Abstract
Purpose
After preoperative chemoradiotherapy followed by total mesorectal excision for locally advanced rectal cancer, patients who experience local or systemic relapse of disease may be eligible for curative salvage surgery, but the benefit of this surgery has not been fully investigated. The purpose of this study was to characterize recurrence patterns and investigate the impact of salvage surgery on survival in patients with rectal cancer after receiving multidisciplinary treatment.
Patients and Methods
Patients with locally advanced (cT3-4 or cN+) rectal cancer who were treated with preoperative chemoradiotherapy followed by total mesorectal excision at our institution during 1993 to 2008 were identified. We examined patterns of recurrence location, time to recurrence, treatment factors, and survival.
Results
A total of 735 patients were included. Tumors were mostly midrectal to lower rectal cancer, with a median distance from the anal verge of 5.0 cm. The most common recurrence site was the lung followed by the liver. Median time to recurrence was shorter in liver-only recurrence (11.2 months) than in lung-only recurrence (18.2 months) or locoregional-only recurrence (24.7 months; P = .001). Salvage surgery was performed in 57% of patients with single-site recurrence and was associated with longer survival after recurrence in patients with lung-only and liver-only recurrence (P < .001) but not in those with locoregional-only recurrence (P = .353).
Conclusion
We found a predilection for lung recurrence in patients with rectal cancer after multidisciplinary treatment. Salvage surgery was associated with prolonged survival in patients with lung-only and liver-only recurrence, but not in those with locoregional recurrence, which demonstrates a need for careful consideration of the indications for resection.
INTRODUCTION
Advances in rectal cancer treatment—of note, total mesorectal excision (TME) and use of preoperative chemoradiotherapy (CRT)—have contributed to marked improvements in outcomes over the past several decades. Since the initial report of the TME technique TME in 1982,1 many studies, including randomized trials, have provided evidence of its benefit in the treatment of rectal cancer, with improved rates of local recurrence and survival.2-6 To further improve local control and survival in these patients, radiation therapy has been administered; addition of preoperative radiation therapy followed by TME has been shown to improve local control compared with surgery alone.7-9 Randomized trials in patients with rectal cancer have also evaluated the benefit of adding chemotherapy to radiation, which has improved survival and local control. In addition, preoperative CRT has been associated with a pathologic complete response in 15% to 20% of treated patients.10-13 Currently, multidisciplinary treatment with preoperative CRT followed by TME is the standard treatment of patients with locally advanced rectal cancer (≥ T3 or ≥ N1).14
Although multidisciplinary treatment of rectal cancer successfully improves outcomes, little is known about the patterns of rectal cancer recurrence after CRT and TME and the potential for and outcomes after salvage surgery for recurrent disease. Understanding these factors in the era of multidisciplinary treastment and TME for rectal cancer would help guide modern surveillance strategies and treatment stratification. Moreover, although there has been extensive discussion about the resection of liver metastases from colorectal cancer,15 the benefit of surgical resection of extrahepatic recurrence has not been well investigated.14,16 The aim of this study was to characterize the patterns and timing of recurrence according to location and to investigate the impact of salvage surgical resection on survival in patients with locally advanced primary rectal cancer who underwent standard multidisciplinary treatment and TME.
PATIENTS AND METHODS
Patients with locally advanced (cT3-4 or cN+) rectal cancer who were treated with preoperative CRT followed by TME at The University of Texas MD Anderson Cancer Center during 1993 to 2008 were identified from the institutional colorectal cancer database and tumor registry. The study period was determined on the basis of our evolving treatment paradigm. Patients who had urgent surgery, a prior history of pelvic radiotherapy, or a prior history of malignancy other than nonskin squamous cell cancer or in situ cervical cancer within 5 years before surgery were excluded. Medical records of patients who met eligibility criteria were retrospectively reviewed. This study was approved by the institutional review board.
After pathologic diagnosis with rectal cancer, preoperative staging included computed tomography scan of the chest, abdomen, and/or pelvis, pelvic magnetic resonance imaging, and/or endoscopic ultrasound. Clinical and pathologic staging was based on the American Joint Committee on Cancer, 6th edition.16a All patients received preoperative external beam radiation (45 Gy to 54 Gy) with concurrent fluorouracil-based chemotherapy.
After surgery, all patients were recommended follow-up with clinical, laboratory, and radiologic examinations, physical examination, carcinoembryonic antigen levels every 3 to 6 months, and radiographic evaluation of the chest, abdomen, and pelvis every 6 to 12 months for 5 years. In some cases, follow-up was coordinated with patients’ local providers with imaging visits performed at MD Anderson Cancer Center. The institutional tumor registry also contacts patients annually to collect information regarding vital status and treatment of disease. Death certifications were also reviewed for verification of given information. Criteria for the diagnosis of recurrence included histologic confirmation or radiologic studies with subsequent clinical progression and supportive biochemical data. The date of recurrence was defined as the date of confirmatory imaging or, in cases that needed tissue diagnosis confirmation, the date of biopsy. Clinicopathologic variables evaluated were age, sex, tumor location within the rectum, pathologic stage, lymphovascular invasion and perineural invasion, site of recurrence, number of recurrent lesions, and nature of treatment of recurrence, including surgical resection with curative intent—that is, salvage surgery. In general, patients who developed metastatic lesion(s) were considered for salvage surgery if such surgery could completely remove lesion(s) for curative intent. The use of preoperative therapy, including chemotherapy and radiation therapy, was determined on a case-by-case basis. Sites of recurrence were categorized into locoregional sites, extraregional lymph nodes, liver, lung, peritoneum, brain, and bone. Overall survival was defined as the time from the date of primary surgery to the date of the last follow-up or death. Time to recurrence (TTR) was defined as the time from the date of surgical resection of the primary tumor to the date of recurrence, and time to second recurrence was defined as the time from the date of resection of the first recurrence to the date of second recurrence.
