Abstract
Purpose
To assess the role of participant-reported symptoms on long-term adherence to preventive therapy in the United Kingdom sample of the International Breast Cancer Intervention Study (IBIS-I). IBIS-I was a randomized controlled trial that investigated the effectiveness of tamoxifen in reducing the risk of breast cancer among women at increased risk of the disease.
Participants and Methods
Women were randomly assigned to tamoxifen versus placebo (20 mg/day; n = 4,279). After 456 exclusions, 3,823 women were included in this analysis. Adherence (< 4.5 years or ≥ 4.5 years) was calculated using data from six monthly clinical visits. Analyses were adjusted for age, Tyrer-Cuzick risk, smoking, use of hormone replacement therapy, menopausal status, baseline menopausal symptoms, and treatment.
Results
Overall, 69.7% of women were adherent for at least 4.5 years (tamoxifen: 65.2% v placebo: 74.0%; P < .001). Differences in adherence between treatment arms were observed from 12 months onward (all P < .01) and were largest at 54 months. Dropout rates were highest in the first 12 to 18 months and decreased thereafter. Women reporting nausea/vomiting were less likely to be adherent in both the tamoxifen (odds ratio [OR], 0.57; 95% CI, 0.37 to 0.86; P = .007) and placebo (OR, 0.58; 95% CI, 0.37 to 0.93; P = .023) arms. Headaches were associated with adherence only in the placebo arm (OR, 0.62; 95% CI, 0.42 to 0.91; P = .016), whereas gynecologic symptoms were significant only in the tamoxifen arm (OR, 0.77; 95% CI, 0.62 to 0.97; P = .024). Effect sizes for each symptom on adherence were not significantly different between the treatment groups (P > .05). In both treatment arms, we observed significant trends for lower adherence with increasing severity for all symptoms (P < .01) except headaches (P = .054).
Conclusion
In the IBIS-I trial, experiencing predefined symptoms in the first 6 months reduced long-term adherence. Effects were similar between treatment arms, suggesting that women were attributing age-related symptoms to preventive therapy. Interventions were required to support symptom management.
INTRODUCTION
Breast cancer remains the most commonly diagnosed cancer in women from developed countries.1 In 2013, more than 50,000 women were diagnosed with breast cancer in the United Kingdom, and 11,643 deaths were recorded in 2012.2 Incidence of the disease has rapidly increased since the 1970s,3,4 with one in eight women now expected to be diagnosed with breast cancer in her lifetime.5 Efforts to prevent the disease are therefore taking on greater significance.6
Preventive therapy is an option for women at increased risk of breast cancer resulting from known risk factors such as having a family history of the disease. Individual participant data from nine primary prevention trials showed a reduction in breast cancer incidence of at least 30% among women who used selective estrogen receptor modulators.7 These data also show an increased risk of thromboembolic events and endometrial cancer among women receiving preventive therapy, tamoxifen in particular. Data from the International Breast Cancer Intervention Study (IBIS-I) showed that the preventive effects of tamoxifen last at least 20 years.8 However, uptake of preventive therapy is low, with only 16% of women opting to use chemoprevention after it has been offered.9 Patient concern about adverse effects of drugs is a major deterrent to initiating preventive therapy.10-14
Women who adhere to the full course of medication are more likely to experience benefit; however, adherence to the recommended course of therapy is suboptimal.9 Among preventive therapy trials reporting 5-year follow-up, 5-year adherence ranges from 61%15 to 81%.16 Menopausal symptoms such as hot flashes and irregular bleeding are common among women taking selective estrogen receptor modulators17-19 and may reduce adherence.9 Studies that collected off-therapy forms report that more than half of all women who drop out of preventive therapy trials attribute their decision to adverse effects of medication.20-23 However, retrospectively assessing decisions to discontinue medications may be a biased approach.
Data from the National Surgical Adjuvant Breast and Bowel Project, Prevention-1 (NSABP P-1) trial, also called the Breast Cancer Prevention Trial (BCPT), show that after 12 months, 84% of participants were considered to be adherent, which is defined as taking at least 76% of their medication.17 In models adjusted for participant characteristics and participant-reported outcomes, women who had a higher quality of life after 3 months of therapy, as assessed by the mental component of the 36-Item Short Form Health Survey (SF-36), were more likely to be adherent. Women who experienced gynecologic, vasomotor, or sexual symptoms were less likely to be adherent after 1 year. No studies have examined associations between these symptoms and adherence using 5-year follow-up data.
The aim of this analysis was to assess the role of predefined symptoms on long-term adherence in the United Kingdom sample from IBIS-I. We hypothesized that there would be associations between nausea/vomiting, headaches, hot flashes, irregular bleeding, vaginal dryness, and vaginal discharge recorded at the 6-month follow-up and lower adherence to the full course of therapy. These effects were expected to be similar across treatment arms. Because these symptoms occur primarily in the first 12 months of treatment,24 we anticipated a higher rate of dropout in the first year compared with the remaining years of the trial. We hypothesized that the effect of the predefined symptoms on adherence would be larger among women reporting more severe symptoms.
