Abstract
Purpose
Survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for neurocognitive deficits that are associated with treatment, individual, and environmental factors. This study examined the impact of different methotrexate (MTX) and corticosteroid treatment strategies on neurocognitive functioning in children with high-risk B-lineage ALL.
Methods
Participants were randomly assigned to receive high-dose MTX with leucovorin rescue or escalating dose MTX with PEG asparaginase without leucovorin rescue. Patients were also randomly assigned to corticosteroid therapy that included either dexamethasone or prednisone. A neurocognitive evaluation of intellectual functioning (IQ), working memory, and processing speed (PS) was conducted 8 to 24 months after treatment completion (n = 192).
Results
The method of MTX delivery and corticosteroid assignment were unrelated to differences in neurocognitive outcomes after controlling for ethnicity, race, age, gender, insurance status, and time off treatment; however, survivors who were age < 10 years at diagnosis (n = 89) had significantly lower estimated IQ (P < .001) and PS scores (P = .02) compared with participants age ≥ 10 years. In addition, participants who were covered by US public health insurance had estimated IQs that were significantly lower (P < .001) than those with US private or military insurance.
Conclusion
Children with high-risk B-lineage ALL who were age < 10 years at diagnosis are at risk for deficits in IQ and PS in the absence of cranial radiation, regardless of MTX delivery or corticosteroid type. These data may serve as a basis for developing screening protocols to identify children who are at high risk for deficits so that early intervention can be initiated to mitigate the impact of therapy on neurocognitive outcomes.
INTRODUCTION
Children who are treated for acute lymphoblastic leukemia (ALL) without cranial radiation are at risk for neurocognitive late effects, including deficits in processing speed (PS), visual-motor abilities, attention, working memory (WM), and executive function.1-6 Intellectual functioning (IQ) commonly remains within the average range,7,8 although mean IQs may decline significantly within the average range.9 Younger age at diagnosis,1,4 female gender,3,10 Hispanic/Latino ethnicity,11 and lower socioeconomic status (SES)12 have emerged as risk factors for poorer neurocognitive outcomes. Although many survivors exhibit stable functioning, 20% to 40% develop cognitive difficulties over time that impact overall adaptive functioning, learning, and adjustment.13
Intravenous (IV) methotrexate (MTX) is an important component of therapy, although doses and schedules of MTX infusions and leucovorin rescue vary across regimens. Some evidence suggests that higher doses of MTX increase the risk for neurocognitive impairment14-16; however, children who received extremely high-dose MTX (HDMTX; 33.6 g/m2) have exhibited stable verbal IQs and improvements in performance IQ over time,17 which suggests that dose alone does not predict outcome. When examining the impact of dexamethasone versus prednisone, few significant differences have been found in IQ, attention, PS, or WM, although a higher percentage of children who are treated with dexamethasone receive special education services.18 Thus, the differential impact of variations in MTX and corticosteroid delivery on neurocognitive outcome is unclear. Much of the published data that examine these variables have been derived from single- or limited institution trials, or from retrospective samples in which key components of therapy vary or were not randomized. The Children’s Oncology Group (COG) AALL023219 trial randomly assigned patients with high-risk B-lineage ALL (HR B-ALL) to receive therapy that included a 2-month block of either HDMTX with leucovorin rescue or a lower, escalating dose MTX without leucovorin rescue, plus asparaginase.
Results demonstrated a significant increase in the 5-year event-free survival for participants who received HDMTX (79.6%) versus the escalating dose regimen (75.2%).19 Children age 1 to 9 years were also randomly assigned to receive 14 days of dexamethasone versus 28 days of prednisone during induction therapy, with superior survival observed for those randomly assigned to receive dexamethasone plus HDMTX compared with other regimens. This trial provided an opportunity to evaluate the relative impact of two different approaches to MTX and corticosteroid delivery while examining the moderating role of age on neurocognitive functioning.
The primary aim of this study was to evaluate the differences in estimated IQ, WM, and PS between children who were randomly assigned to receive HDMTX with leucovorin rescue versus those who received escalating dose MTX with asparaginase. The effect of the corticosteroid—dexamethasone versus prednisone—delivered during induction therapy was also evaluated. An exploratory aim was to identify germline host polymorphisms that may predict which individuals are at increased risk for neurocognitive toxicity. This study represents the first evaluation of patients randomly assigned to different MTX and corticosteroid regimens within a common therapeutic trial.
METHODS