Categorical data were summarized by frequency and comparisons were performed by using χ2 or Fisher’s exact tests. Continuous data were compared by using Wilcoxon rank-sum test or one-way analysis of variance, as appropriate. Survival end points were examined by using the Kaplan-Meier method and log-rank test. Adjusted hazard ratio (HR) was estimated by multivariable Cox proportional hazards regression analysis. All patient characteristics that were significant in univariable models at P = .10 were included in the multivariable model. The terms were also assessed for interaction, which was addressed in the final model. Cumulative incidences of lung, liver, and locoregional recurrence were compared among tumor location groups that were stratified by the length from anal verge (lower rectum [< 6.0 cm], middle rectum [6.0 to 9.9 cm], and upper rectum groups [≥ 10 cm]). A P value of < .05 was considered significant. Statistical analyses were performed by using STATA 14.1 (STATA, College Station, TX; Computing Resource Center, Santa Monica, CA).
RESULTS
Demographic and Primary Tumor Characteristics
Seven hundred thirty-five patients with rectal cancer met study criteria (Table 1). Median distance of the primary tumor from the anal verge was 5.0 cm. Sphincter-preserving procedures were performed in 528 (71.8%) patients. One hundred twenty-two (16.6%) patients required multivisceral resection.
Table 1.
Demographic and Primary Tumor Characteristics
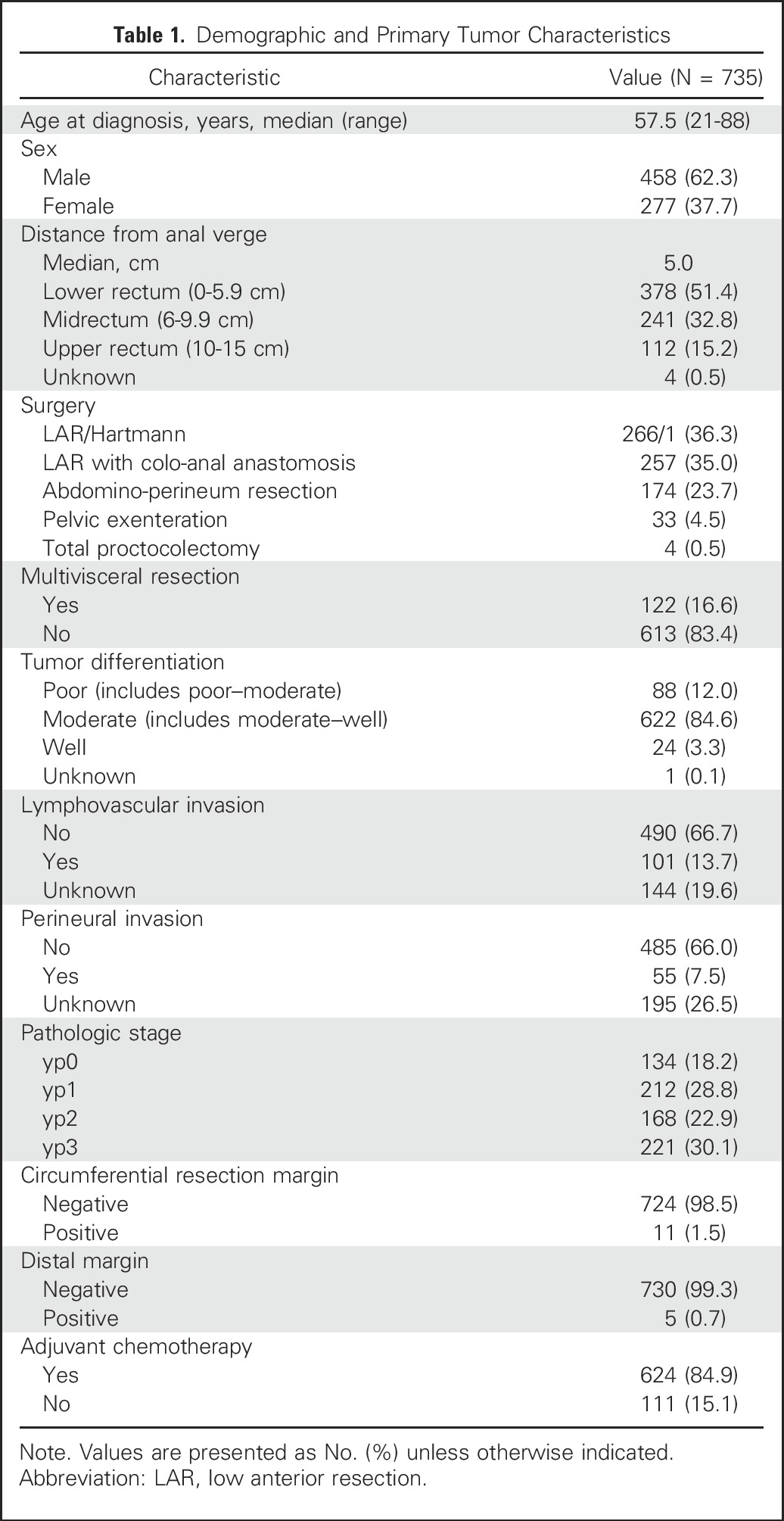
First Recurrence
Median follow-up time was 96 months (interquartile range [IQR], 66 to 141 months). Nine international patients were lost to follow-up shortly after surgical resection and were excluded from survival analysis. Follow-ups with complete information for vital status and disease treatment were available for 5 years after primary surgery for nearly all patients, with two patients censored as a result of loss.
