Abstract
IMPORTANCE
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated the benefit of lowering systolic blood pressure (SBP) to 120 mm Hg, yet other trials, such as Heart Outcomes Prevention Evaluation–3 (HOPE-3), did not find consistent benefit. How to incorporate these results into the treatment of those with elevated SBP in the general population is not clear.
OBJECTIVES
To assess the representativeness of SPRINT and HOPE-3 relative to patients in the United States and to explore the cardiovascular disease (CVD) risk profiles of various populations with elevated SBP.
DESIGN, SETTING, AND PARTICIPANTS
The study examined data from nonpregnant adults aged 20 to 79 years participating in the 2007–2012 National Health and Nutrition Examination Survey (NHANES) who had complete data available (n = 14 142), representing 206.9 million US adults. The study was performed from October 1, 2015, to August 2, 2016.
MAIN OUTCOMES AND MEASURES
The study estimated the number and characteristics of adults with SBP of 120 mm Hg or higher, including SPRINT and HOPE-3 eligibility, and estimated who may have newly required treatment initiation or intensification if various trial or risk-based criteria were applied.
RESULTS
NHANES included completed clinical evaluations from mobile examination centers on 15 974 adults aged 20 to 79 years (mean [SD] age, 45.9 [15.5] years). The study excluded 182 pregnant women and 1650 adults in whom CVD risk data were unavailable, leaving a final study population of 14 142 (50.5% women [95% CI, 49.6%–51.3%] and 49.5% men [95% CI, 48.6%–50.4%]). An estimated 53.3 million untreated and 19.8 million treated US adults have an SBP in the diagnostic and treatment gray zone (120–139 mm Hg), a small proportion of whom would have been eligible for SPRINT (5.4% untreated, 13.9% treated) or HOPE-3 (13.9% treated, 1.7% untreated). Even among those with prior CVD or high risk of CVD and elevated SBP (120–139 mm Hg), only a few would have qualified for SPRINT (27.0% and 21.9% of untreated and treated patients, respectively) or HOPE-3 (10.6% and 2.1% of untreated and treated, respectively). If blood pressure treatment recommendations were extended to adults with an SBP between 120 and 139 mm Hg, as well as prior CVD or CVD risk of 15% or higher, then 5.8 million untreated adults would be reclassified as treatment eligible; furthermore, 8.5 million treated patients would require medication intensification.
CONCLUSIONS AND RELEVANCE
Millions of US adults have elevated SBP and high CVD risk, most of whom would not have been eligible for SPRINT. Until more definitive evidence becomes available, clinicians should consider a management paradigm based on CVD risk in addition to blood pressure measurements.
High blood pressure (BP), or hypertension, is a well-established risk factor for cardiovascular disease (CVD), but the particular systolic BP (SBP) that signals the need for treatment initiation and SBP treatment goals remains uncertain. Epidemiologic data support a near-continuous association between SBP and an increased risk of stroke and CVD down to an SBP of 115 mm Hg,1 but a meta-analysis2 of prior randomized clinical trials found mixed results, revealing benefit below an SBP of 130 mm Hg. Consequently, the USBP guidelines have defined systolic hypertension based on an SBP of 140 mm Hg or higher and generally focused treatment targets on BP measurement alone.3,4 This approach contrasts with current lipid guidelines that determine treatment eligibility and intensity based on a patient’s overall CVD risk.5
Results from the Systolic Blood Pressure Intervention Trial (SPRINT) challenged prior hypertension treatment goals. SPRINT randomized adults with CVD and high risk of CVD to intensive (<120 mm Hg) vs standard (<140 mm Hg) SBP targets.6 Patients randomized to intensive therapy experienced a 25% reduction in CVD events and lower overall mortality. SPRINT’s findings differ from other trials,7–9 such as Heart Outcomes Prevention Evaluation–3 (HOPE-3), which sought to modestly lower SBP in patients at intermediate risk of CVD but reported nosignificant intervention benefit except among adults with baseline SBPs above 143.5 mm Hg.10
Reconciling the discordance between SPRINT and other intervention trials, such as HOPE-3, has created a dilemma for practicing clinicians. Which patients should be diagnosed as having and treated for hypertension and, once treated, to what target goal? Because SPRINT provides the most compelling evidence to date for more aggressive intervention, one approach would be to apply diagnostic and treatment strategies only to patients who met SPRINT eligibility criteria and avoid intensive goals for patients more closely matching HOPE-3 criteria. However, the SPRINT and HOPE-3 inclusion criteria are complex, somewhat overlapping, and only address a few patients with elevated BP.11
Using data from the National Health and Nutrition Examination Survey (NHANES), we evaluated several population based implications of hypertension diagnosis and management by ( 1) determining the number of US adults in the diagnostic and therapeutic gray zone (ie, SBP ≥120 mm Hg [the intensive target in SPRINT] but <140 mm Hg); (2) evaluating characteristics of patients with treated and untreated elevated BP as a function of their CVD risk profile; (3) comparing population-based estimates of SPRINT and HOPE-3 eligibility, the overlap of their inclusion criteria, the patient characteristics of those eligible, and the number of individuals at high risk for CVD who were not eligible for these trials; and (4) estimating how many patients would need treatment intensification or initiation if SPRINT thresholds were applied to US adults at highest risk for CVD.