PARTICIPANTS AND METHODS
Participants and Procedures
Details of the trial design and entry criteria are described elsewhere.8,25 Briefly, women age 35 to 70 years at increased risk of developing breast cancer were recruited between April 1992 and March 2001 from various centers in Australia/New Zealand, the United Kingdom, and other European countries. Written informed consent to participate was collected after an initial discussion with an IBIS-I physician and a consideration period of at least 24 hours. For this analysis, only data from the United Kingdom were reported because case report forms and adherence data were not as reliably collected in other countries. Women were excluded from the adherence analysis if they died, were diagnosed with any invasive cancer (excluding non-melanoma skin cancer), experienced a thromboembolic event or deep vein thrombosis, or underwent a prophylactic mastectomy during the 5-year active treatment phase. Women who withdrew within the first 6 months after random assignment were also excluded from the main analyses.
On study entry, women were randomly assigned to 5 years of treatment with tamoxifen (20 mg/day) or matching placebo on a 1:1 basis. A baseline case report form was completed for each participant, which assessed baseline demographics (eg, age) and clinical information (eg, baseline menopausal symptoms and family history). All women received follow-up via six monthly visits or telephone calls for the duration of the active treatment period (60 months). During these follow-ups, current use of the assigned medication was assessed, and patient symptoms in the previous 6 months were self-reported using predefined items. Local ethics committees approved the trial at each participating institution.
Measures
Adherence.
Adherence was defined as the period of persistent use of the allocated medication from initiation to cessation.26 In our data, adherence was calculated by using the period of time between the trial randomization date and the date of the final follow-up visit. Trial coordinators recorded whether participants were still using their allocated medication at each time point (yes/no). Notes on adherence were described on the case report forms (eg, if a patient could not be contacted). Predefined rules developed for these analyses were used by two researchers to review all case report forms and assess adherence (see the Appendix, online only). Women who marked “no” for their use of the medication at any point were classified as nonadherent. Agreement between the two raters was high (κ, 0.95). Each participant was assessed for persistent use of the medication for at least 4.5 years (adherent) or stopping before 4.5 years (nonadherent).
Participant symptoms.
Any menopausal symptoms before beginning the trial were recorded in the baseline case report form (yes/no). Subsequent symptoms were assessed every 6 months during clinical visits or telephone calls by using predefined items for nausea, vomiting, headaches, hot flashes, irregular bleeding, vaginal dryness, and vaginal discharge. Because of the low number of women reporting nausea and vomiting and the similarities between these symptoms, these responses were combined. Irregular bleeding, vaginal dryness, and vaginal discharge were more common but were combined because the symptoms are similar (referred to as gynecologic symptoms herein). All symptoms were classified as mild, moderate, or severe as judged by the women.
Demographic and clinical variables.
Baseline demographics included age (< 50, 50 to 60, > 60 years), body mass index (< 25, 25 to 30, > 30, unknown), family history (one affected relative or two or more affected relatives), Tyrer-Cuzick 10-year breast cancer risk score (< 8%, ≥ 8%), smoking status (never, current, ex), hormone replacement therapy (HRT) use (never, current, ex), menopausal status (premenopausal, postmenopausal), baseline menopausal symptoms (yes/no) and whether they had undergone a hysterectomy (yes/no) or oophorectomy (yes [single], yes [bilateral], no).
Statistical Analysis
We used the Kaplan-Meier method27 to estimate adherence at different follow-up times, both overall and by treatment group separately. Baseline factors, treatment arm, and the occurrence of symptoms at month 6 were analyzed for the prediction of adherence by using logistic regression. Predictors were included in the multivariable analyses if they were significant in the univariable models. Tests of heterogeneity were used to determine whether the effects of the predefined symptoms on adherence were similar between the treatment groups. Changes in likelihood ratio statistics were used to determine the effect of treatment and symptoms at 6 months on adherence. The analyses testing the effect of symptom severity on adherence were also based on comparisons of proportions. We used a nonparametric test of trend for ranks across ordered groups. All P values were two-sided and all CIs were at the 95% level. All calculations were performed by using STATA version 13.1 (STATA, College Station, TX).
RESULTS
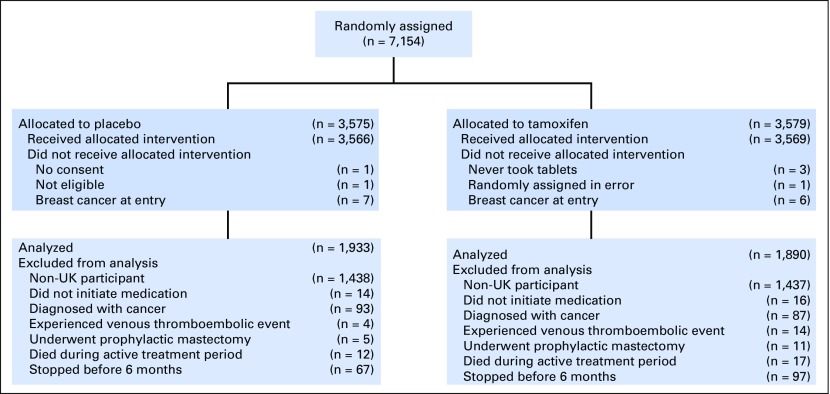
A total of 7,152 women were enrolled in the IBIS-I trial, 4,279 of whom were from the United Kingdom and were included in this analysis (CONSORT diagram; Fig 1). Women were excluded from the adherence analysis if they did not consent (n = 1), were ineligible (n = 1), had breast cancer on entry (n = 13), were randomly assigned in error (n = 1), or did not take tablets (n = 3). We then inspected case report forms and further excluded all data from 273 women who were diagnosed with a cancer, including breast cancer (n = 180), experienced a venous thromboembolic event (n = 18), had undergone a prophylactic mastectomy (n = 16), did not initiate any medication (n = 30), or died during the active treatment period (n = 29). An additional 164 women withdrew from the study within 6 months of random assignment and were also excluded (97, tamoxifen arm; 67, placebo arm). Of these, 32 women reported experiencing a symptom before the 6-month visit (19, tamoxifen arm; 13, placebo arm). This left 3,823 women to be included in the adherence analysis (1,890, tamoxifen arm; 1,933, placebo arm). Baseline demographic and clinical factors were balanced between treatment groups (Table 1).