COG AALL06N1 was designed to evaluate the neurocognitive impact of treatment delivered in AALL0232. Slow accrual led to several amendments that were designed to facilitate enrollment, including changing from a longitudinal design to a cross-sectional design, expanding the assessment window to 8 to 24 months after completion of AALL0232 therapy, and revising the evaluation from a ≥ 4-hour comprehensive neuropsychological battery to a screening battery (approximately 1 hour) administered by a psychometrist, as in COG ALTE07C1.20
As amended, eligibility criteria for AALL06N1 included enrollment in COG AALL0232, age at diagnosis of 1 to 18 years, and a primary language of English or Spanish. Exclusion criteria included preexisting neurodevelopmental disability, significant sensory impairment, central nervous system (CNS) involvement, treatment with cranial radiation, or recurrent disease. AALL06N1 was approved by the National Cancer Institute and the institutional review boards of participating institutions. Informed consent was obtained in accordance with Department of Health and Human Services guidelines. All participants had also enrolled in AALL03B1. This trial included the procurement of remission peripheral blood samples for the determination of host polymorphisms. Single-nucleotide polymorphism (SNP) genotyping was performed on germline DNA by using the Illumina Human Exome BeadChip v1.1 (Illumina, San Diego, CA) and Affymetrix GeneChip Human Mapping Array 6.0 (Affymetrix, Santa Clara, CA), and imputation was performed according to the 1000 Genome Project as previously described.21
Participants
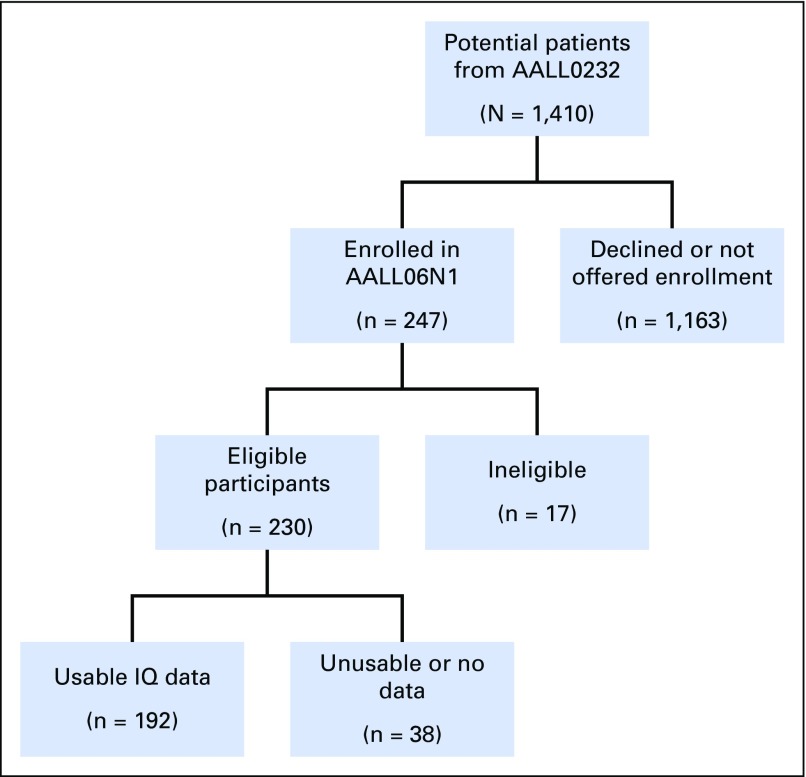
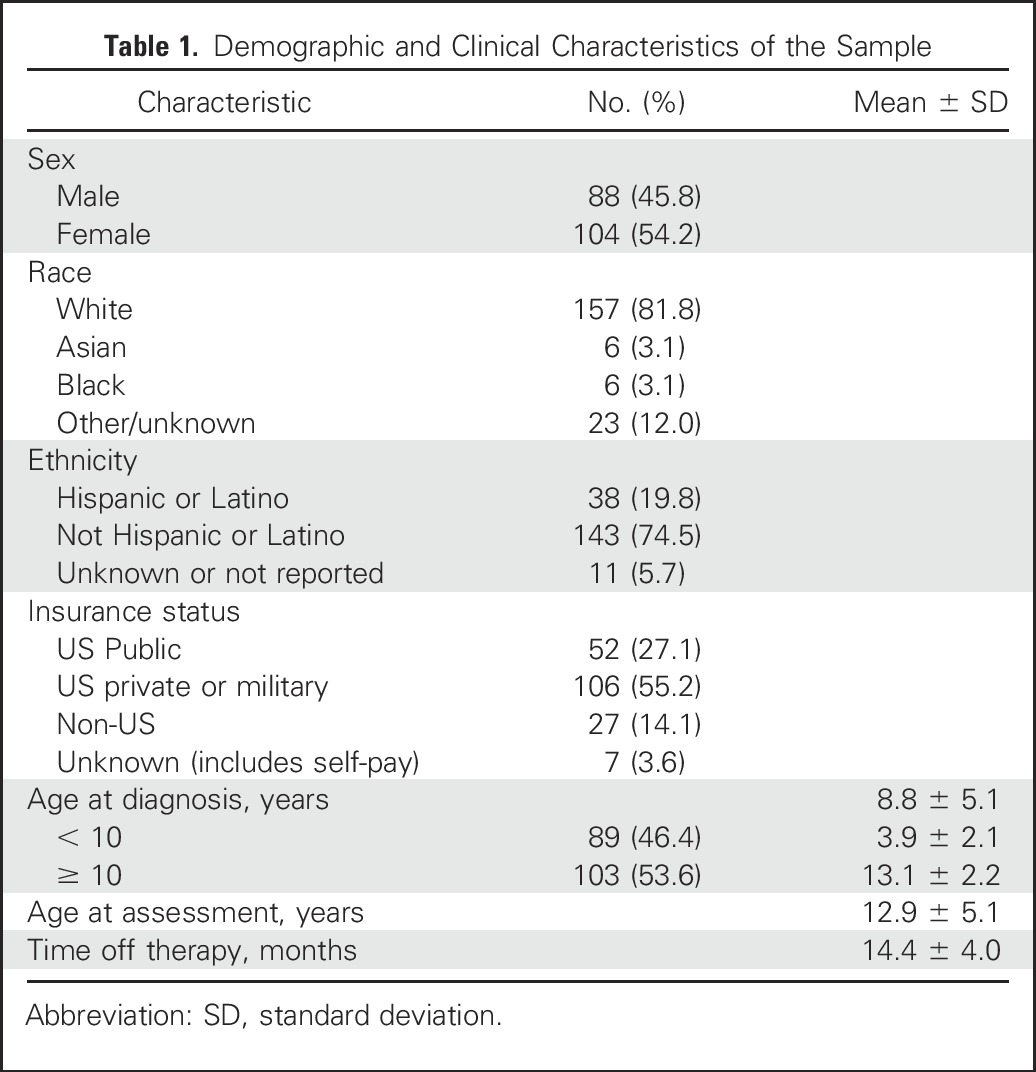
The study enrolled 230 eligible participants (Fig 1), with a final sample of 192 participants (83.5%) who submitted valid neurocognitive data. Fifty-four percent were female (n = 104), 82% (n = 157) self-identified as White, 20% (n = 38) Hispanic, and 46% (n = 89) were age < 10 years at diagnosis (mean age, 8.8 years; standard deviation, 5.1). Insurance status was used as a proxy for SES. Insurance designations were collapsed into the following categories for analysis: US public (n = 52), US private or military (n = 106), non-US (n = 27), and unknown or self-pay (n = 7; Table 1).
Fig 1.
AALL06N1 flow diagram (excluding two enrollments from AALL0434). IQ, intelligence quotient.
Table 1.
Demographic and Clinical Characteristics of the Sample
Treatment
A detailed treatment schema for AALL0232 has been published.19 In brief, eligible consenting patients were randomly assigned to 14 days of dexamethasone or 28 days of prednisone (induction), and to four courses of HDMTX with leucovorin rescue or five doses of escalating dose MTX with PEG asparaginase (interim maintenance). Both random assignments were restricted or halted before study closure on the basis of response data accrued during the trial.19 Approximately 4 years after study activation, excessive osteonecrosis among patients who were age > 10 years at diagnosis led to the non–random assignment of these patients to prednisone; younger patients continued to be randomly assigned. Girls received 23 doses and boys 27 doses of intrathecal MTX, with one dose of intrathecal cytarabine on day 1 of therapy for CNS prophylaxis. Patients with overt CNS leukemia and those with a slow early response to induction therapy received cranial radiation and were excluded from AALL06N1.
Procedures
Neurocognitive assessments were conducted 8 to 24 months after completion of therapy with widely used clinical measures with well-established validity and reliability, normalized on large representative samples. To assess the primary domains of estimated IQ, WM, and PS, age-appropriate versions of the Wechsler Intelligence Scales were used.22-24 Estimated IQ was derived by using vocabulary and block design subtests. Within the Wechsler series, this short-form combination correlates highly with full-scale IQ, with validity coefficients of 0.85 to 0.88.25 WM was assessed with digit span, and PS was assessed with symbol search and coding from the Wechsler scales.
Statistical Analysis
Descriptive statistics were calculated for clinical and demographic characteristics, including gender, race, ethnicity, insurance status, age at diagnosis, age at assessment, MTX delivery method, corticosteroid type, time off therapy, estimated IQ, PS, and WM. Primary analysis was performed with multiple linear regression that included the covariates gender, age at diagnosis (age >10 years or age < 10 years), race, ethnicity, insurance status, and time between completion of treatment and assessment. Primary independent variables were MTX delivery method and type of corticosteroid, and the primary outcome was post-treatment estimated IQ score. PS and WM were analyzed as secondary outcome variables using the same model. Models with interactions terms for MTX dosing and the type of corticosteroid as well as for age at diagnosis and MTX dosing or corticosteroid were also considered on the basis of prior outcome results.19 Statistical significance was defined as P < .05. A sample size of 192 gives 79% and 78% power, respectively, to detect a 0.4 standard deviation difference in post-treatment estimated IQ for MTX and corticosteroid comparisons on the basis of attained sample sizes per group. All analyses were performed by using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC).