Methods
We evaluated SBP in nonpregnant adults 20 years or older and younger than 80 years who completed a mobile examination center visit and were participating in the 2007–2012 continuous NHANES. The study was performed from October 1, 2015, to August 2, 2016. NHANES survey data are available publically, and the survey has been approved by the National Center for Health Statistics Ethics Review Board. All participants provided written informed consent. NHANES analyses have been approved by the Duke University Institutional Review Board.
Blood pressures were measured by trained examiners; SBP was calculated by averaging up to 3 BP readings. The BP treatment status was determined by self-report. Medication type was defined by prescription review for those who reported taking prescription medications in the last 30 days. The BP medications were classified as aldosterone antagonists, angiotensin-converting enzyme inhibitors, aldosterone receptor blockers, peripherally acting antiadrenergic agents, centrally acting antiadrenergic agents, long-acting nitrates, β-blockers, calcium channel blockers, thiazide diuretics, nonthiazide diuretics, renin antagonists, and direct vasodilators. A patient’s number of medications was defined by the number of medication classes used. Possibly resistant hypertension was defined as taking medication from 3 or more medication classes, with at least 1 being a thiazide or nonthiazide diuretic. Laboratory data were measured on all participants regardless of fasting status. Diabetes was defined by self-report or a hemoglobin A1c level of 6.5% or higher (to convert to proportion of hemoglobin, multiply by 0.01). Smoking status was based on self-reported current cigarette use. Angina was defined by self report or positive result on the World Health Organization Rose angina questionnaire. Race was self-reported.
We calculated 10-year CVD risk estimates using pooled cohort equations12 for all patients free of CVD. We estimated the proportion and number of adults in the United States undergoing BP treatment and evaluated the distribution of SBP among those not undergoing treatment. To understand the magnitude of the gray zone (120–139 mm Hg), we estimated the number and characteristics of patients with an SBP of 140 mm Hg or higher and an SBP of 120 to 139 mm Hg by treatment status for all adults and adults at high risk for CVD or with prevalent CVD. We considered patients high risk if they had a 10-year CVD risk of 15% or higher, thereby paralleling the inclusion criteria for SPRINT, which defined high risk using a 15% Framingham risk score.13 Among adults in each BP group, we evaluated SPRINT and HOPE-3 eligibility as detailed in the eAppendix in the Supplement. The CVD prevalence and CVD risk distribution among adults in each BP category, stratified by treatment, was estimated.
To account for nonresponse and differential sampling in the survey design, we used NHANES survey weights per NHANES analytic guidelines.14 Participants missing data for BP, cholesterol level, diabetes, smoking status, or BP treatment were excluded to allow for calculation of CVD risk. Missing data were considered missing at random if no single variable was missing for more than 10%. Population totals were generated by applying national proportion estimates to the overall number represented by the mobile examination center subsample. Population estimates were generated to depict 206.9 million nonpregnant US adults representing the midpoint of NHANES 2007–2012.
Results
NHANES included completed clinical evaluations from mobile examination centers on 15 974 adults aged 20 to 79 years. We excluded 182 pregnant women and 1650 adults in whom CVD risk data were unavailable, leaving a final study population of 14 142 (50.5% women [95% CI, 49.6%–51.3%] and 49.5% men [95% CI, 48.6%–50.4%], with a mean [SD] age of 45.9 [15.5] years).
Magnitude of the Gray Area
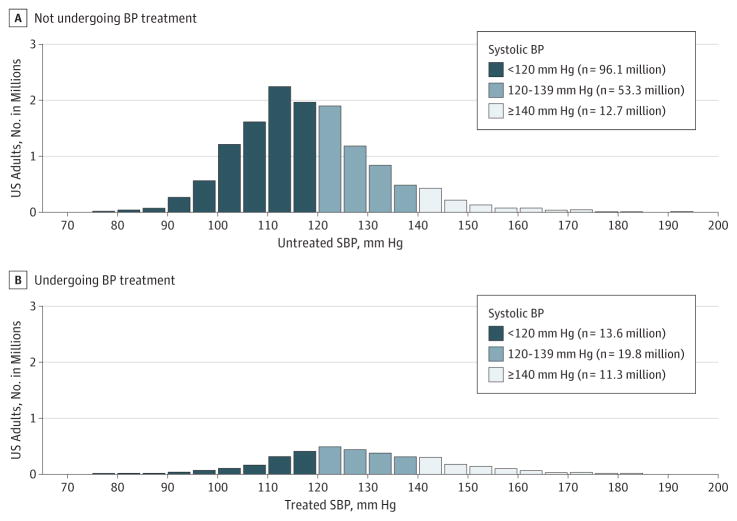
Figure 1 shows the SBP distribution among those who were and were not taking BP-lowering medication. On the basis of population extrapolations from the NHANES subsample, of the total 206.9 million US adults aged 20 to 79 years, 162.2 million (78.4%; 95% CI, 77.0%–79.8%) were not taking medication; of these, 12.7 million (6.1% of US adults; 95% CI, 5.6%–6.7%) had an SBP of 140 mm Hg or higher, thereby traditionally classified as having untreated hypertension. An additional 53.3 million (25.8% of US adults; 95% CI, 24.5%–27.1%) had an elevated SBP (120–139 mm Hg) and were not undergoing treatment, representing the gray area for treatment initiation.