During the follow-up period, 151 (20.8%) patients developed recurrence. Of those 151 patients, 129 (85.4%) patients had single-site recurrence, whereas 22 (14.6%) patients had multiple-site recurrence. The most common site of recurrence among all patients was the lung (n = 70; 9.6%) followed by the liver (n = 43; 5.9%), and locoregional sites (n = 37; 5.1%), including four patients with iliac lymph nodes, extraregional inguinal, aortic, or retroperitoneal lymph nodes (n = 11; 1.5%), peritoneum (n = 6; 0.8%), brain (n = 4; 0.6%), and bone (n = 4; 0.6%). The 5-year cumulative incidence of lung recurrence was 10.2% (11.1% in lower rectum, 10.0% in middle rectum, and 7.7% in upper rectum groups; Ptrend = .268), that of liver recurrence was 6.3% (5.6% in lower rectum, 5.0% in middle rectum, and 11.4% in upper rectum; Ptrend = .133), and that of locoregional recurrence was 4.6% (5.6% in lower rectum, 4.6% in middle rectum, and 1.0% in upper rectum; Ptrend = .032).
Characteristics of patients who developed recurrence at a single site were examined (Table 2), and the development of single-site recurrences by site over time is shown in Figure 1. Mean distance of the primary tumor from the anal verge was lowest in patients with locoregional-only recurrence (4.4 cm; standard deviation [SD], 2.4 cm), followed by patients with lung-only recurrence (5.3 cm; SD, 2.7 cm) and those with liver-only recurrence (6.4 cm; SD, 3.4 cm; P = .033); however, the site of recurrence was not associated with any of the other factors that are traditionally associated with risk of recurrence in general, including advanced pathologic stage, presence of lymphovascular or perineural invasion, and/or high grade.
Table 2.
Demographic, Tumor, and Outcome Characteristics by Recurrence Site for Patients With Single-Site Recurrence
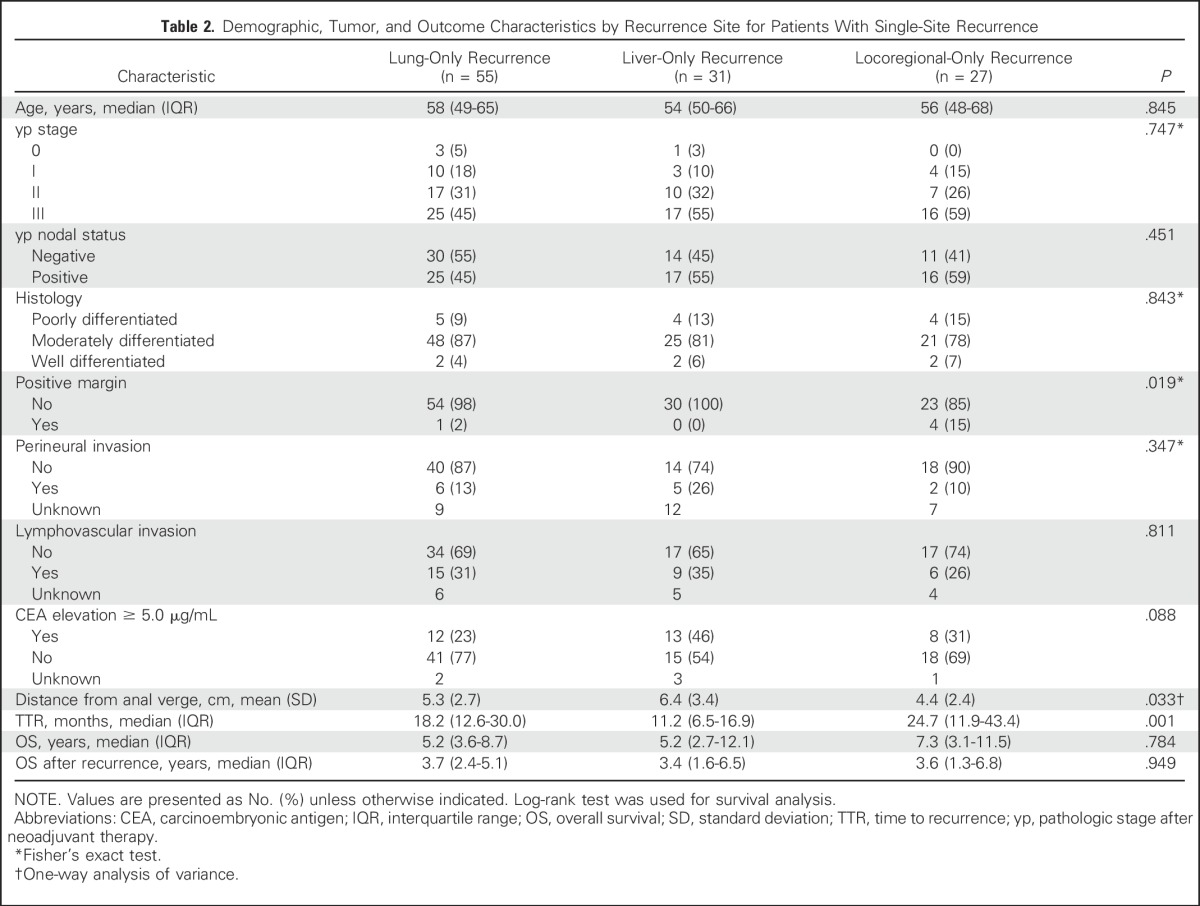
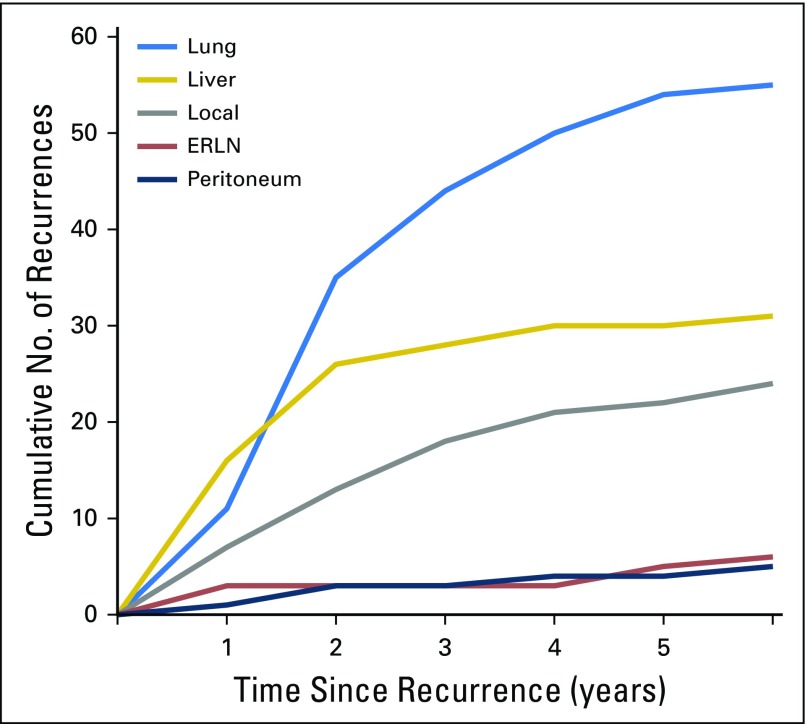
Fig 1.
Cumulative number of recurrences by site. ERLN, extraregional lymph node.