Fig 1.
CONSORT diagram for medication adherence analysis within International Breast Cancer Intervention I trial.
Table 1.
Baseline Demographic and Clinical Characteristics by Study Arm
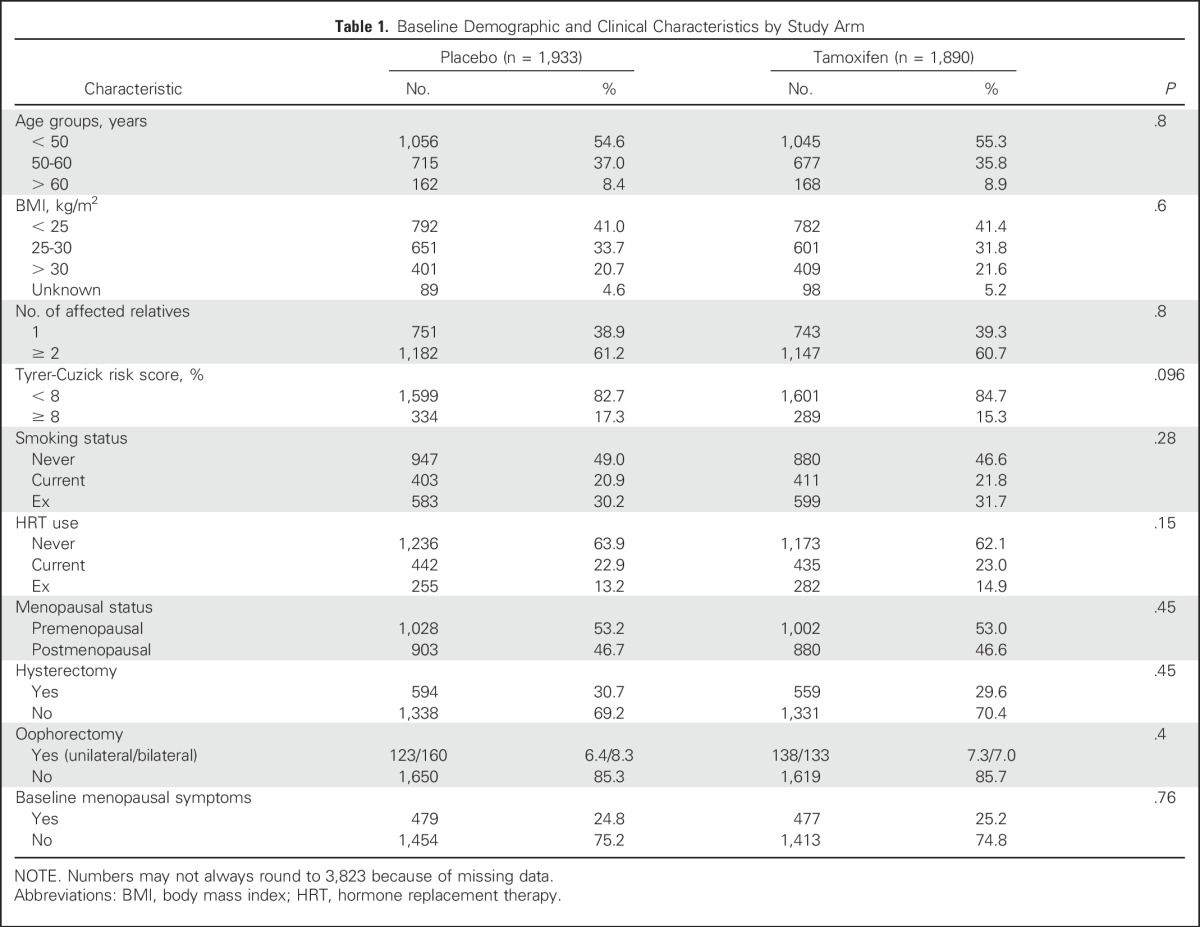
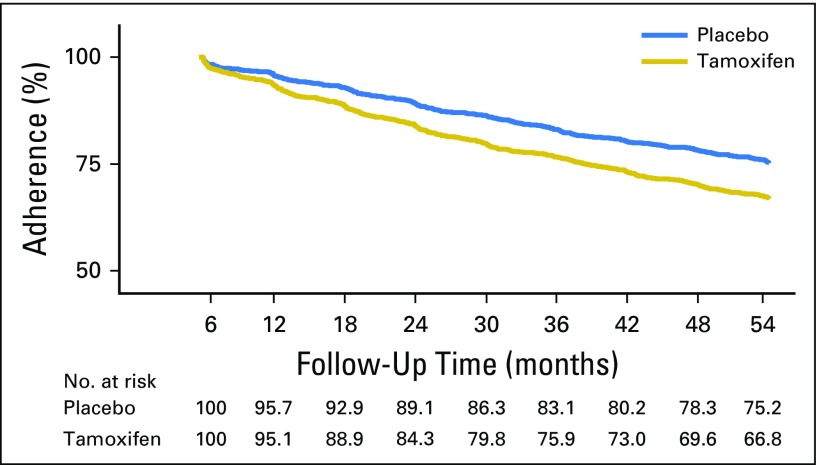
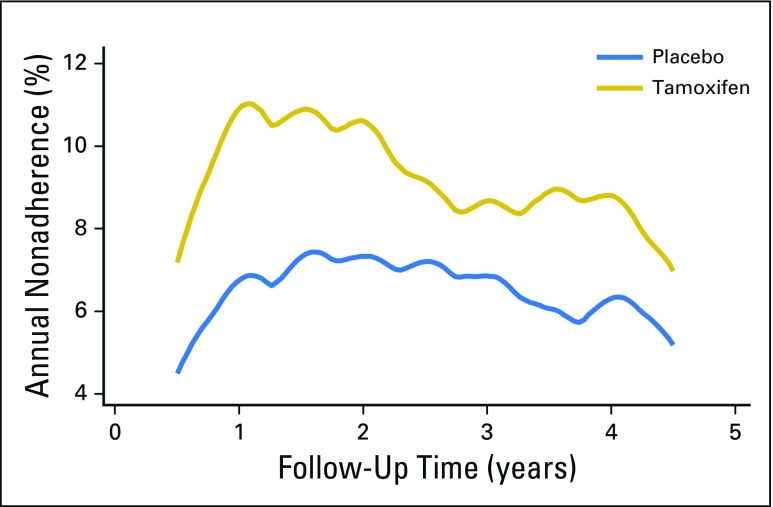
Overall, 69.7% of women were adherent for at least 4.5 years. Kaplan-Meier estimates for adherence were significantly higher among women taking placebo (75.2%) than among those taking tamoxifen (66.8%; hazard ratio [HR], 1.43; 95% CI, 1.27 to 1.60; P < .001; Fig 2). The mean time on treatment was 4.3 years (standard deviation [SD], 