Figure 1. Distribution of Untreated and Treated Systolic Blood Pressure (SBP) Measurements in US Adults Aged 20 to 79 Years.
Histogram of SBP distribution in US adults not undergoing blood pressure (BP) treatment (A) and undergoing treatment (B). Population estimates are calculated from application of weighted National Health and Nutrition Examination Survey estimates to population totals of nonpregnant US adults at the midpoint between 2007 and 2012.
Overall, an estimated 44.7 million (21.6% of US adults; 95% CI, 20.2%–23.0%) reported taking at least 1 BP-lowering medication; of these, 11.3 million (5.4% of US adults; 95% CI, 5.0%–5.9%) had an SBP of 140 mm Hg or higher, therefore requiring medication intensification under traditional guidelines. In addition, there were 19.8 million undergoing BP treatment (9.6% of US adults; 95% CI, 8.8%–10.3%) with an SBP of 120 to 139 mm Hg who were potentially eligible for medication intensification if SPRINT treatment targets of less than 120 mm Hg were applied.
Characteristics of Adults With Elevated BP
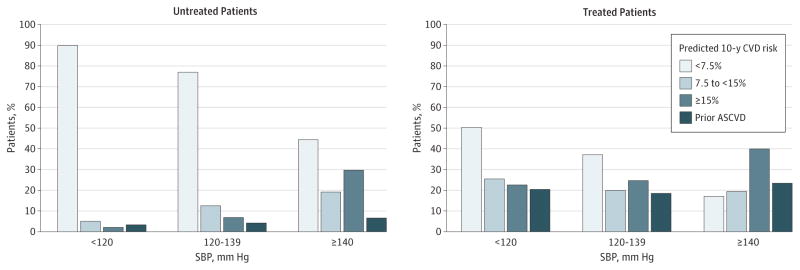
Figure 2 shows the distribution of CVD risk profiles among US adults by BP category. As BP increased, the CVD risk and proportion of those with prior CVD increased markedly among treated and untreated adults. Among patients with an untreated SBP of 120 to 139 mm Hg, only 10.8% (95% CI, 9.4%–12.5%; n = 5.8 million) had high CVD risk (10-year CVD risk ≥15% or prior CVD) vs 76.7% (95% CI, 74.5%–78.7%; n = 40.9 million) with low risk (10-year risk <7.5%, the treatment threshold for current cholesterol guidelines5). In contrast, among those with an untreated SBP of 140 mm Hg or higher, 36.3% (95% CI, 31.8%–41.1%; n = 4.6 million) had high CVD risk or prior CVD vs 44.5% (95% CI, 40.3%–48.7%; n = 5.7 million) with low risk. Among those undergoing treatment (vs not), CVD risk estimates were higher for each BP category. For patients with an SBP of 120 to 139 mm Hg while receiving treatment, 37.3% (95% CI, 33.2%–41.6%; n = 7.4 million) had a low 10-year risk profile (<7.5%), and 43.0% (95% CI, 39.6%–46.5; n = 8.5 million) had high CVD risk or prior CVD.
Figure 2. Cardiovascular Disease (CVD) Risk Profiles by Systolic Blood Pressure (SBP) and Treatment Status.
Prevalence of prior CVD and predicted 10-year CVD risk for those free of CVD by SBP category in those not undergoing treatment and those undergoing treatment. ASCVD indicates atherosclerotic cardiovascular disease.
Table 1 and Table 2 give the clinical characteristics of adults aged 20 to 79 years with untreated and treated SBPs of 120 mm Hg or higher in the overall population and for those at high CVD risk (≥15% or prior CVD). Among all untreated adults aged 20 to 79 years, only 5.4% (95% CI, 4.5%–6.5%) with an SBP of 120 to 139 mm Hg would have been eligible for SPRINT and only 4.4% (95% CI, 3.6%–5.4%) for HOPE-3 (Table 1). Even among those with high CVD risk and an untreated SBP of 120 to 139 mm Hg, only a few would have been eligible for SPRINT (27.0%; 95% CI, 22.5%–32.0%) or HOPE-3 (10.6%; 95% CI, 8.1%–13.8%). Similarly, relatively few high-risk adults undergoing BP treatment would have qualified for SPRINT: 21.9% (95% CI, 18.3%–26.1%) with an SBP of 120 to 139 mm Hg and 36.3% (95% CI, 31.3%–41.6%) with an SBP of 140 mm Hg or higher (Table 2). Few high-risk adults undergoing treatment would have qualified for HOPE-3: 2.1% (95% CI, 1.2%–3.8%) with an SBP of 120 to 139 mm Hg and 3.5% (95% CI, 1.9%–6.6%) with an SBP of 140 mm Hg or higher.