Genome-wide association study was performed with estimated IQ score or PS as dependent variables and using linear regression models that adjusted for covariates using PLINK (Version 1.9, Center for Human Genetic Research, Boston, MA). Covariates included age, corticosteroid use (dexamethasone v prednisone), MTX use (HDMTX v escalating dose), insurance status, and ancestry treated as a continuous variable (percent European, African, Asian, or Native American). Ancestry was determined by using STRUCTURE (Version 2.2.3).21
RESULTS
We compared demographic characteristics between eligible participants in AALL06N1 and eligible individuals from AALL0232 who did not enroll in AALL06N1. Compared with patients in AALL0232 (n = 1,163), participants in AALL06N1 were younger—at diagnosis—by an average of 1.2 years (P < .01) and fewer identified as Hispanic/Latino (19% v 22%; P = .02).
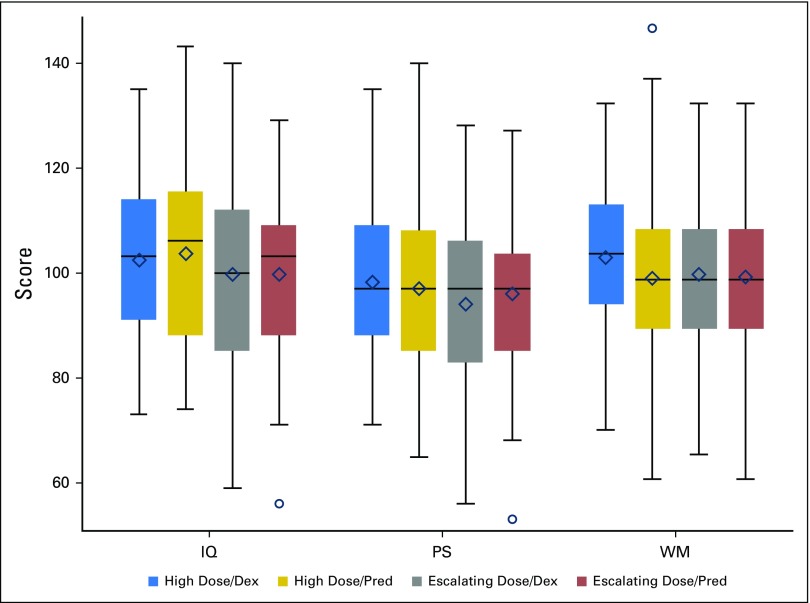
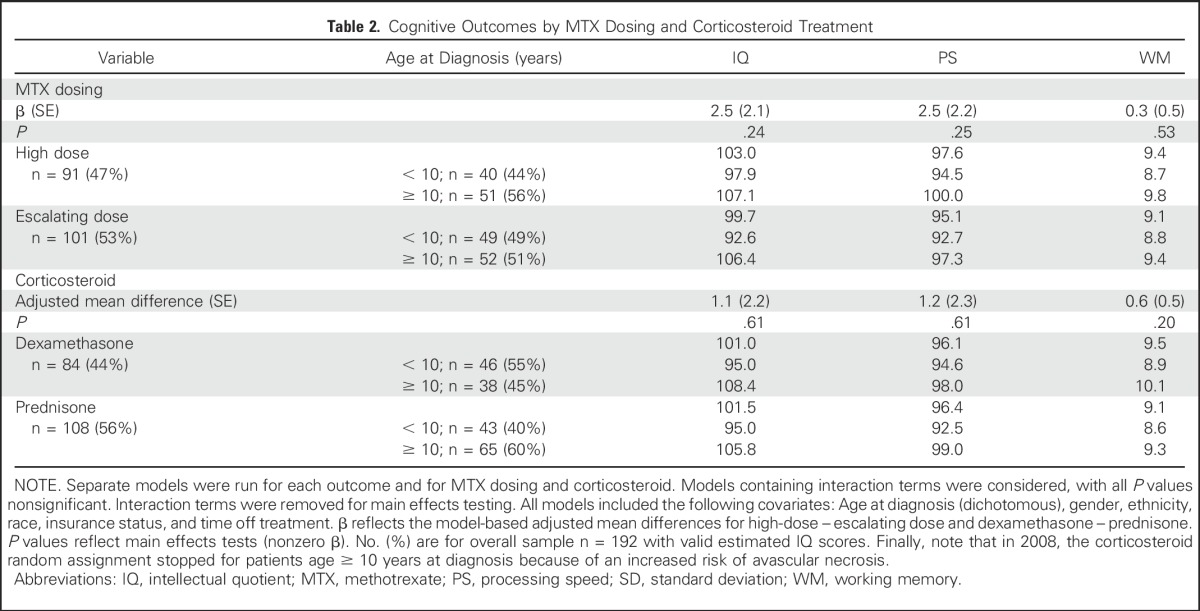
After controlling for age, gender, race, ethnicity, time since diagnosis, and type of insurance, there were no significant differences in estimated IQ, PS, or WM scores 8 to 24 months postcompletion of therapy for children who received HDMTX versus escalating dose MTX, nor were there significant differences on the basis of induction corticosteroid type (all P ≥ .20; Table 2). The interaction between MTX dosing and the type of corticosteroid reflected no significant differences for any of the cognitive outcomes (all P ≥ .45), including when analysis was restricted to only patients whose corticosteroid treatment was randomly assigned (Appendix Table A1, online only). Interaction between age at diagnosis and MTX dosing or corticosteroid was also examined with nonsignificant results (all P ≥ .17).
Table 2.