Median TTR for all patients was 17.5 months (IQR, 10.4 to 34.1 months) and significantly differed between liver-only (11.2 months; IQR, 6.5 to 16.9 months), lung-only (18.2 months; IQR, 12.6 to 30.0 months), and locoregional-only recurrence (24.7 months; IQR, 11.9 to 43.4 months; P = .001). The liver was the predominant site of recurrence during the first year, whereas the lung was the predominant site of recurrence after the second year. Of all recurrences, 63.6% (96 of 151) occurred within the first 2 years, and 75.5% (114 of 151) occurred within the first 3 years. Eleven (7.3%) additional recurrences—five locoregional, one lung, liver, extraregional lymph nodes, peritoneal, bone, and multiple-site recurrence—each occurred after five years.
Salvage Surgery for Recurrence
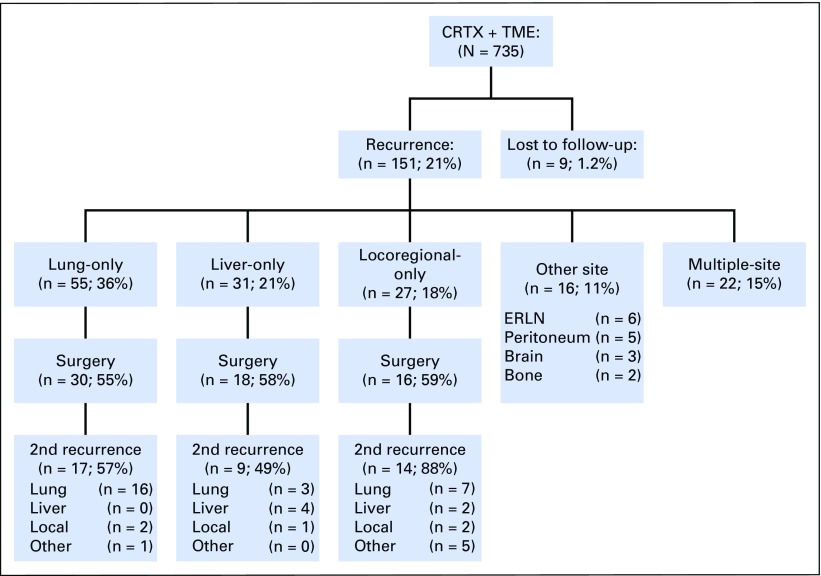
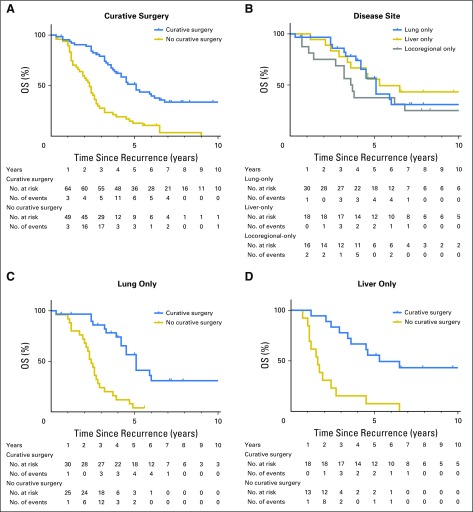
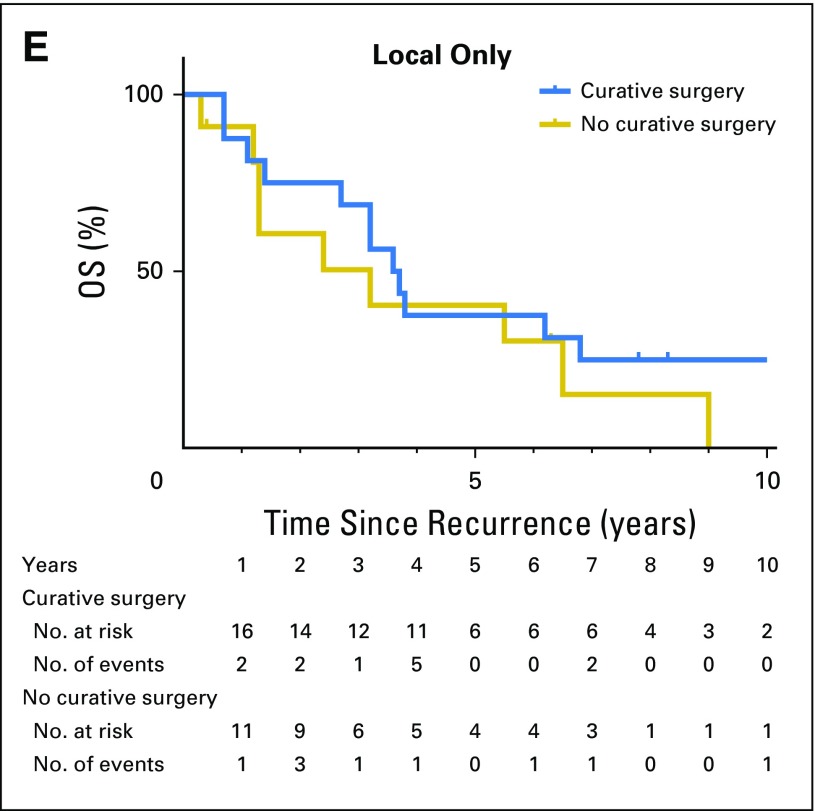
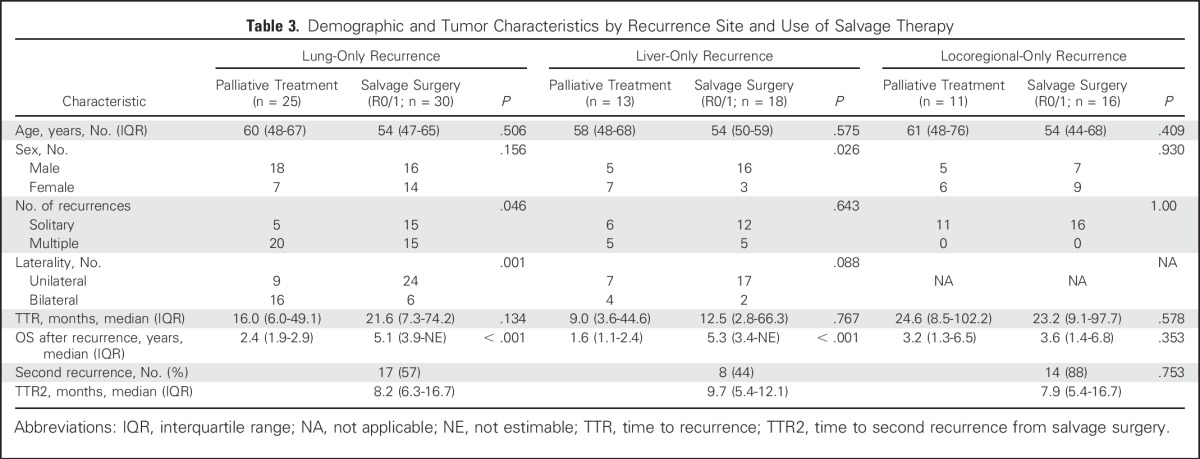
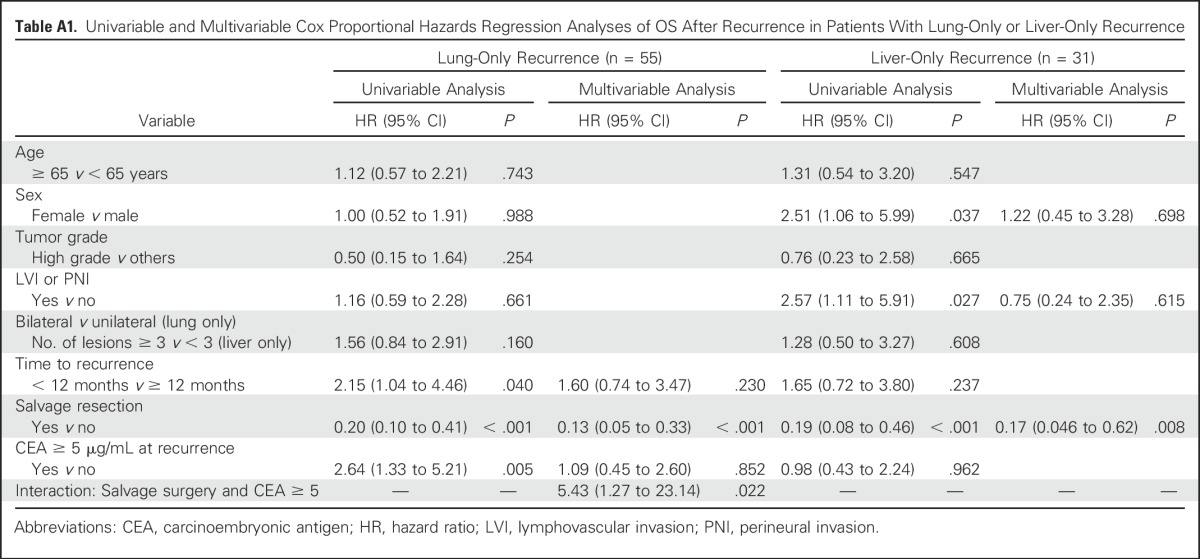
A total of 64 (56.6%) of 113 patients with single-site recurrence at the liver, lung, or locoregional sites underwent salvage surgery (Fig 2). Radiofrequency ablation or stereotactic radiation therapy were not regarded as salvage surgery. The indication for salvage surgery was the ability to achieve complete (ie, R0) resection on the basis of preoperative evaluation. The total salvage rate, including other single-site recurrences and multiple-site recurrences, was 46.4% (70 of 151). The overall rate of R0 resection was 90%, 89%, and 63% for lung, liver, and locoregional recurrences, respectively. Of those 113 patients with single-site recurrence, 84 deaths (40, 23, and 21 patients with lung-only, liver-only, and locoregional-only recurrence, respectively) were observed during follow-up. Patients who underwent salvage surgery had significantly longer overall survival after recurrence than did those who did not receive salvage surgery (estimated median survival after recurrence, 5.1 years v 2.3 years; 5-year survival after recurrence, 51% v 13%; P < .001; Fig 3A). Of patients with lung-only recurrences, those who underwent salvage surgery were more likely to have solitary and unilateral disease (Table 3). Survival times after recurrence in patients who underwent salvage surgery for lung-only recurrence and liver-only recurrence were similar (estimated median survival after recurrence, 5.1 years for lung-only v 5.3 years for liver-only; P = .39; Fig 3B). In both groups, salvage surgery was associated with prolonged overall survival (lung-only recurrence: adjusted HR 0.25; 95% CI, 0.12 to 0.51; P < .001; liver-only recurrence: adjusted HR, 0.17; 95% CI, 0.05 to 0.62; P = .008; Figs 3C and 3D; and Appendix Table A1, online only). In contrast, salvage surgery was not associated with improved survival in patients with locoregional-only recurrence (estimated median survival after recurrence, 3.6 years with surgery v 3.2 years without surgery; P = .353; Fig 3E).