Table 1.
Characteristics of Adults Aged 20 to 79 Years With Elevated SBP Not Undergoing Blood Pressure Treatmenta
| Characteristic | All Adults
|
Adults With Prior CVD or Risk ≥15%
|
||
|---|---|---|---|---|
| SBP 120–139 mm Hg | SBP ≥140 mm Hg | SBP 120–139 mm Hg | SBP ≥140 mm Hg | |
| Total NHANES population, No. | 3472 | 1052 | 515 | 441 |
|
| ||||
| US population, No. in millions | 53.3 | 12.7 | 5.8 | 4.6 |
|
| ||||
| % Of US populationb | 25.8 (24.5–27.1) | 6.1 (5.6–6.7) | 2.8 (2.4–3.2) | 2.2 (1.9–2.6) |
|
| ||||
| SPRINT eligible | 5.4 (4.5–6.5) | 33.7 (30.1–37.5) | 27.0 (22.5–32.0) | 58.1 (51.3–64.6) |
|
| ||||
| HOPE-3 eligible | 4.4 (3.6–5.4) | 10.6 (8.2–13.5) | 10.6 (8.1–13.8) | 15.9 (11.7–21.3) |
|
| ||||
| Eligible for both HOPE-3 and SPRINT | 1.2 (0.9–1.7) | 7.5 (5.4–10.3) | 5.2 (3.5–7.7) | 13.8 (9.8–19.1) |
|
| ||||
| Age, mean (IQR), y | 45.3 (34.0–56.0) | 54.8 (46.0–65.0) | 62.3 (53.0–73.0) | 65.3 (59.0–73.0) |
|
| ||||
| Male sex | 61.6 (59.8–63.4) | 56.8 (53.4–60.1) | 71.2 (65.2–76.7) | 64.8 (58.2–70.9) |
|
| ||||
| African American | 10.1 (8.0–12.8) | 14.8 (11.3–19.1) | 6.9 (4.8–9.7) | 11.1 (7.5–16.2) |
|
| ||||
| Prior MI or angina | 3.5 (2.8–4.3) | 5.5 (3.9–7.8) | 32.0 (26.3–38.3) | 15.3 (11.1–20.6) |
|
| ||||
| Prior stroke | 1.1 (0.8–1.6) | 1.8 (1.1–3.1) | 10.3 (7.5–14.1) | 5.0 (2.9–8.5) |
|
| ||||
| Diabetes | 7.7 (6.8–8.7) | 13.7 (11.0–16.9) | 28.6 (23.0–34.9) | 28.0 (22.4–34.4) |
|
| ||||
| Heart failure | 0.9 (0.6–1.4) | 1.9 (1.0–3.4) | 7.2 (4.4–11.5) | 3.7 (1.9–7.0) |
|
| ||||
| BMI, mean (IQR) | 29.1 (24.7–32.4) | 29.2 (24.2–32.6) | 29.6 (25.2–33.1) | 29.1 (24.3–32.4) |
|
| ||||
| GFR, mean (IQR), mL/min/1.72 m2 | 91.4 (77.9–102.9) | 87.8 (74.0–99.9) | 81.5 (68.0–93.3) | 81.9 (68.3–96.5) |
|
| ||||
| Smoking | 26.5 (24.5–28.7) | 26.2 (22.8–29.8) | 38.1 (32.6–43.9) | 32.9 (27.0–39.4) |
|
| ||||
| Total cholesterol level, mean (IQR), mg/dL | 203.4 (175.0–228.0) | 209.0 (180.0–236.0) | 210.1 (177.0–237.0) | 212.9 (176.0–247.0) |
|
| ||||
| HDL-C, mean (IQR), mg/dL | 51.4 (40.0–59.0) | 54.3 (42.0–64.0) | 46.5 (37.0–54.0) | 52.2 (41.0–61.0) |
|
| ||||
| 10-y CVD riskc | 4.8 (0.7–6.2) | 11.6 (3.3–17.4) | 23.3 (17.3–27.3) | 24.7 (17.8–28.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HOPE-3, Heart Outcomes Prevention Evaluation–3; HR, hazard ratio; IQR, interquartile range; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
SI conversion factors: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259.
Data are presented as percentage (95% CI) of column total, and continuous variables are presented as mean (IQR).
Percentage of population of nonpregnant US adults aged 20 to 79 years based on population extrapolations from the NHANES sample.