Cognitive Outcomes by MTX Dosing and Corticosteroid Treatment
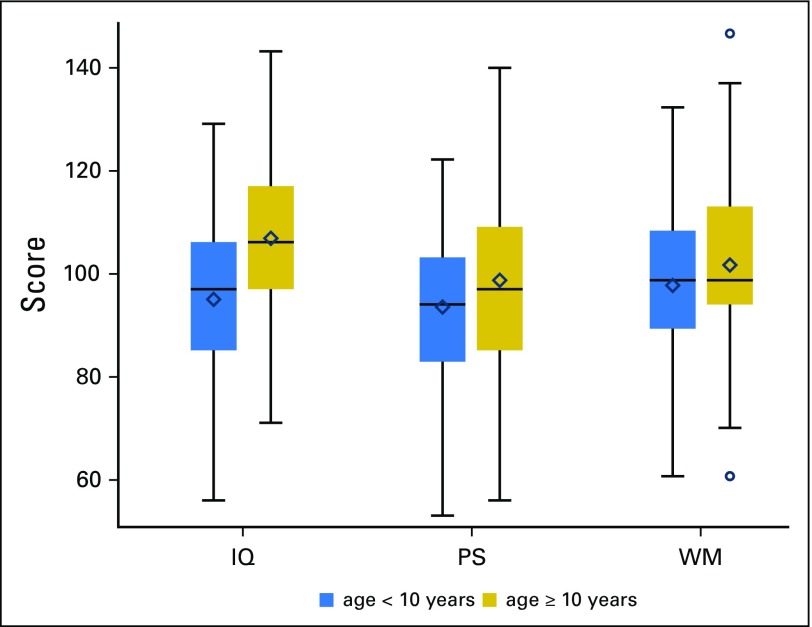
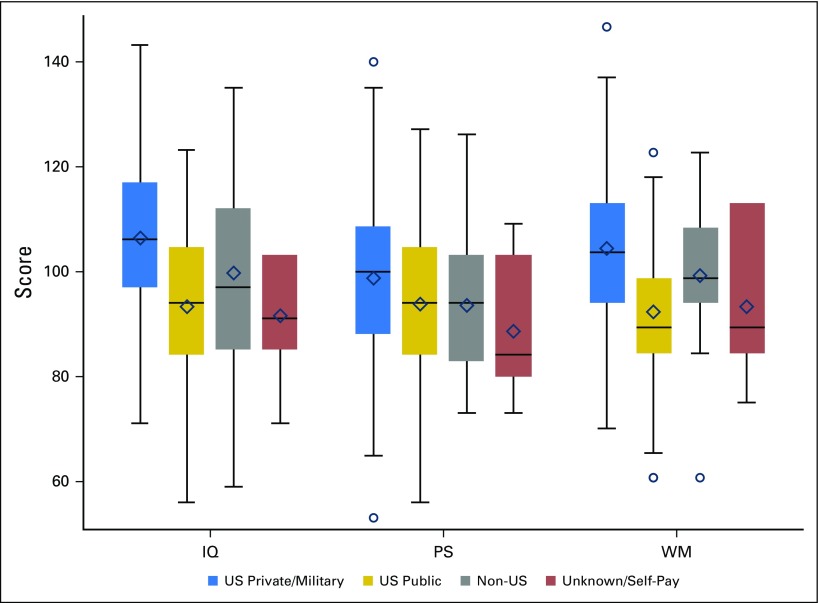
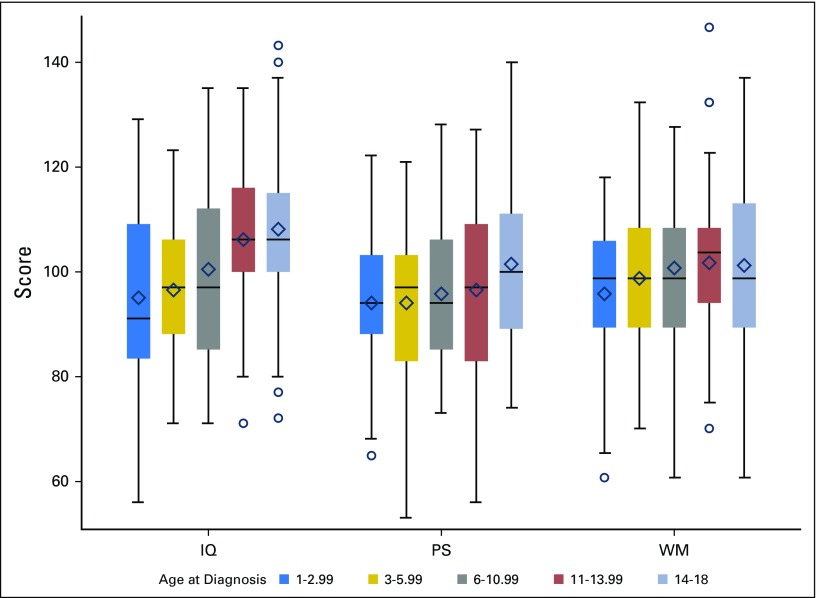
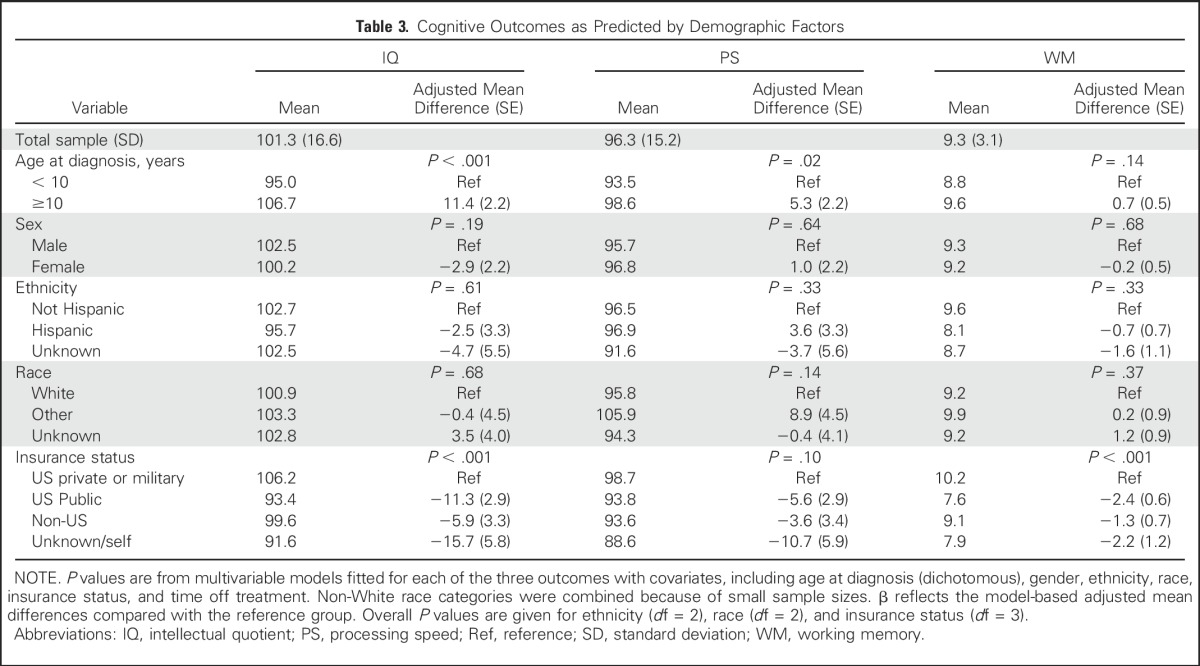
In multivariable models with the other covariates, both age and insurance status were found to be significant predictors of post-treatment estimated IQ (both P < .001); age was predictive of post-treatment PS (P = .02; Fig 2); and insurance type was predictive of post-treatment WM (P < .001; Fig 3). Specifically, children who were age < 10 years at diagnosis exhibited significantly lower post-treatment estimated IQ (adjusted difference, 11.4; standard error [SE], 2.2) and PS (adjusted difference, 5.3; SE, 2.2) scores compared with those age ≥ 10 years (Table 3). Using age at diagnosis as a continuous variable in a multivariable logistic model, a single year of increase in age at diagnosis was associated with a 14% decrease in the odds of having an IQ score ≥ 1 standard deviation below the normative mean. In addition, US public insurance was associated with lower post-treatment IQ scores (adjusted difference, −11.3; SE, 2.9) and WM (adjusted difference, −2.4; SE, 0.6) compared with US private and military insurance (Table 3).