Fig 2.
Recurrence patterns and salvage surgery. CRTx, chemoradiation therapy; ERLN, extraregional lymph node; TME, total mesorectal excision.
Fig 3.
Overall survival after recurrence (A) by curative surgery, (B) among patients who had salvage surgery by disease site, (C) in lung-only recurrence, (D) in liver-only recurrence, and (E) in locoregional-only recurrence by curative surgery. OS, overall survival.
Table 3.
Demographic and Tumor Characteristics by Recurrence Site and Use of Salvage Therapy
Second Recurrence—After Salvage Surgery
Patterns of second recurrence were investigated in 64 patients who underwent salvage surgery for lung-only, liver-only, and locoregional-only recurrence (Fig 2). Among these patients, 40 (62.5%) experienced second recurrence, with a median time to second recurrence of 8.2 months (IQR 5.4 to 16.7 months). Time to second recurrence did not significantly differ by site of recurrence (P = .753; Table 3). The lung continued to be the most common site for second recurrence (26 [65%] of 40). Overall, recurrence rates after salvage surgery were 57% among patients with lung-only, 49% with liver-only, and 88% with locoregional-only recurrence. Among 16 patients with locoregional-only recurrence, the majority (12 [86%] of 14) of second recurrences occurred at a distant site, whereas the other two (14%) second recurrences were repeat local recurrences.
DISCUSSION
In patients with rectal cancer who underwent preoperative CRT and standardized TME at a single tertiary referral center, the lung was the most common site of recurrence, which contrasted with the liver-dominant pattern that has been reported in previous studies. Median TTR differed by site of recurrence, but the overwhelming majority occurred within 3 years. Curative-intent salvage surgery was associated with improved survival in patients with lung-only recurrence and those with liver-only recurrence; however, secondary recurrences occurred in approximately one half of these patients. Among patients with locoregional-only recurrence, distant recurrence after salvage surgery was even more common and determined long-term prognosis.