The 10-year risk of CVD by the pooled cohort equations.
Table 2.
Characteristics of Adults Aged 20 to 79 Years Undergoing Blood Pressure Treatment With SBPs of 120 mm Hg or Highera
| Characteristic | All Adults
|
Adults With CVD or Risk ≥15%
|
||
|---|---|---|---|---|
| SBP 120–139 mm Hg | SBP ≥140 mm Hg | SBP 120–139 mm Hg | SBP ≥140 mm Hg | |
| Total NHANES, No. | 1565 | 1073 | 840 | 799 |
|
| ||||
| Population total, No. in millions | 19.7 | 11.3 | 8.5 | 7.1 |
|
| ||||
| % Of US populationb | 9.6 (8.8–10.3) | 5.4 (5.0–5.9) | 4.1 (3.7–4.6) | 3.5 (3.1–3.9) |
|
| ||||
| SPRINT eligible | 13.9 (11.6–16.6) | 34.3 (29.7–39.2) | 21.9 (18.3–26.1) | 36.3 (31.3–41.6) |
|
| ||||
| HOPE-3 eligible | 1.7 (1.0–2.9) | 2.6 (1.5–4.4) | 2.1 (1.2–3.8) | 3.5 (1.9–6.6) |
|
| ||||
| Eligible for both HOPE-3 and SPRINT | 0.2 (0.1–0.5) | 2.3 (1.2–4.1) | 0.4 (0.2–1.2) | 3.2 (1.6–6.2) |
|
| ||||
| Age, mean (IQR), y | 58.9 (51.0–69.0) | 61.6 (54.0–71.0) | 67.3 (63.0–74.0) | 66.3 (60.0–74.0) |
|
| ||||
| Male sex | 48.2 (44.7–51.8) | 46.1 (42.9–49.2) | 55.4 (51.1–59.6) | 52.2 (47.2–57.2) |
|
| ||||
| African American | 14.0 (10.8–17.8) | 18.0 (13.7–23.4) | 14.3 (11.1–18.3) | 20.3 (15.3–26.4) |
|
| ||||
| Prior MI or angina | 14.4 (12.4–16.5) | 18.6 (15.1–22.7) | 33.4 (29.1–38.0) | 29.3 (24.5–34.6) |
|
| ||||
| Prior stroke | 5.9 (4.5–7.8) | 8.0 (6.4–10.0) | 13.8 (10.5–17.9) | 12.7 (10.2–15.5) |
|
| ||||
| Diabetes | 26.6 (23.2–30.3) | 31.4 (27.4–35.6) | 40.1 (35.6–44.7) | 43.1 (37.5–49.0) |
|
| ||||
| Heart failure | 5.5 (4.3–7.1) | 5.8 (4.2–7.8) | 11.1 (8.3–14.9) | 7.7 (5.8–10.3) |
|
| ||||
| BMI, mean (IQR) | 32.1 (27.2–36.0) | 31.6 (26.4–35.2) | 31.5 (27.0–34.9) | 30.9 (26.4–34.0) |
|
| ||||
| GFR, mean (IQR), mL/min/1.72 m2 | 80.2 (66.1–92.0) | 77.0 (62.6–90.4) | 74.1 (59.0–85.8) | 73.6 (58.4–86.5) |
|
| ||||
| Smoking | 14.2 (12.6–16.0) | 13.8 (11.5–16.4) | 17.4(15.0–20.2) | 17.3 (14.2–20.9) |
|
| ||||
| Total cholesterol level, mean (IQR), mg/dL | 192.5 (164.0–218.0) | 202.0 (170.0–228.0) | 186.6 (156.0–211.0) | 198.6 (162.0–220.0) |
|
| ||||
| HDL-C level, mean (IQR), mg/dL | 51.0 (40.0–58.0) | 51.5 (40.0–60.0) | 48.6 (39.0–56.0) | 50.2 (40.0–59.0) |
|
| ||||
| No. of medications, HR (IQR) | 1.9 (1.0–2.0) | 1.9 (1.0–3.0) | 2.1 (1.0–3.0) | 2.0 (1.0–3.0) |
|
| ||||
| 10-y CVD risk, mean (IQR)c | 12.1 (3.3–17.2) | 20.6 (8.1–30.2) | 25.8 (18.8–30.3) | 32.3 (20.4–40.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HOPE-3, Heart Outcomes Prevention Evaluation–3; HR, hazard ratio; IQR, interquartile range; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
SI conversion factors: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259.
Data are presented as percentage (95% CI) of column total, and continuous variables are presented as mean (IQR).
Percentage of population of US adults aged 20 to 79 years.
The 10-year risk of CVD by the pooled cohort equations.