Fig 2.
Cognitive outcomes by age at diagnosis. Colored boxes represent the middle 50% of the sample with the median represented as a horizontal line and mean as diamond. The ends of the whiskers are the minimum and maximum values discounting outliers that are beyond 1.5 interquartile ranges above or below the 75th and 25th percentiles, respectively, which are delineated separately as circles. For presentation purposes, working memory (WM) was scaled to have a sample mean of 100 and a sample standard deviation of 15. IQ, intellectual quotient; PS, processing speed.
Fig 3.
Cognitive outcomes by insurance status. Colored boxes represent the middle 50% of the sample with the median represented as a horizontal line and mean as diamond. The ends of the whiskers are the minimum and maximum values discounting outliers that are beyond 1.5 interquartile ranges above or below the 75th and 25th percentiles, respectively, which are delineated seprately as circles. For presentation purposes, working memory (WM) was scaled to have a sample mean of 100 and a sample standard deviation of 15. IQ, intellectual quotient; PS, processing speed.
Table 3.
Cognitive Outcomes as Predicted by Demographic Factors
As a group, these survivors showed estimated IQ, PS, and WM scores within the average range (Table 3). Regardless, 21.4% of participants demonstrated impairment in IQ and 28.6% had impaired PS as defined by scores of ≥ 1 standard deviation below the mean—versus 15.9% of individuals in the normative samples for each Wechsler measure (two-sided P = 2.04 and < .01, respectively). In contrast, the number of participants with WM impairment was not different than expectations on the basis of the standardization sample (15.9% vs. 15.9; P > 0.99). Figures 2-4, Appendix Figure A1 (online only) and the Data Supplement show the distributions of outcomes by treatment and key demographic features.
Fig 4.
Cognitive outcomes by methotrexate dosing and corticosteroid treatment. Colored boxes represent the middle 50% of the sample with the median represented as a horizontal line and mean as diamond. The ends of the whiskers are the minimum and maximum values discounting outliers that are beyond 1.5 interquartile ranges above or below the 75th and 25th percentiles, respectively, which are delineated separately as circles. For presentation purposes, working memory (WM) was scaled to have a sample mean of 100 and a sample standard deviation of 15. Dex, dexamethasone; IQ, intellectual quotient; Pred, prednisone; PS, processing speed.
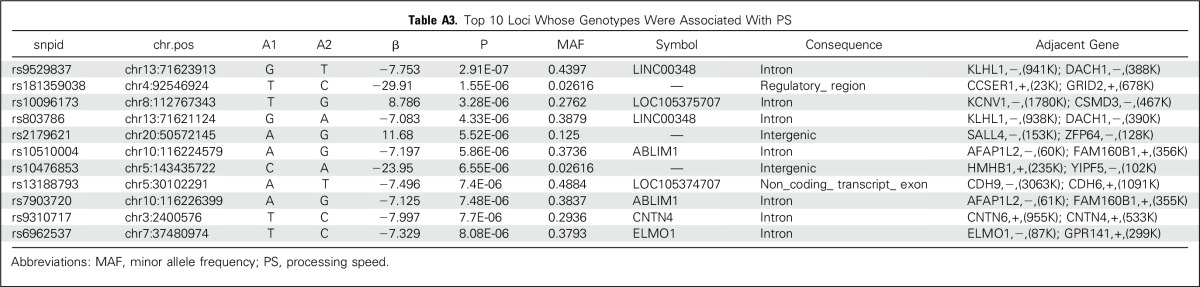
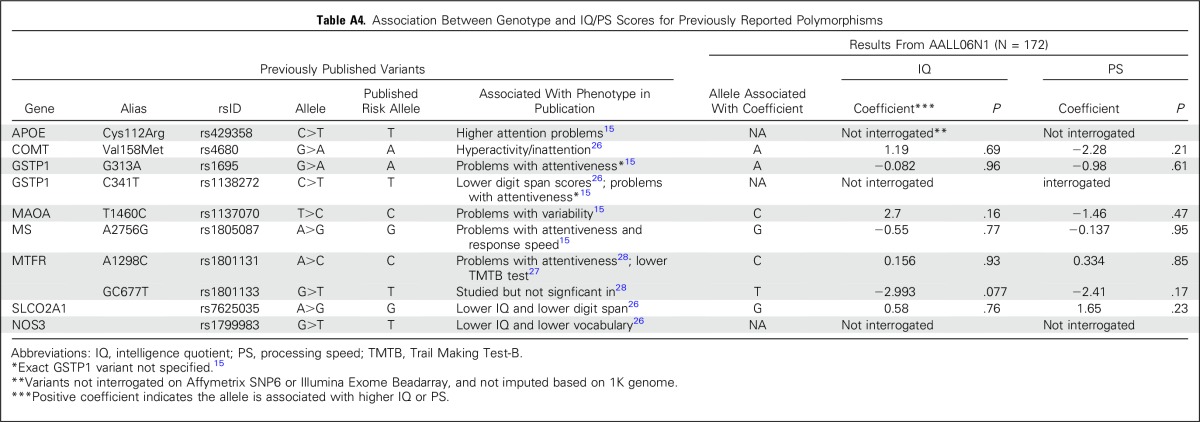
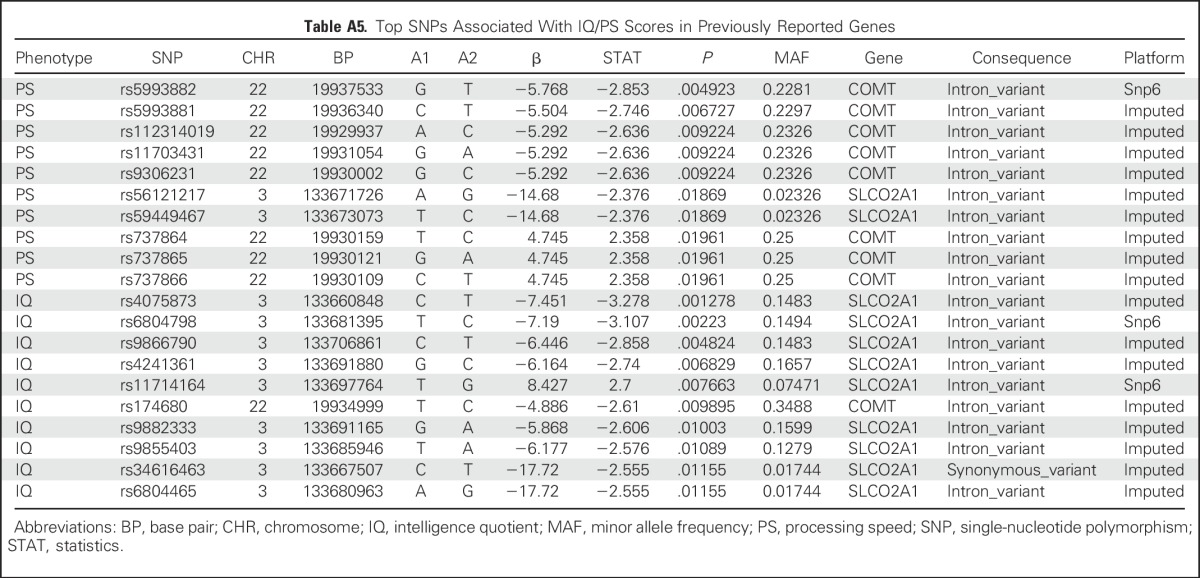
An exploratory aim sought to determine whether neurocognitive outcomes were influenced by identifiable germline SNPs. We tested the association between germline genotypes and estimated IQ or PS scores in 172 patients with genotypes available at more than 6 million SNPs. Our sample size was significantly underpowered for a genome-wide association study; thus, there were no germline SNPs associated with estimated IQ or PS that reached a genome-wide significance threshold of P < 5 × 10−8. The top 10 loci are listed in Appendix Tables A2 and A3 (online only). We also examined SNPs in candidate genes that were previously associated with decreased attention (COMT, MTHFR, GSTP, MS, MAOA),15,26-28 slower PS (MAOA, MTHFR, MS),15,27,28 and decreases in IQ (SLCO2A1).26 None of these SNPs was significant at the P < .05 level in our study (Appendix Table A4, online only). We additionally examined all 530 SNPs—75 independent loci, on the basis of r2 for linkage disequilibrium of < 0.5 in our study population—that were located within 5,000 base pairs of these candidate genes (Appendix Table A5, online only). No germline genomic variants were associated with IQ or PS at a genome-wide level of significance, although COMT variants rs5993882 and rs174680 were associated with PS and IQ at P = .005 and .01, respectively.