Our findings are consistent with recent studies that have demonstrated a predilection for lung recurrence among patients with rectal cancer.17-19 This lung predilection, in contrast with the liver-dominant recurrence patterns reported in previous studies of colorectal cancer,20-23 is likely explained by anatomic drainage pathways in the rectum; the low-lying rectum below the peritoneal reflection has drainage to the systemic circulation via the iliac system and subsequent dissemination to the lung.20 In fact, we observed a liver-dominant distant recurrence pattern in the upper rectum group, whereas the lower and middle rectum groups had a lung-dominant distant recurrence pattern. In analysis of patients with recurrences, an association of the site of recurrence and mean distance from the anal verge of the primary tumor was observed, with patients with lung-only recurrence having lower-lying tumors than patients with liver-only recurrence. A prior retrospective study of 593 patients with rectal cancer (median length from anal verge, 7 cm) also described a lung-dominant recurrence pattern of rectal cancer after multidisciplinary therapy (69% of all recurrences were in the lung, 20% in the liver), although further comparison was limited as recurrence patterns were not stratified by tumor location.19
Of note, median TTR was 11.2 months for liver-only and 18.2 months for lung-only recurrence, and recurrence within the liver after 3 years was rare in this study, which may have implications for surveillance recommendations. Relatively longer TTR in lung-only metastasis compared with other site recurrence has also been previously reported.19 We have previously shown that the response to preoperative therapy is associated with recurrence-free survival, with a low risk for recurrence among good responders and a higher risk among poor responders.10 Although optimal surveillance should be individualized by the overall underlying risk, on the basis of the results of our study, the period of highest yield for surveillance testing can be predicted to be the first 3 years, beyond which there may be a more limited benefit of routine surveillance imaging.