SPRINT and HOPE-3: Population-Based Estimates and Overlap
Overall, 14.2% (95% CI, 13.1%–15.3%; n = 13.8 million) of adults with an untreated or a treated SBP of 120 mm Hg or higher would have been eligible for SPRINT and 4.5% (95% CI, 3.9%–5.1%; n = 4.3 million) for HOPE-3. There was overlap between trial eligibility. Of those with an SBP of 120 mm Hg or higher eligible for HOPE-3, 43.6% (95% CI, 37.3%–50.1%; n = 1.9 million) would have also been eligible for SPRINT.
Table 3 gives the clinical characteristics of adults who would have been SPRINT and HOPE-3 eligible and those actually enrolled. Ten-year CVD risk estimates are similar between SPRINT-enrolled and SPRINT-eligible adults, but SPRINT-eligible adults were younger and had higher BPs than those enrolled. HOPE-eligible adults included more males with lower mean BPs and total cholesterol levels than those enrolled. SPRINT-eligible adults had higher mean 10-year CVD risk estimates than HOPE-3–eligible adults (18.1% and 12.9%, respectively).
Table 3.
SPRINT and HOPE-3 Trial Actual vs Potentially Eligible Patients in US Population
| Characteristic | SPRINT Trial | SPRINT Eligiblea | HOPE-3 Trial | HOPE-3 Eligiblea |
|---|---|---|---|---|
| Age, mean, y | 67.9 | 65.2 (59.0–72.0) | 65.8 | 65.1 (5.0–70.0) |
| Male sex, % | 64.0 | 61.1 (58.1–64.1) | 54.2 | 64.4 (59.6–68.8) |
| SBP, mean, mm Hg | 139.7 | 144.8 (135.3–152.7) | 138.2 | 128.4 (116.0–139.3) |
| DBP, mean, mm Hg | 78.2 | 75.3 (68.0–83.3) | 82.0 | 71.5 (65.3–78.7) |
| BMI, mean | 29.9 | 29.1 (25.1–32.0) | 27.1 | 28.0 (23.5–31.6) |
| Smoker, % | NA | 21.5 (18.8–24.4) | 28.0 | 26.7 (22.3–31.6) |
| GFR, mean, mL/min/1.72 m2 | 71.8 | 77.7 (64.6–89.8) | NA | 79.3 (66.2–91.1) |
| Total cholesterol level, mean, mg/dL | 190.2 | 211.8 (183.0–237.0) | 201.4 | 201.0 (176.0–224.0) |
| HDL-C level, mean, mg/dL | 52.9 | 51.8 (41.0–60.0) | 44.9 | 56.7 (44.0–68.0) |
| Prior CVD, % | 16.7 | 13.5 (10.5–17.3) | NA | NA |
| Framingham risk, % | 20.1 | 24.6 (17.5–29.3) | NA | 16.6 (10.5–20.3) |
| 10-y CVD risk, % | NA | 18.1 (11.1–22.9) | NA | 12.9 (7.4–16.3) |
Abbreviations: BMI, body mass index (calculated as a measure of weight in kilograms divided by square of height in meters); CVD, cardiovascular disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HOPE-3, Heart Outcomes Prevention Evaluation–3; NA, not applicable; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
SI conversion factors: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259.
Data are presented as percentage (95% CI) of column total, and continuous variables are presented as mean (interquartile range).
High-Risk Patients Who Were Not Trial Eligible
Of the estimated 26.0 million adults with prior CVD or CVD risk of 15% or greater and an SBP of 120 mm Hg or higher, 66.6% (95% CI, 64.2%–68.9; n = 17.3 million) would not have been SPRINT eligible; diabetes disqualified more than half of high-risk adults (54.4%; 95% CI, 50.6%–58.2%), followed by prior stroke (16.8%; 95% CI, 14.6%–19.1%), age older than 50 years (12.8%; 95% CI, 10.8%–15.0%), and congestive heart failure (12.0%; 95% CI, 9.9%–14.5%).
Treatment Gaps and Potential for Intensification
Currently, 5.4% (95% CI, 5.0%–5.9%; n = 11.3 million) of US adults have an SBP of 140 mm Hg or higher, are taking BP medication, and require treatment intensification; of these, 21.9% (95% CI, 17.9%–26.5%) are already taking 3 or more medications (with at least 1 being a diuretic) and may have resistant hypertension. In addition, 1.3% (95% CI, 1.1%–1.6%; 2.8 million) of US adults have an SBP of 120 to 139 mm Hg, are currently undergoing BP treatment, and would have been SPRINT eligible; of these, 18.9% (95% CI, 13.4%–26.0%) are already taking 3 or more medications (with at least 1 being a diuretic). If we moved hypertension treatment targets to 120 mm Hg for patients with prior CVD and/or at high risk of CVD (≥15% 10-year atherosclerotic CVD risk), then an additional 3.2% (95% CI, 2.8%–3.6%; n = 6.6 million) of the US population would require treatment intensification; of these, 27.2% (95% CI, 22.8%–32.2%) may have resistant hypertension.
Discussion
SPRINT provides the most compelling evidence to date that more intensive SBP targets can reduce CVD events and all-cause mortality in adults with an elevated SBP who are at high risk for CVD. Nonetheless, it remains unclear how broadly the results of SPRINT can and should be applied in community practice.