DISCUSSION
The majority of children with HR B-ALL will be cured, which makes it imperative that we understand how components of therapy impact long-term neurocognition. This trial is the first to our knowledge to assess neurocognitive function 8 to 24 months after the completion of therapy among patients with HR B-ALL who were treated without cranial irradiation and randomly assigned to two different methods of MTX and corticosteroid delivery. Neither MTX delivery method nor corticosteroid assignment was associated with neurocognitive outcome 3 to 5 years after diagnosis; however, regardless of treatment arm, patients who were age < 10 years at diagnosis were at a greater risk for neurocognitive toxicity, with significantly lower estimated IQ and PS scores compared with older participants after controlling for ethnicity, race, gender, insurance status, and time off treatment. In addition, participants covered by US public insurers had estimated IQs that were approximately three fourths of a standard deviation lower than participants with US private or military insurance.
Detailed meta-analysis, reviews, and large single-institution trials have demonstrated neurocognitive deficits in survivors of ALL who were treated without cranial radiation.6,8,29 An integral component of all curative regimens for patients with ALL, MTX has been associated with acute, subacute, and chronic neurotoxicities.6,8,29-33 Acute neurotoxicity and leukoencephalopathy31,34 have been described after repetitive exposure to low doses of oral MTX and after IV doses of only 1 g/m2 per dose, with the frequency of toxicity lessened by increasing leucovorin rescue.31,34 Similarly, infants who were treated with IV infusions of MTX 33.6 g/m2 had little acute neurotoxicity and stable verbal IQs, with improvements in performance IQ over time,17 when therapy included early, high-dose leucovorin (200 mg/m2 at hour 36; 12 mg/m2 every 3 hours × 6, then every 6 hours until [MTX]plasma < 0.08 μM). Thus, the impact of MTX dose is influenced by the timing and quantity of leucovorin. Our finding of no significant difference in neurocognitive outcomes among patients who were randomly assigned to HDMTX with leucovorin versus escalating dose MTX with PEG asparaginase does not support an association between MTX dose and neurocognitive effects, although this is confounded by the use of leucovorin rescue in one arm and PEG asparaginase in the other arm.
There were also no significant corticosteroid-related differences in estimated IQ, PS, or WM. Similarly, no significant difference in overall neurocognitive function was observed among children who were randomly assigned to dexamethasone versus prednisone in two prior studies, although one found a difference in word reading35 and the other, a difference in fluid reasoning.18 Considering all available data, there seems to be no robust corticosteroid-related differences in neurocognitive outcomes, although there may be differences in specific cognitive domains that merit further study.
Although treatment-related variables did not predict neurocognitive outcomes in our sample, demographic factors significantly predicted differences in estimated IQ and PS. Insurance status—used as a proxy for SES—correlated with both estimated IQ and WM, with an increased risk of lower scores associated with US public insurance status. This relationship between socioeconomic disadvantage and cognitive outcome has been established for typically developing children.36-40 Future work would benefit from the careful assessment of SES, including parental education, resource insecurity, caregiver occupation, and family structure41 as potential moderators that interact with disease or treatment factors to mitigate or enhance neurocognitive deficits among children with ALL. Regardless of etiology, it may be that children with lower cognitive reserve, particularly younger children, may benefit from cognitive enrichment strategies during and after chemotherapy. Indeed, there is some evidence to indicate that enhancement of cognitive or academic skills during this period can mitigate declines over time in children with ALL.42 It is also possible that interventions that have been known to remediate difficulties in the survivorship period could be effective in preventing or delaying the onset of cognitive difficulties.43-45
In this small sample, there were no germline SNPs associated with estimated IQ or PS that reached a genome-wide level of significance. COMT encodes for catechol-O-methyltransferase and its substrates include neurotransmitters. Nonsynonymous variants in COMT (eg, rs4680) have been associated with neuropsychiatric difficulties46-48 and, specifically, with attention and hyperactivity in survivors of ALL.26 Although rs4680 was not associated with estimated IQ or PS in our study (Appendix Table A3), there were other COMT variants (all intronic) that were marginally associated with PS and estimated IQ (Appendix Table A4). Likewise, a variant (rs7625035) in SLCO2A1—a prostaglandin transporter—was associated with lower scores of tests of IQ, digit span, and block design in a prior study of ALL,26 but not in our study. Additional functional studies or replication with larger samples are required to better understand the relationship between specific SNPs and neurocognition.
The data presented here must be interpreted in light of the limitations of this trial. The population enrolled in AALL06N1 represents less than 20% of those who were enrolled in AALL0232 who were eligible for this trial. Comparing those who did and did not enroll, those evaluated in AALL06N1 were younger and less ethnically diverse; however, among the patients who were evaluable for AALL06N1, sociodemographic characteristics among those who were randomly assigned HDMTX versus escalating dose MTX were comparable, thus preserving the primary aim of the trial—to determine whether MTX or corticosteroid random assignment was predictive of outcome. The cross-sectional design of the trial is also a limitation, as we could not assess a change in neurocognitive function over time. Thus, the finding that public insurance status—used as a proxy for SES—was associated with a lower mean estimated IQ and WM may reflect a premorbid discrepancy that stems from long-term socioeconomic disadvantage. Longitudinal data are needed to determine whether cognitive losses are greater for children who begin treatment with an SES disadvantage. Finally, we present findings that pertain to estimated intellectual functioning only. It will be important to analyze additional measures that evaluate memory, functional outcomes, and psychosocial functioning completed by this sample to have a fuller picture of possible late effects and contributing risk factors. Despite these limitations, the identification of deficits in younger patients, regardless of therapy delivered, led to the funding of an ongoing, longitudinal, computer-based assessment of neurocognitive functioning of patients enrolling in the current COG therapeutic trial for children who are diagnosed with HR B-ALL (AALL1131) with the aim of clarifying the timing and trajectory of neurocognitive deficits in this population.