1.4), and this was higher on the placebo arm (mean, 4.4 years [SD, 1.3 years]) than on the tamoxifen arm (mean, 4.1 years [SD, 1.5 years]; P < .001). Significant differences in the proportion of women who were classified as adherent between treatment arms were observed after 12 months (P < .003), and differences were largest at 54 months. Overall, annual dropout rates were highest within the first 12 to 18 months of follow-up (12.2%, tamoxifen arm; 7.4%, placebo arm) and decreased thereafter (Fig 3).
Fig 2.
Kaplan-Meier curve for adherence over 4.5 years of follow-up according to treatment arm.
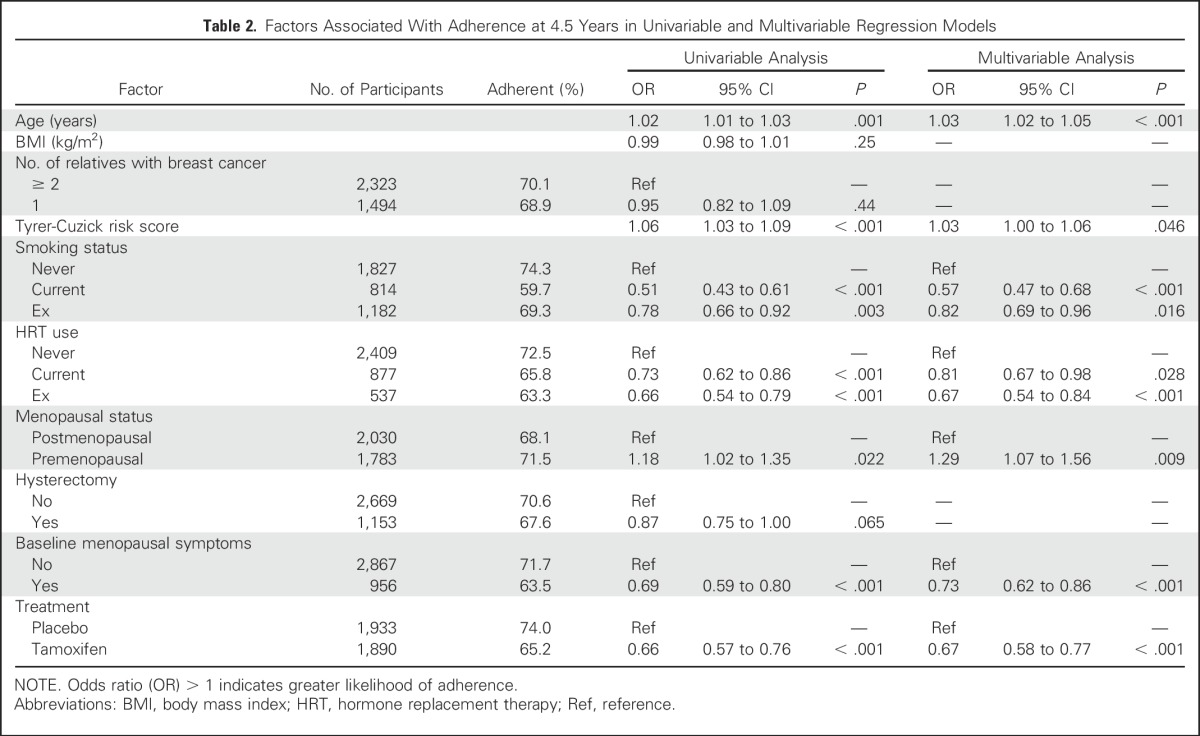
Fig 3.