The study had several important discoveries. First, there are huge public health implications surrounding how to define and treat hypertension. More than 53 million US adults with an elevated SBP (120–139 mm Hg) are not being treated, and nearly 20 million US adults are undergoing treatment but still have a modestly elevated SBP (120–139 mm Hg); these patients are in an uncertain diagnostic and therapeutic gray zone but represent a substantial portion of those potentially at risk for CVD morbidity. Priorwork15 has found that more than half of excess CVD deaths occur in adults with an SBP of 120 to 139 mm Hg. Attempts to lower BP via medication dosages do not fully mitigate CVD risk in those with elevated BP, highlighting the importance of hypertension prevention.16 Second, exclusive use of SPRINT inclusion and exclusion criteria to define those likely to benefit from more intensive BP control may not be an ideal approach; this strategy may overemphasize the clinical relevance of certain enrollment criteria. Third, there is a difference between patients who appear SPRINT eligible and who SPRINT actually enrolled. Fourth, there is some overlap in trial inclusion criteria for SPRINT (a positive study) and HOPE-3 (a neutral one in adults with normal starting BPs). Fifth, and perhaps most important, most patients with an elevated SBP would not have qualified for SPRINT; many of them were high risk and, therefore, may have benefitted from more intensive treatment.
Recent trials call into question keeping an SBP goal of less than 140 mm Hg in groups excluded from SPRINT. For example, adults with diabetes were originally excluded from SPRINT because the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial did not find an intensive BP-lowering strategy to be beneficial (hazard ratio, 0.9; 95% CI, 0.75–1.07). Nonetheless, recent long-term follow-up (8.8 years) of ACCORD reveals a statistically significant benefit to intensive BP targets in patients randomized to the standard glycemic therapy arm.17 Similarly, the Secondary Prevention of Small Subcortical Strokes study,18 which was not completed at the time of SPRINT enrollment, has since found that lower SBP targets in those with prior stroke may reduce the risk of recurrent stroke.
Because applying clinical trial findings to community practice is challenging, we propose that clinicians consider an individual patient’s CVD risk profile instead of relying on BP measurement alone. Such a risk-based strategy is consistent with current cholesterol treatment guidelines5 and encourages approaching hypertension control as part of an overall CVD risk reduction strategy. As a meta-analysis19 of clinical trials has demonstrated, if the relative benefits of intensive BP lowering are generally constant across the CVD-risk spectrum, then absolute risk reduction is greatest in those with the highest CVD risk and perhaps the most to gain from more intensive treatment.
Although a risk-based hypertension treatment strategy seems reasonable, optimal treatment goals have not been validated by randomized intervention trials and will need to be individualized. Relative to HOPE-3, patients in SPRINT had significantly higher CVD risk profiles and event rates. Consequently, patients with elevated SBP, as well as prior CVD or a 10-year CVD risk profile of 15% or higher, should first be considered for an intensive intervention. Moving forward, other cut points for initiating treatment goals could be considered for specific groups. For example, some have questioned aggressive BP control in frail or elderly populations; however, a recent secondary analysis of SPRINT found the benefits of intensive SBP goals to be similar or greater in older (vs younger) patients and in those with frailty (vs without).20 Nevertheless, patients who received intensive BP management in SPRINT required more medications and had slightly greater risk for hypotension and other adverse effects.
Younger adults with a high BP and even several other cardiovascular risk factors rarely have a 10-year risk of 15% or greater but often have high lifetime CVD risk; therefore, selecting treatment goals based on 10-year CVD risk alone may not be ideal. Studies21–23 have found that prolonged exposure to an elevated SBP (120–139 mm Hg) in early adulthood increases the long-term risk of CVD; thus, one could consider using a lower 10-year CVD risk profile or calculate the patient’s lifetime risk. Future trials should explore the possible long-term benefits of treating younger patients with an SBP of 120 to 139 mm Hg.
Regardless of which treatment threshold is applied, the data demonstrate the enormous need for better BP control at the population level. Even at the current SBP treatment goal of less than 140 mm Hg, 11.6% of all US adults taking medication have uncontrolled hypertension, including 25.2% currently undergoing treatment and 6.1% not taking any medication despite having SBPs of 140 mm Hg or higher. Fortunately, there appears to be room for tighter BP control among those currently undergoing BP therapy, regardless of how low the BP goals are set. Among patients currently treated whose SBP is 120 mm Hg or higher, most are taking fewer than 3 medications or are not taking a diuretic; therefore, these patients have room for treatment intensification.