Among patients with recurrence, salvage surgery was performed for 57% of with single-site recurrence—the highest rate among reported studies.20,24-30 Our study showed significantly improved outcomes among patients who underwent lung or liver metastasectomy compared with patients with recurrences at those sites who did not undergo salvage surgery, which supports the accumulating evidence that salvage surgery for liver-only or lung-only recurrence may improve survival in selected patients. Salvage surgery was performed in 59% of patients with locoregional-only recurrence, but our study failed to show a significant survival benefit for surgery among these patients.
The Intergroup 0114 study randomly assigned patients with rectal cancer to different protocols of postoperative CRT, and in a secondary analysis of 123 patients with rectal cancer with local recurrence, of whom 37% underwent resection, the 5-year survival rate was 20% in patients who underwent resection and 10% in those who did not have resection (P = .053).20 However, in that multi-institutional study, the local recurrence rate was 14% and there was no standardized surgical technique, namely TME, which was standard in the current study for which the local recurrence rate was 5%. Most of the patients in our study who underwent salvage surgery subsequently developed a second recurrence within a year after salvage surgery. Among these patients, the site of second recurrence was predominantly distant—for example, the liver or the lung—but a high rate of local control was achieved.
After multimodality therapy and high-quality TME, salvage surgery for local recurrence is more difficult; it may be more likely to occur outside of the central pelvis and often requires extensive resection, such as pelvic exenteration and extravascular lateral pelvic sidewall resection. Unlike many central recurrences that may be attributed to technical failure after incomplete TME, recurrences after optimal multimodal management and TME surgery may represent more biologically aggressive disease. Thus, there may be greater potential for salvage surgery of local recurrence when the recurrence is a result of technical failure after suboptimal surgery than when local recurrence occurs after standardized TME. These results after salvage surgery contrast with our broader experience for surgery for recurrent rectal cancer, including patients after prereferral primary resection, which demonstrates a durable benefit with salvage resection for local recurrence.31 Moreover, a recently published multi-institutional retrospective study of 533 patients who underwent pelvic exenteration surgery for locally recurrent rectal cancer also showed that the 5-year cancer-specific survival was 44% after R0 resection, achieved in 59% of patients.32 Thus, patients with locoregional recurrence may benefit from improved selection, perhaps with initial systemic therapy to identify patients with more favorable tumor biology for resection. In addition, the decision of salvage surgery for pelvic recurrence should take into account the impact on both oncologic outcomes and quality of life. In a prospective study of 105 patients who were treated for local recurrence of rectal cancer, we have previously shown that patient-reported quality of life was preserved after salvage resection for locally recurrent disease, but this rapidly deteriorated in patients who did not undergo salvage resection.33
The current retrospective study was subject to several limitations. Although the approach to follow-up and subsequent treatment were standardized, the decision of salvage surgery was not randomized and factors that are associated with surgery selection, such as response to preoperative systemic therapy before metastasectomy, may have also influenced outcomes. We did not have specific information about patient comorbidity or performance status that may have affected the decision for surgery at the time of recurrence, although performance of salvage surgery in the majority of patients with recurrence is among the highest reported. Although there was a standard follow-up strategy, we cannot exclude individual variation among those who underwent resection or not, which would have subjected the analysis to the potential for lead time bias. Outcomes after salvage resection, particularly for local recurrence, likely depend on the completeness of resection after initial surgery and on tumor biology; therefore, these findings may be less generalizable to patients who were not initially treated within specialized high-volume units. For example, salvage surgery may still yield a survival benefit in cases where primary resection did not achieve complete TME and local recurrence occurred within the residual mesorectum.
In conclusion, this study demonstrated that lung recurrence is the most common site of rectal cancer after preoperative multidisciplinary treatment. Median TTR was longer in lung-only recurrence than in liver-only recurrence, but shorter than locoregional recurrence. More than 90% of liver recurrences were identified within 3 years, which supports a tailored approach to surveillance. In patients with lung-only and liver-only recurrences, salvage surgery was associated with improved survival. The lack of benefit for salvage surgery for local recurrence after prior high-quality TME as a result of a high rate of secondary distant recurrence suggests that biologic determinants of disease play an important role in these patients. On the basis of these data, all patients with locally or distantly recurrent rectal cancer should be carefully evaluated by a multidisciplinary team with consideration of salvage surgery.
Appendix
Table A1.
Univariable and Multivariable Cox Proportional Hazards Regression Analyses of OS After Recurrence in Patients With Lung-Only or Liver-Only Recurrence
Footnotes
Supported in part by the National Institutes of Health Grant No. P30-CA016672 via The University of Texas MD Anderson Cancer Center and by the Aman Trust for Colorectal Cancer Research and Education (G.J.C.).