Annual hazard rates for nonadherence according to treatment arm.
In univariable analysis, family history, smoking status, HRT use, menopausal status, occurrence of hysterectomy, baseline menopausal symptoms, and treatment arm were significantly associated with adherence and were entered into the multivariable model (Table 2). In the multivariable analysis, women in the tamoxifen arm (OR, 0.67; 95% CI, 0.58 to 0.77; P < .001) and women with any baseline menopausal symptoms (OR, 0.73; 95% CI, 0.62 to 0.86; P < .001) were less likely to be adherent at follow-up. Significantly lower odds of adherence were also observed in current smokers (OR, 0.57; 95% CI, 0.47 to 0.68; P < .001), ex-smokers (OR, 0.82; 95% CI, 0.69 to 0.96; P = .016), users of HRT at baseline (OR, 0.81; 95% CI, 0.67 to 0.98; P = .028), and ex-users of HRT (OR, 0.67; 95% CI, 0.54 to 0.84; P < .001). Higher odds of adherence were noted with increasing age (OR, 1.03 per year; 95% CI, 1.02 to 1.05 per year; P < .001), premenopausal women (OR, 1.29; 95% CI, 1.07 to 1.56; P = .009), and those with a higher Tyrer-Cuzick risk score (OR, 1.03 per 1% increase; 95% CI, 1.00 to 1.06 per 1% increase; P = .046).
Table 2.
Factors Associated With Adherence at 4.5 Years in Univariable and Multivariable Regression Models
Baseline menopausal symptoms were reported by 25.0% of women, but the difference between treatment arms was not statistically significant (P = .74). At 6-month follow-up, a low proportion of women experienced nausea/vomiting (5.6% tamoxifen arm v 4.5% placebo arm; P = .14) and headaches (7.3% tamoxifen arm v 6.8% placebo arm; P = .53), whereas gynecologic symptoms (28.9% tamoxifen arm v 14.2% placebo arm; P < .001) and hot flashes (44.2% tamoxifen arm v 20.5% placebo arm; P < .001) were more common and significantly different between treatment arms. More than half (53%) of nonadherence is explained by treatment group (P < .001) and an additional 22% of nonadherence is explained by reporting any of the aforementioned symptoms at 6 months (P = .002).
Women reporting nausea/vomiting were less likely to be adherent in both the tamoxifen (OR, 0.57; 95% CI, 0.37 to 0.86; P = .007) and placebo (OR, 0.58; 95% CI, 0.37 to 0.93; P = .023) arms (Table 3). Headaches were associated with adherence in the placebo arm only (OR, 0.62; 95% CI, 0.42 to 0.91; P = .016), whereas gynecologic symptoms were significant in the tamoxifen arm (OR, 0.77; 95% CI, 0.62 to 0.97; P = .024; Table 3). Hot flashes were not associated with adherence in either treatment group. Tests of heterogeneity showed that the effect sizes for each symptom on adherence were not significantly different between the treatment groups (nausea/vomiting, P = .96; headaches, P = .25; hot flashes, P =.53; gynecologic symptoms, P = .24).
Table 3.