The study has several technical limitations. First, NHANES relies on BP measurements taken at a mobile examination center, whereas a clinical hypertension diagnosis requires 2 measurements at different time points. Second, data for estimating CVD risk were missing for approximately 10% of the population (ie, approximately 20 million US adults). Third, we were unable to apply the exact technical specifications used in SPRINT and HOPE-3 to determine eligibility (eg, HOPE-3 used waist to hip ratio to define obesity, where as we used body mass index). Other inclusion criteria are impossible to directly model (eg, clinician determination that a patient is recommended for treatment with a statin, angiotensin-converting enzyme inhibitor, aldosterone receptor blocker, or thiazide); however, the criteria were designed to match the general clinical phenotypes included and excluded in SPRINT and HOPE-3 and provide a reasonable approximation of eligibility. Fourth, SPRINT demonstrated the benefits of BP control among individuals with high CVD risk based on the Framingham risk score; we used pooled cohort equations to reflect current lipid guidelines, which model a slightly different end point. Going forward, dynamic thresholds (by age, sex, or race) could be considered and would be similar to what has been proposed for lipid guidelines.24
Conclusions
A large proportion of US adults have an SBP of 120 to 139 mm Hg, many of whom do not meet clinical trial criteria to easily identify a treatment target. Further trial data will ideally clarify how, when, and whom to treat more intensively. In the interim, we propose a diagnosis-based approach that takes into consideration not only a patient’s BP but also the overall CVD risk.
Key Points.
Question
How should results from recent blood pressure intervention trials be applied to appropriately treat patients with elevated systolic blood pressure?
Findings
More than 73.1 million US adults have elevated systolic blood pressure (120–139 mm Hg), many of whom are at high risk for cardiovascular disease (CVD); however, only a few high-risk adults would have qualified for the Systolic Blood Pressure Intervention Trial. If hypertension treatment were initiated for systolic blood pressure of 120 mm Hg or higher in adults with prior CVD or at high risk for CVD (risk ≥15%), 5.8 million untreated adults would require treatment initiation and 8.5 million would require medication intensification.
Meaning
Clinicians should consider CVD risk in addition to blood pressure measurements alone to guide blood pressure treatment recommendations.
Acknowledgments
Funding/Support: This study was supported internally by the Duke Clinical Research Institute.
Footnotes
Additional Contributions: Erin Hanley, MS, the Duke Clinical Research Institute, Duke University Medical Center, made editorial contributions to this article. Ms Hanley did not receive compensation for her contributions apart from her employment at the institution where this study was conducted.
Author Contributions: Dr Navar had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Navar.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Navar.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Navar.
Administrative, technical, or material support: Peterson.
Study supervision: Navar, Peterson.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Navar reported receiving research funding from Sanofi and Regeneron. Dr Pencina reported receiving research funding to Duke from Bristol-Myers Squibb and Regeneron/Sanofi. Dr Peterson reported receiving grant support from American College of Cardiology, American Heart Association, and Janssen and consulting fees from Bayer, Boehringer Ingelheim, Merck, Valeant, Sanofi, AstraZeneca, Janssen, Regeneron, and Genentech. No other disclosures were reported.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension, 2: effects at different baseline and achieved blood pressure levels: overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2296–2304. doi: 10.1097/HJH.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Jr, Williamson JD, Whelton PK, et al. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogihara T, Saruta T, Rakugi H, et al. Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension Study. Hypertension. 2010;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 9.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 10.Lonn EM, Bosch J, López-Jaramillo P, et al. HOPE-3 Investigators. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 11.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the US adult population. J Am Coll Cardiol. 2016;67(5):463–472. doi: 10.1016/j.jacc.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 15.Karmali KN, Ning H, Goff DC, Lloyd-Jones DM. Identifying individuals at risk for cardiovascular events across the spectrum of blood pressure levels. J Am Heart Assoc. 2015;4(9):e002126. doi: 10.1161/JAHA.115.002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K, Colangelo LA, Daviglus ML, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2015;4(9):e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman WC, Evans GW, Cutler JA. Long-term cardiovascular effects of 4.9 years of intensive blood pressure control in type 2 diabetes mellitus: the Action to Control Cardiovascular Risk in Diabetes Follow-On Blood Pressure Study. [Accessed June 20, 2016];American Heart Association Professional Heart Daily website. https://professional.heart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_478891.pdf.
- 18.Benavente OR, Coffey CS, Conwit R, et al. SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial [published correction appears in Lancet. 2013;382(9891):506] Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundström J, Arima H, Woodward M, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384(9943):591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 20.Williamson JD, Supiano MA, Applegate WB, et al. SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years. JAMA. 2016;315(24):2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsia J, Heiss G, Ren H, et al. Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Ma H, Xiang MX, Wang JA. Meta-analysis of cohort studies of baseline prehypertension and risk of coronary heart disease. Am J Cardiol. 2013;112(2):266–271. doi: 10.1016/j.amjcard.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Navar-Boggan AM, Peterson ED, D’Agostino RB, Sr, Pencina MJ, Sniderman AD. Using age- and sex-specific risk thresholds to guide statin therapy: one size may not fit all. J Am Coll Cardiol. 2015;65(16):1633–1639. doi: 10.1016/j.jacc.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]