AUTHOR CONTRIBUTIONS
Conception and design: Naruhiko Ikoma, Y. Nancy You, George J. Chang
Provision of study materials or patients: Y. Nancy You, Brian K. Bednarski, Miguel A. Rodriguez-Bigas, Cathy Eng, Scott Kopetz, Craig Messick, John M. Skibber, and George J. Chang
Collection and assembly of data: Naruhiko Ikoma, George J. Chang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Recurrence and Salvage Surgery on Survival After Multidisciplinary Treatment of Rectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Naruhiko Ikoma
No relationship to disclose
Y. Nancy You
No relationship to disclose
Brian K. Bednarski
Travel, Accommodations, Expenses: Intuitive Surgical
Miguel A. Rodriguez-Bigas
Patents, Royalties, Other Intellectual Property: UpToDate, author
Cathy Eng
Consulting or Advisory Role: Genentech, Bayer Schering Pharma, Sirtex Medical, Advaxis, Forty-Seven
Research Funding: Keryx, Advaxis, Genentech, Forty-Seven
Travel, Accommodations, Expenses: Genentech, Bayer
Prajnan Das
Honoraria: Springer International Publishing
Scott Kopetz
Stock or Other Ownership: MolecularMatch
Consulting or Advisory Role: Amgen, Roche, Merrimack Pharmaceuticals, Bayer, Taiho Pharmaceutical, Sanofi, Array BioPharma, Genentech, MolecularMatch
Research Funding: Amgen (Inst), Sanofi (Inst), Biocartis (Inst), Guardant Health (Inst), Array BioPharma (Inst), Genentech (Inst), EMD Serono (Inst), MedImmune (Inst), Novartis (Inst)
Craig Messick
No relationship to disclose
John M. Skibber
No relationship to disclose
George J. Chang
Consulting or Advisory Role: Johnson & Johnson
REFERENCES
- 1.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 2.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 3.Arbman G, Nilsson E, Hallböök O, et al. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg. 1996;83:375–379. doi: 10.1002/bjs.1800830326. [DOI] [PubMed] [Google Scholar]
- 4.Maurer CA, Renzulli P, Kull C, et al. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: Long-term results. Ann Surg Oncol. 2011;18:1899–1906. doi: 10.1245/s10434-011-1571-0. [DOI] [PubMed] [Google Scholar]
- 5.Enker WE, Thaler HT, Cranor ML, et al. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–346. [PubMed] [Google Scholar]
- 6.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, et al. ; Improved survival with preoperative radiotherapy in resectable rectal cancer N Engl J Med 336980–987.1997 [DOI] [PubMed] [Google Scholar]
- 8.Frykholm GJ, Glimelius B, Påhlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–572. doi: 10.1007/BF02049863. [DOI] [PubMed] [Google Scholar]
- 9.Cammà C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 10.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 12.Bosset JF CL, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 13.Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. doi: 10.1245/s10434-013-3198-9. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network NCCN guidelines: Rectal cancer. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- 15.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–466, discussion 466-467. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 16.Subbiah IM, Blackmon SH, Correa AM, et al. Preoperative chemotherapy prior to pulmonary metastasectomy in surgically resected primary colorectal carcinoma. Oncotarget. 2014;5:6584–6593. doi: 10.18632/oncotarget.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a. Greene FL, Page DL, Fleming ID, et al (eds): AJCC Cancer Staging Manual, 6th edition. Chicago, IL, American Joint Committee on Cancer, 2002.
- 17.Pucciarelli S, Gagliardi G, Maretto I, et al. Long-term oncologic results and complications after preoperative chemoradiotherapy for rectal cancer: A single-institution experience after a median follow-up of 95 months. Ann Surg Oncol. 2009;16:893–899. doi: 10.1245/s10434-009-0335-6. [DOI] [PubMed] [Google Scholar]
- 18.Arredondo J, Baixauli J, Rodríguez J, et al. Patterns and management of distant failure in locally advanced rectal cancer: A cohort study. Clin Transl Oncol. 2016;18:909–914. doi: 10.1007/s12094-015-1462-0. [DOI] [PubMed] [Google Scholar]
- 19.Ding P, Liska D, Tang P, et al. Pulmonary recurrence predominates after combined modality therapy for rectal cancer: An original retrospective study. Ann Surg. 2012;256:111–116. doi: 10.1097/SLA.0b013e31825b3a2b. [DOI] [PubMed] [Google Scholar]
- 20.Tepper JE OCM, O’Connell M, Hollis D, et al. Analysis of surgical salvage after failure of primary therapy in rectal cancer: Results from Intergroup Study 0114. J Clin Oncol. 2003;21:3623–3628. doi: 10.1200/JCO.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Chau I, Allen MJ, Cunningham D, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol. 2004;22:1420–1429. doi: 10.1200/JCO.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Roth ES FD, Fetzer DT, Barron BJ, et al. Does colon cancer ever metastasize to bone first? A temporal analysis of colorectal cancer progression BMC Cancer 9274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38:448–454. doi: 10.1016/j.canep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Kim AW FL, Faber LP, Warren WH, et al. Repeat pulmonary resection for metachronous colorectal carcinoma is beneficial. Surgery. 2008;144:712–717, discussion 717-718. doi: 10.1016/j.surg.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Ogata Y, Matono K, Hayashi A, et al. Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg. 2005;29:363–368. doi: 10.1007/s00268-004-7537-7. [DOI] [PubMed] [Google Scholar]
- 26.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 27.Abdalla EK VJ, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825, discussion 825-827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K, Shimada H, Ueda M, et al. Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14:1336–1346. doi: 10.1245/s10434-006-9071-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee WS, Yun SH, Chun HK, et al. Pulmonary resection for metastases from colorectal cancer: Prognostic factors and survival. Int J Colorectal Dis. 2007;22:699–704. doi: 10.1007/s00384-006-0218-2. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto T, Tsubota N, Iwanaga K, et al. Pulmonary resection for metastases from colorectal cancer. Chest. 2001;119:1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 31.You YN, Skibber JM, Hu CY, et al. Impact of multimodal therapy in locally recurrent rectal cancer. Br J Surg. 2016;103:753–762. doi: 10.1002/bjs.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris CA, Solomon MJ, Heriot AG, et al. The outcomes and patterns of treatment failure after surgery for locally recurrent rectal cancer. Ann Surg. 2016;264:323–329. doi: 10.1097/SLA.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 33.You YN, Habiba H, Chang GJ, et al. Prognostic value of quality of life and pain in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2011;18:989–996. doi: 10.1245/s10434-010-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]