Presence or Absence of Symptoms Reported at 6 Months Associated With Adherence by Study Arm in Multivariable Model
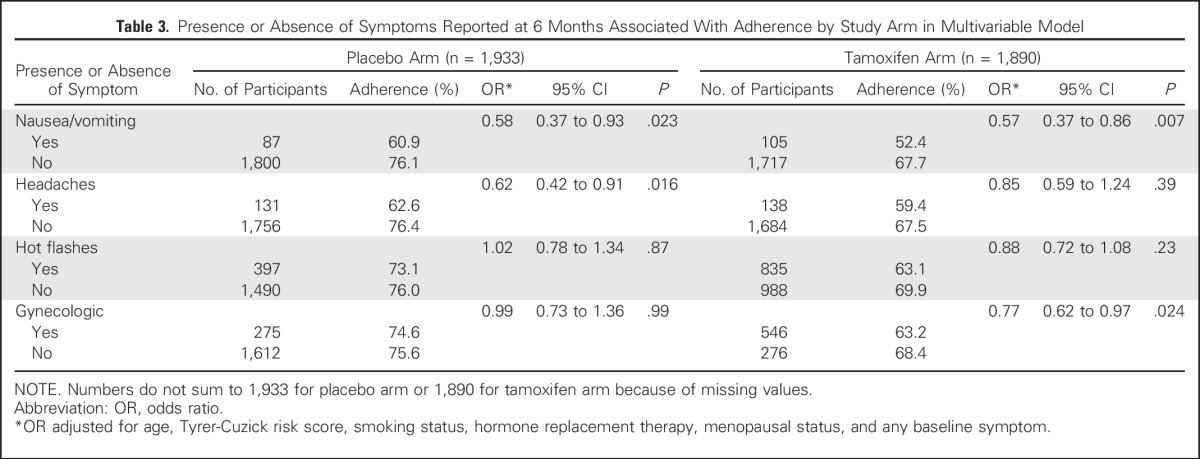
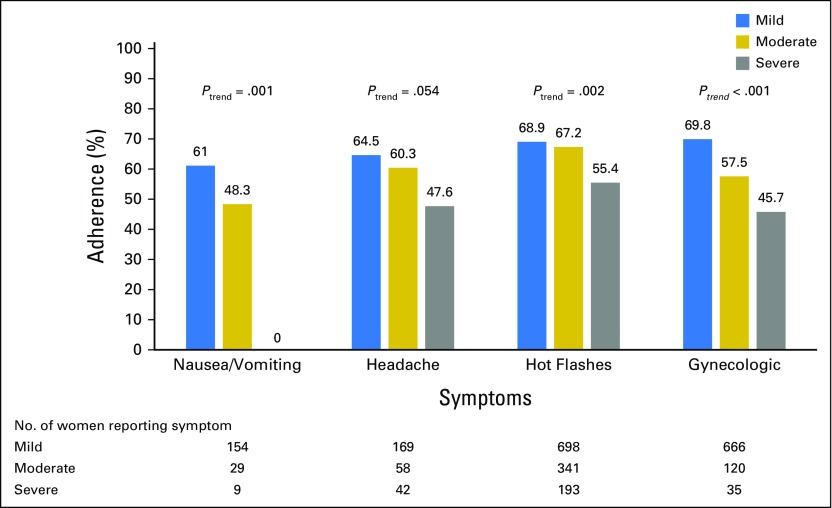
The majority of symptoms reported at 6 months among both treatment groups were of mild or moderate severity (Fig 4). There were significant trends for lower adherence with increasing severity for all symptoms (P < .002 to P < .001), with the exception of headaches (P = .054).
Fig 4.
Adherence for 4.5 years according to severity of symptoms.
DISCUSSION
In the IBIS-I primary prevention trial, two thirds of women were adherent to tamoxifen or matching placebo for the duration of therapy. Dropout rates were highest in the tamoxifen arm, particularly after the first 12 months of follow-up, which coincides with the period when women are most susceptible to experiencing adverse effects from the drug.24 The association between nausea/vomiting, headaches, hot flashes, and gynecologic symptoms was largely similar between the tamoxifen and placebo arms, and tests of heterogeneity indicated no significant differences between the arms. Women may therefore be attributing age-related symptoms to their assigned medication. This is suggestive of the nocebo response, whereby seemingly inert substances cause adverse symptoms or effects.28
Bodily symptoms such as dizziness, headache, and fatigue are frequently misattributed by clinical trial participants as adverse effects of medications, and this also extends to participants on the placebo arm.29-31 For example, as expected in the International Breast Cancer Intervention Study II (IBIS II) trial, a large proportion (64%) of women taking anastrozole reported musculoskeletal problems; however, only slightly lower proportions of women experiencing this symptom (58%) were noted in the placebo arm.32 Such symptoms are also common among healthy people,33 demonstrating that trial participants may be misattributing normal bodily changes to adverse effects of drugs. In line with findings in the adjuvant setting, our data suggest that misattributing non-medication symptoms as adverse effects can affect adherence.34
Our observations have implications for communicating with women who are considering initiating preventive therapy and for those already using it. Research from the literature on chronic disease has shown people with more negative general attitudes toward medication at baseline are more likely to misattribute non-medication symptoms as adverse effects.35 Addressing such concerns in women before preventive therapy is initiated may reduce symptom misattribution and have consequent positive effects on medication adherence.36 Discussing the prevalence and natural history of symptoms with women before therapy is initiated may also affect their interpretation of normal bodily changes. This information will be particularly important for women who are expected to experience menopause while taking preventive therapy. These discussions may encourage more realistic expectations of the likelihood of experiencing adverse effects, which have been shown to be powerful drivers of subsequent experience37 and adherence in the adjuvant setting.38
Although it is commonly thought that treatment-related adverse effects affect adherence to preventive therapy for breast cancer, the evidence supporting this assumption is weak. A recent systematic review of adherence to preventive therapy for breast cancer included studies that suggested an association between adverse effects of treatment and persistent use of medication.9 However, the design of these studies was subject to bias because of retrospective recall when completing the off-therapy forms and an inability to compare adherence rates between those who did and did not experience adverse effects.20-23 Data from the NSABP P-1 trial has demonstrated the role of gynecologic, vasomotor, and sexual symptoms on adherence at 1-year follow-up.17 Our analysis adds to these data by demonstrating that more than one fifth of 5-year nonadherence can be explained by participant-reported early symptoms.