In summary, our findings suggest that more than 70% of children with HR B-ALL who are treated with contemporary therapies that do not include cranial radiation have estimated IQ, PS, and WM scores within the average range. Neither MTX nor corticosteroid random assignment impacted outcomes, but when evaluated in the context of age, those who were age < 10 years at diagnosis were found to be at risk for lower estimated IQ and PS scores. Future work must focus on identifying patients for whom therapy can be reduced further without eroding the excellent event-free survival that has been achieved with contemporary therapy as well as collecting longitudinal data that include sensitive evaluations of IQ, PS, WM, and SES, so that trajectories can be examined. Although complex, simultaneous assessments of SES, host genetics, and chemotherapy-induced metabolic insults would provide information that is critical to understanding the etiology of neurocognitive dysfunction and identifying children at risk.
Appendix
Fig A1.
Cognitive outcomes by age at diagnosis. Colored boxes represent the middle 50% of the sample with the median represented as a horizontal line and the mean as a diamond. The ends of the whiskers are the minimum and maximum values discounting outliers that are beyond the 1.5 interquartile ranges above or below the 75th and 25th percentiles, respectively, which are delineated separately as circles. For presentation purposes, WM was scaled to have a sample mean of 100 and a sample SD = 15. IQ, intelligence quotient; PS, processing speed; SD, standard deviation; WM, working memory.
Table A1.
Cognitive Outcomes by Corticosteroid Treatment Using Only Corticosteroid Randomly Assigned Patients
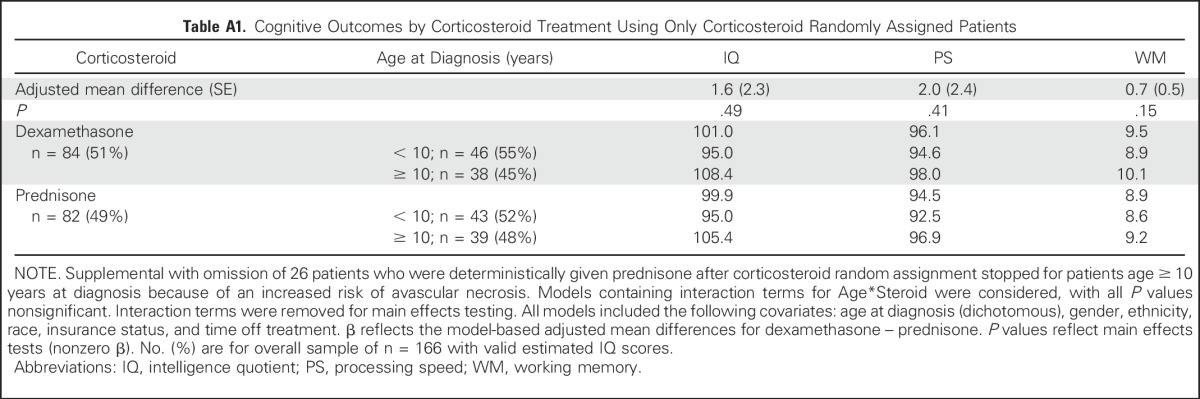
Table A2.
Top 10 Loci Whose Genotypes Are Associated With IQ Score
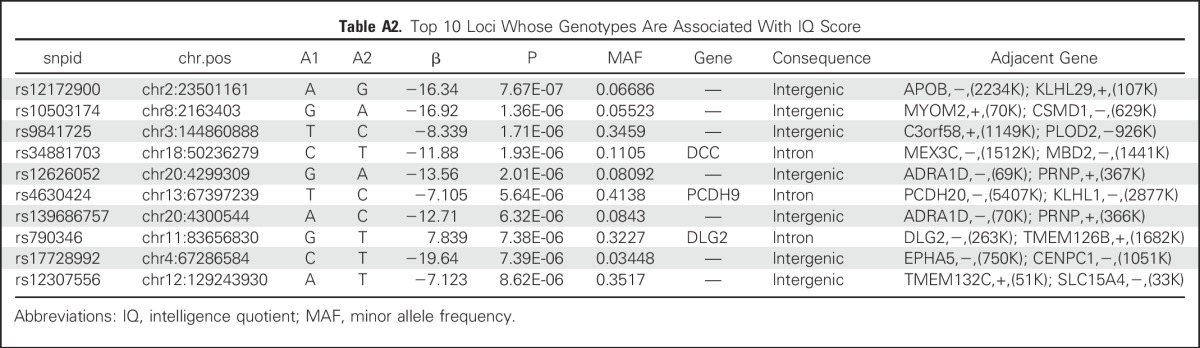
Table A3.
Top 10 Loci Whose Genotypes Were Associated With PS
Table A4.
Association Between Genotype and IQ/PS Scores for Previously Reported Polymorphisms
Table A5.
Top SNPs Associated With IQ/PS Scores in Previously Reported Genes
Footnotes
This work was supported by COG Group Chair’s grant 3 U10 CA098543-03S1; COG Chair’s Operations grants U10 CA98543-06S1, U10 CA98543-07S1, and U10 CA180886; COG Statistics and Data Center grants U10 CA098413 and U10 CA180899, P50 GM115279, RC CA56449, P30 CA21765; and American Lebanese Syrian Association Charities.
These results were presented in part, in abstract form, at the 2014 meeting of the American Society of Clinical Oncology.
Clinical trial information: NCT00437060.
Listen to the podcast by Dr Spiegler at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Leanne Embry, John A. Kairalla, Meenakshi Devidas, Daniel Armstrong, Stephen Hunger, William L. Carroll, Eric Larsen, Elizabeth A. Raetz, Mignon L. Loh, Mary V. Relling, Robert B. Noll, Naomi Winick
Collection and assembly of data: Kristina K. Hardy, Leanne Embry, John A. Kairalla, Shanjun Helian, Meenakshi Devidas, Wenjian Yang, Mary V. Relling, Robert B. Noll
Data analysis and interpretation: Kristina K. Hardy, Leanne Embry, John A. Kairalla, Shanjun Helian, Meenakshi Devidas, Stephen Hunger, William L. Carroll, Wenjian Yang, Mary V. Relling, Robert B. Noll, Naomi Winick
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neurocognitive Functioning of Children Treated for High-Risk B-Acute Lymphoblastic Leukemia Randomly Assigned to Different Methotrexate and Corticosteroid Treatment Strategies: A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kristina K. Hardy
No relationship to disclose
Leanne Embry
No relationship to disclose
John A. Kairalla
Stock or Other Ownership: Sophiris Bio, Johnson & Johnson, Bristol-Myers Squibb
Shanjun Helian
No relationship to disclose
Meenakshi Devidas
Honoraria: Pfizer, Novartis
Daniel Armstrong
No relationship to disclose
Stephen Hunger
Stock or Other Ownership: Express Scripts, Amgen, Merck (I), Amgen (I), Pfizer (I)
Honoraria: Jazz Pharmaceuticals, Spectrum Pharmaceuticals, ERYTECH Pharma
Consulting or Advisory Role: Novartis
Patents, Royalties, Other Intellectual Property: Coinventor on US patent #8,568,974 B2 Identification of novel subgroups of high-risk pediatric precursor-B acute lymphoblastic leukemia, outcome correlations and diagnostic and therapeutic methods related to same. It has not been licensed and there is no income.
