research Funding: Incyte, Merck
Travel, Accommodations, Expenses: AstraZeneca, Abbvie, Genetech (a member of the Roche group)
Samuel A. Jacobs
No relationship to disclose
Nicholas J. Robert
Employment: McKesson Subspecialty Health
Leadership: McKesson Subspecialty Health
Consulting or Advisory Role: New Century Health, Paradigm Cancer Diagnostics
Research Funding: Side-Out Foundation (Inst)
Judith O. Hopkins
Consulting or Advisory Role: AIM Specialty Health
Joyce A. O'Shaughnessy
Honoraria: AstraZeneca, Lilly, Abbvie, Arno Therapeutics, Celgene, Nektar, Eisai, Novartis, Pfizer, Genentech (a member of the Roche group)
Consulting or Advisory Role: Novartis, Pfizer, Lilly, Arno Therapeutics, Abbvie, AstraZeneca, Celegene, Nektar, Genentech (a member of the Roche group), Eisai
Travel, Accommodations, Expenses: Celgene, Nektar, Lilly, Novartis, Pfizer, Abbvie
Chau T. Dang
Research Funding: GlaxoSmithKline (Inst), Genentech (a member of the Roche group) (Inst), Puma Biotechnology (Inst)
Travel, Accommodations, Expenses: Genentech BioOncology
Henry Leonidas Gómez
No relationship to disclose
Louis Fehrenbacher
Research Funding: Genentech (a member of the Roche group) (Inst)
Svetislava J. Vukelja
Employment: Texas Oncology–Tyler
Alan P. Lyss
Consulting or Advisory Role: Verdi Oncology
Devchand Paul
No relationship to disclose
Adam M. Brufsky
No relationship to disclose
Jong-Hyeon Jeong
No relationship to disclose
Linda H. Colangelo
No relationship to disclose
Sandra M. Swain
Honoraria: Roche, Clinigen Group, AstraZeneca, Pfizer
Consulting or Advisory Role: Genentech (a member of the Roche group), Clinigen Group, OncoPlex Diagnostics, Lilly, Pieris Pharmaceuticals
Research Funding: Puma Biotechnology (Inst), Roche (Inst), Genentech (Inst), Pfizer (Inst), Merrimack, Lilly (Inst)
Travel, Accommodations, Expenses: Genentech (a member of the Roche group), Genentech (I)
Eleftherios P. Mamounas
Honoraria: Genentech (a member of the Roche group), Genomic Health
Consulting or Advisory Role: Genomic Health, Celgene, Pfizer, GlaxoSmithKline, Novartis, Ventana Medical Systems, Macrogenics, GRAIL, Celcuity, Bayer, bioTheranostics
Speakers' Bureau: Genomic Health, Genentech (a member of the Roche group)
Travel, Accommodations, Expenses: Genomic Health, Genentech (a member of the Roche group), Celgene
Stephen E. Jones
Consulting or Advisory Role: Pfizer
Speakers' Bureau: Pfizer, Amgen, Celegene
Norman Wolmark
Research Funding: Abbvie (Inst), Puma (Inst), Bayer (Inst)
REFERENCES
- 1.Peto R, Davies C, Godwin J, et al. : Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432-444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Fisher ER, Redmond C: Ten-year results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trial evaluating the use of L-phenylalanine mustard (L-PAM) in the management of primary breast cancer. J Clin Oncol 4:929-941, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Bonadonna G, Valagussa P, Moliterni A, et al. : Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. N Engl J Med 332:901-906, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Henderson IC, Berry DA, Demetri GD, et al. : Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976-983, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Mamounas EP, Bryant J, Lembersky B, et al. : Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol 23:3686-3696, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Citron ML, Berry DA, Cirrincione C, et al. : Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 21:1431-1439, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Wang M, Martino S, et al. : Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663-1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparano JA, Zhao F, Martino S, et al. : Long-term follow-up of the E1199 phase III trial evaluating the role of taxane and schedule in operable breast cancer. J Clin Oncol 33:2353-2360, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin M, Pienkowski T, Mackey J, et al. : Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302-2313, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Swain SM, Tang G, Geyer CE, Jr, et al. : Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol 31:3197-3204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SE, Savin MA, Holmes FA, et al. : Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381-5387, 2006[Erratum: J Clin Oncol 25:1819, 2007] [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Holmes FA, O’Shaughnessy J, et al. : Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research trial 9735. J Clin Oncol 27:1177-1183, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Giordano SH, Lin Y-L, Kuo YFR, et al. : Decline in the use of anthracyclines for breast cancer. J Clin Oncol 30:2232-2239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White SJ, Freedman LS: Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 37:849-857, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efron B: Forcing a sequential experiment to be balanced. Biometrika 58:403-441, 1971 [Google Scholar]
- 16.Wieand S, Schroeder G, O’Fallon JR: Stopping when the experimental regimen does not appear to help. Stat Med 13:1453-1458, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gennari A, Sormani MP, Pronzato P, et al. : HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst 100:14-20, 2008 [DOI] [PubMed] [Google Scholar]