To ensure that women experience the full benefit of preventive therapy, interventions such as that used in the MENOS1 (Randomised Controlled Trial of a Cognitive Behavioural Intervention for Women Who Have Menopausal Symptoms Following Breast Cancer Treatment)39 trial have been successful in helping to manage symptoms. Physical activity has also been shown to reduce the impact of adverse effects experienced in the adjuvant breast cancer setting, including fatigue,40 arthralgia,41 and menopausal symptoms.42 Medication-taking behavior was not assessed as an outcome within these trials, and the extent to which improving symptoms and adverse effects by physical activity translates to improved adherence remains an unexplored hypothesis.
This study had notable strengths and limitations. We are among the first to prospectively investigate the association between these predefined symptoms and subsequent long-term adherence in women taking breast cancer preventive therapy. The analysis used data from the IBIS-I trial, which includes a large sample of well-characterized women who regularly completed clinic visits during which adherence was assessed. However, there is no current gold standard for assessing adherence. Although there are notable advantages to using clinician-assessed case report forms, this method may be subject to bias, which likely led us to overestimate adherence. Adherence was defined as the time between initiating and stopping the medication.26 However, we did not report the use of medications on a day-to-day basis, and different patterns of associations between our exposure and outcome variables may be expected if adherence was defined differently. Our data were collected in the context of a clinical trial with women who may have a stronger motivation to participate than those taking tamoxifen as part of usual care. Our adherence estimates may therefore be higher than achievable in routine practice, and additional research is needed to investigate adherence outside a trial context.43 Our analysis focused on the relationship between early symptoms and long-term adherence because the majority of the symptoms we assessed occur within the first year of therapy.24 However, the symptoms experienced by some women may have been transient, which may have weakened the relationship between experiencing a symptom and subsequent adherence. Reporting an early symptom may also be a marker for subsequent experience, and the evolution of these symptoms was not investigated as part of this analysis. Late-onset symptoms may be contributing to the 25% of unexplained variance in 5-year adherence that we identified within these data. Finally, because of time constraints in the clinic visits, we assessed the experience of symptoms by using single severity measures for each item. These measures do not assess the effect of symptoms on physical, mental, or sexual quality of life. Participant-reported outcome measures assessing these factors may be more closely associated with adherence.17
In conclusion, these data from the IBIS-I breast cancer preventive therapy trial show that the effect of nausea/vomiting, headaches, hot flashes, and gynecologic symptoms on adherence was similar between tamoxifen and placebo arms, suggesting that women are attributing non-medication–related bodily changes to preventive therapy. Our data have implications for communicating with prospective users of preventive therapy, particularly with regard to encouraging accurate symptom expectations and correcting potential misattributions. Intervention strategies are needed to promote adherence, as well as to effectively communicate the harms and benefits of preventive therapy to participants.
ACKNOWLEDGMENT
This work was supported by Cancer Research UK (C569/A16891 [J.C.], C42785/A17965 [S.S.]). AstraZeneca supplied tamoxifen and matching placebo without charge. We also acknowledge the support of Madeleine Freeman in the inter-rater reliability analysis.
Appendix
International Breast Cancer Intervention I Rules for Adherence
Time between initiation and cessation is less than 4.5 years = nonadherent
Time between initiation and cessation is 4.5 years or more = adherent
Described in notes as:
Off study = nonadherent
Poor compliance = nonadherent
Cannot be contacted = nonadherent
Substantial (25+) pills remaining = nonadherent
Stopped for 1 month or less = adherent
Stopped for more than 1 month = nonadherent
Took on alternate days for < 6 months = adherent
Took on alternate days for > 6 months/no timeframe given = nonadherent
Missed a few days = adherent
If participants did not attend appointment, but they attended subsequent appointments, assume they were sent medication = adherent
If participants did not attend appointment and did not attend any subsequent appointments, assume study dropout = nonadherent
Footnotes
Clinical trial information: ISRCTN91879928.
AUTHOR CONTRIBUTIONS
Conception and design: Samuel George Smith, Ivana Sestak, John Forbes, Jack Cuzick
Collection and assembly of data: Ivana Sestak, John Forbes, Jack Cuzick
Data analysis and interpretation: Samuel George Smith, Ivana Sestak, Anthony Howell, Jack Cuzick
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Samuel George Smith
No relationship to disclose
Ivana Sestak
No relationship to disclose
Anthony Howell
No relationship to disclose
John Forbes
No relationship to disclose
Jack Cuzick
Research Funding: AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Myriad Genetics to Queen Mary University of London of which I receive a share for development of cell cycle progression score (Prolaris)
REFERENCES
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. : Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 378:1461-1484, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Cancer Research UK: Breast cancer statistics. 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer.