William L. Carroll
No relationship to disclose
Eric Larsen
No relationship to disclose
Elizabeth A. Raetz
No relationship to disclose
Mignon L. Loh
No relationship to disclose
Wenjian Yang
No relationship to disclose
Mary V. Relling
No relationship to disclose
Robert B. Noll
No relationship to disclose
Naomi Winick
No relationship to disclose
REFERENCES
- 1.Campbell LK, Scaduto M, Sharp W, et al. : A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer 49:65-73, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Mennes M, Stiers P, Vandenbussche E, et al. : Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatr Blood Cancer 44:478-486, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Jacola LM, Krull KR, Pui C-H, et al. : Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol 34:1239-1247, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson CC, Johnson CE, Ramirez LY, et al. : A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer 51:99-104, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Knight S, McCarthy M, Anderson V, et al. : Visuomotor function in children treated for acute lymphoblastic leukaemia with chemotherapy only. Dev Neuropsychol 39:101-112, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Iyer NS, Balsamo LM, Bracken MB, et al. : Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: A review and meta-analysis. Blood 126:346-353, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Pui C-H, Campana D, Pei D, et al. : Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360:2730-2741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conklin HM, Krull KR, Reddick WE, et al. : Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst 104:1386-1395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halsey C, Buck G, Richards S, et al. : The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients: Results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol 4:42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain N, Brouwers P, Okcu MF, et al. : Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer 115:4238-4245, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Patel SK, Lo TTY, Dennis JM, et al. : Neurocognitive and behavioral outcomes in Latino childhood cancer survivors. Pediatr Blood Cancer 60:1696-1702, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler RW, Fairclough DL, Katz ER, et al. : Intellectual functioning and multi-dimensional attentional processes in long-term survivors of a central nervous system related pediatric malignancy. Life Sci 93:611-616, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. : Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 57:1197-1203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aukema EJ, Caan MWA, Oudhuis N, et al. : White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys 74:837-843, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Krull KR, Bhojwani D, Conklin HM, et al. : Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 31:2182-2188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krawczuk-Rybak M, Grabowska A, Protas PT, et al. : Intellectual functioning of childhood leukemia survivors--relation to Tau protein--A marker of white matter injury. Adv Med Sci 57:266-272, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Nathan PC, Whitcomb T, Wolters PL, et al. : Very high-dose methotrexate (33.6 g/m2) as central nervous system preventive therapy for childhood acute lymphoblastic leukemia: Results of National Cancer Institute/Children’s Cancer Group trials CCG-191P, CCG-134P and CCG-144P. Leuk Lymphoma 47:2488-2504, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Waber DP, McCabe M, Sebree M, et al. : Neuropsychological outcomes of a randomized trial of prednisone versus dexamethasone in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute All Consortium Protocol 00-01. Pediatr Blood Cancer 60:1785-1791, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen EC, Devidas M, Chen S, et al. : Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group Study AALL0232. J Clin Oncol 34:2380-2388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Embry L, Annett RD, Kunin-Batson A, et al. : Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: Can it be done? Pediatr Blood Cancer 59:536-539, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Karol SE, Yang W, Van Driest SL, et al. : Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood 126:1770-1776, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D: Wechsler Adult Intelligence Scale (ed 3). San Antonio, TX, The Psychological Corporation, 1997 [Google Scholar]
- 23.Wechsler D: Wechsler Preschool and Primary Scale of Intelligence (ed 3). San Antonio, TX, The Psychological Corporation, 2002 [Google Scholar]
- 24.Wechsler D: Wechsler Intelligence Scale for Children (ed 3). San Antonio, TX, The Psychological Corporation, 2003 [Google Scholar]
- 25.Sattler JM: Resource Guide to Accompany Assessment of Children: Cognitive Foundations (ed 5). San Diego, CA, Jerome M. Sattler Publisher, 2008 [Google Scholar]
- 26.Cole PD, Finkelstein Y, Stevenson KE, et al. : Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 33:2205-2211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamdar KY, Krull KR, El-Zein RA, et al. : Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer 57:454-460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krull KR, Brouwers P, Jain N, et al. : Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr 152:101-105, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Cheung YT, Krull KR: Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev 53:108-120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhojwani D, Sabin ND, Pei D, et al. : Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol 32:949-959, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winick NJ, Bowman WP, Kamen BA, et al. : Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst 84:252-256, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Dicuonzo F, Salvati A, Palma M, et al. : Posterior reversible encephalopathy syndrome associated with methotrexate neurotoxicity: Conventional magnetic resonance and diffusion-weighted imaging findings. J Child Neurol 24:1013-1018, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Parasole R, Petruzziello F, Menna G, et al. : Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk Lymphoma 51:1063-1071, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. : Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: An association with intermediate-dose intravenous methotrexate and intrathecal triple therapy—A Pediatric Oncology Group study. J Clin Oncol 16:1712-1722, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Brouwers P, Breiger D, et al. : A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood 114:1746-1752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardila A, Rosselli M, Matute E, et al. : The influence of the parents’ educational level on the development of executive functions. Dev Neuropsychol 28:539-560, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Gale CR, O’Callaghan FJ, Godfrey KM, et al. : Critical periods of brain growth and cognitive function in children. Brain 127:321-329, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Shaw P, Greenstein D, Lerch J, et al. : Intellectual ability and cortical development in children and adolescents. Nature 440:676-679, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Brooks-Gunn J, Duncan GJ: The effects of poverty on children. Future Child 7:55-71, 1997 [PubMed] [Google Scholar]
- 40.Duncan GJ, Brooks-Gunn J, Klebanov PK: Economic deprivation and early childhood development. Child Dev 65:296-318, 1994 [PubMed] [Google Scholar]
- 41.Shavers VL: Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 99:1013-1023, 2007 [PMC free article] [PubMed] [Google Scholar]
- 42.Moore IM, Hockenberry MJ, Anhalt C, et al. : Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer 59:278-284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conklin HM, Ogg RJ, Ashford JM, et al. : Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled trial. J Clin Oncol 33:3894-3902, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy KK, Willard VW, Allen TM, et al. : Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology 22:1856-1865, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler RW, Copeland DR, Fairclough DL, et al. : A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol 76:367-378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S-G, Joo Y, Kim B, et al. : Association of Ala72Ser polymorphism with COMT enzyme activity and the risk of schizophrenia in Koreans. Hum Genet 116:319-328, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Shifman S, Bronstein M, Sternfeld M, et al. : A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 71:1296-1302, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmatier MA, Pakstis AJ, Speed W, et al. : COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol Psychiatry 9:859-870, 2004 [DOI] [PubMed] [Google Scholar]