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Weir HK, Thompson TD, Soman A, et al. : The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 121:1827-1837, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis C, Ma J, Bryan L, et al. : Breast cancer statistics, 2013. CA Cancer J Clin 64:52-62, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Umar A, Dunn BK, Greenwald P: Future directions in cancer prevention. Nat Rev Cancer 12:835-848, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Bonanni B, et al. : Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet 381:1827-1834, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzick J, Sestak I, Cawthorn S, et al. : Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 16:67-75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SG, Sestak I, Forster A, et al. : Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann Oncol 27:575-590, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bober SL, Hoke LA, Duda RB, et al. : Decision-making about tamoxifen in women at high risk for breast cancer: Clinical and psychological factors. J Clin Oncol 22:4951-4957, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Yeomans-Kinney A, Vernon SW, Frankowski RF, et al. : Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer 76:46-56, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Altschuler A, Somkin CP: Women’s decision making about whether or not to use breast cancer chemoprevention. Women Health 41:81-95, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Donnelly LS, Evans DG, Wiseman J, et al. : Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br J Cancer 110:1681-1687, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heisey R, Pimlott N, Clemons M, et al. : Women’s views on chemoprevention of breast cancer: Qualitative study. Can Fam Physician 52:624-625, 2006 [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel VG, Costantino JP, Wickerham DL, et al. : Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 3:696-706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powles TJ, Jones AL, Ashley SE, et al. : The Royal Marsden Hospital pilot tamoxifen chemoprevention trial. Breast Cancer Res Treat 31:73-82, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Land SR, Walcott FL, Liu Q, et al. : Symptoms and QOL as predictors of chemoprevention adherence in NRG Oncology/NSABP Trial P-1. J Natl Cancer Inst 108:djv365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallowfield L, Fleissig A, Edwards R, et al. : Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized controlled trials. J Clin Oncol 19:1885-1892, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Land SR, Wickerham DL, Costantino JP, et al. : Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2742-2751, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Palva T, Ranta H, Koivisto AM, et al. : A double-blind placebo-controlled study to evaluate endometrial safety and gynaecological symptoms in women treated for up to 5 years with tamoxifen or placebo: A substudy for IBIS I Breast Cancer Prevention Trial. Eur J Cancer 49:45-51, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Powles TJ, Hardy JR, Ashley SE, et al. : A pilot trial to evaluate the acute toxicity and feasibility of tamoxifen for prevention of breast cancer. Br J Cancer 60:126-131, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Eeles R, Ashley S, et al. : Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352:98-101, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Razzaboni E, Toss A, Cortesi L, et al. : Acceptability and adherence in a chemoprevention trial among women at increased risk for breast cancer attending the Modena Familial Breast and Ovarian Cancer Center (Italy). Breast J 19:10-21, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Sestak I, Kealy R, Edwards R, et al. : Influence of hormone replacement therapy on tamoxifen-induced vasomotor symptoms. J Clin Oncol 24:3991-3996, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Forbes J, Edwards R, et al. : First results from the International Breast Cancer Intervention Study (IBIS-I): A randomised prevention trial. Lancet 360:817-824, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Vrijens B, De Geest S, Hughes DA, et al. : A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73:691-705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 28.Colloca L, Miller FG: The nocebo effect and its relevance for clinical practice. Psychosom Med 73:598-603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rief W, Avorn J, Barsky AJ: Medication-attributed adverse effects in placebo groups: Implications for assessment of adverse effects. Arch Intern Med 166:155-160, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al. : Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: A systematic review and meta-analysis. Drug Saf 32:1041-1056, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Amanzio M, Corazzini LL, Vase L, et al. : A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain 146:261-269, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Sestak I, Forbes JF, et al. : Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 383:1041-1048, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Tan K, Petrie KJ, Faasse K, et al. : Unhelpful information about adverse drug reactions. BMJ 349:g5019, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Ammassari A, Murri R, Pezzotti P, et al. : Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr 28:445-449, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Heller MK, Chapman SCE, Horne R: Beliefs about medication predict the misattribution of a common symptom as a medication side effect: Evidence from an analogue online study. J Psychosom Res 79:519-529, 2015 [DOI] [PubMed] [Google Scholar]
- 36.von Blanckenburg P, Schuricht F, Albert US, et al. : Optimizing expectations to prevent side effects and enhance quality of life in breast cancer patients undergoing endocrine therapy: Study protocol of a randomized controlled trial. BMC Cancer 13:426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohl SJ, Schnur JB, Montgomery GH: A meta-analysis of the relationship between response expectancies and cancer treatment-related side effects. J Pain Symptom Manage 38:775-784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nestoriuc Y, von Blanckenburg P, Schuricht F, et al. : Is it best to expect the worst? Influence of patients’ side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol 27:1909-1915, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Mann E, Smith MJ, Hellier J, et al. : Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): A randomised controlled trial. Lancet Oncol 13:309-318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vulpen JK, Peeters PH, Velthuis MJ, et al. : Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas 85:104-111, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Irwin ML, Cartmel B, Gross CP, et al. : Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 33:1104-1111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duijts SFA, van Beurden M, Oldenburg HS, et al. : Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol 30:4124-4133, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Roetzheim RG, Lee JH, Fulp W, et al. : Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast 24:51-56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]