Abstract
Immune checkpoint blockade using antibodies to cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) benefits a limited number of cancer patients. SS1P and LMB-100 are immunotoxins that target mesothelin. We observed delayed responses to SS1P in patients with mesothelioma suggesting that anti-tumor immunity was induced. Our goal was to stimulate anti-tumor immunity by combining SS1P or LMB-100 with anti-CTLA-4. We constructed a BALB/c breast cancer cell line expressing human mesothelin (66C14-M) which was implanted in one or two locations. SS1P or LMB-100 was injected directly into established tumors and anti-CTLA-4 administered i.p. In mice with two tumors, one tumor was injected with immunotoxin and the other was not. The complete regression rate was 86% for the injected tumors and 53% for the uninjetced tumors. No complete regressions occurred when drugs were given separately. In regressing tumors, dying and dead tumor cells were intermingled with PMNs and surrounded by a collar of admixed eosinophils and mononuclear cells. Tumor regression was associated with increased numbers of tumor infiltrating CD8+ cells and blocked by administration of antibodies to CD8. Surviving mice were protected from tumor rechallenge by 66C14 cells not expressing mesothelin, indicating the development of anti-tumor immunity. The anti-tumor effect was abolished when a mutant non-cytotoxic variant was used instead of LMB-100, showing that the anti-tumor response is not mediated by recognition of a foreign bacterial protein. Our findings support developing a therapy composed of immunotoxins and checkpoint inhibitors for patients.
Keywords: Anti-CTLA-4, CD8+ T cells, LMB-100, SS1P, Mesothelin
Introduction
Most human tumors harbor mutant proteins that are capable of becoming targets for the immune system (1–3). However, regulatory mechanisms disable the immune system and prevent tumor rejection (4). Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) functions as an immune checkpoint regulator. Antibodies blocking CTLA-4 (anti-CTLA-4) prolong the survival of patients with metastatic melanoma. However, the response rate is low, ranging from 11–19% (5, 6). Combination therapies are being pursued to increase the number of patients benefiting from anti-CTLA-4 therapy.
Mesothelin is a cell surface 40kDa glycoprotein that is being pursued as a target for antibody based therapies, because it is highly expressed on ductal pancreatic carcinoma (80–85%), mesothelioma (85–90%), ovarian cancers (60–65%), lung cancers (60–65%) and other cancers (7). Mesothelin is not expressed on any vital tissues; its expression is limited to mesothelial cells lining the pleura, peritoneum and pericardium (8).
Recombinant immunotoxins (RITs) are therapeutic agents composed of an antibody fragment attached to a protein toxin. To target the toxin to solid tumors expressing mesothelin, we have constructed RITs composed of an antibody fragment targeting mesothelin and a portion of Pseudomonas exotoxin A (PE). After entering the cell by endocytosis, the RIT reaches the cytosol, where it inactivates elongation factor 2, arrests protein synthesis and induces apoptotic cell death (9).
SS1P is an anti-mesothelin immunotoxin that has been evaluated for cancer therapy in several clinical trials. When given by itself, SS1P is well tolerated, but has low activity (10, 11), mainly because it is immunogenic and can be given for only 1 cycle before neutralizing antibodies develop. When SS1P was combined with pentostatin and cyclophosphamide to modulate the immune system and delay antibody formation, SS1P produced delayed and prolonged regressions in several patients with advanced drug-refractory mesothelioma (12). These responses were accompanied by increased metabolic activity in the tumor, documented by PET scan, indicating that the tumors were infiltrated with immune cells. The ability of RITs to induce anti-tumor immunity was also indicated in a clinical study in patients with brain tumors receiving local immunotoxin therapy, where regressions began and progressed long after therapy had ended (13, 14). Because a direct anti-tumor effect is expected to occur close to the time of treatment, an additional immune-mediated effect was suggested.
LMB-100 (previously named RG7787) is an improved anti-mesothelin RIT designed to be more active, less immunogenic, and better tolerated by patients compared to SS1P (15, 16). It contains a humanized anti-mesothelin Fab fused to a 24kDa truncated PE fragment with mutations that suppress B- and T-cell epitopes. Clinical trials with LMB-100 in patients with mesothelioma and pancreatic cancer are in progress at the National Institutes of Health. (https://clinicaltrials.gov/ct2/show/NCT02810418).
Based on clinical data, we hypothesized that the anti-tumor effect of SS1P or LMB-100 can be potentiated by combining it with anti-CTLA-4. To examine this hypothesis, we used a 66C14 BALB/c mouse breast cancer cell line (17) transfected with a cDNA encoding human mesothelin to create cell line 66C14–M. Since tumor cells expressing human mesothelin are rejected by normal BALB/c mice, the cells were grown in BALB/c mice expressing a human mesothelin transgene. We find that the combination of SS1P or LMB-100 injected directly into tumors with anti-CTLA-4 given i.p. causes complete regressions of 86% of injected tumors and 53% of uninjected tumors and induces anti-tumor immunity.
Materials and Methods
Establishment of 66C14 luc cells expressing human mesothelin
We engineered a plasmid encoding a truncated human mesothelin (Supplementary Figs. S1A and S1B ). The expression plasmid contains a ferritin promoter and an IG κ leader sequence attached to truncated mesothelin (amino acids 296 to 585). The megakaryocyte-potentiating factor (MPF) portion of full-length mesothelin was deleted and the glycosyl phosphatidyl inositol membrane attachment site was replaced with the transmembrane domain of a murine transferrin receptor. The mouse mammary cancer cell line 66Cl4 luc was transfected using lipofectamine LTX & Plus (Invitrogen) according to the manufacturer’s instructions. A puromycin resistance gene enabled selection of the transfected clones. After the cells were grown in 3 μg/ml puromycin for 1 month, cells expressing high levels of mesothelin were sorted by flow cytometry using 5 μg/ml MN-1 anti-mesothelin antibody (Rockland Immunochemicals).
Establishment of BALB/c transgenic mice expressing human mesothelin
To express human mesothelin in mice, a cDNA that consists of full-length mesothelin (Accession Number: NM_005823) under the control of a CAG promoter was produced (Supplementary Fig. S1C). The pronucleus of fertilized oocytes from BALB/c mice were microinjected with the plasmid. Founder animals carrying a human mesothelin transgene were identified by Southern blot analysis followed by PCR screening to establish the founder lines. The founder lines were further characterized for protein expression by ELISA assay of the blood. After screening four founder lines, we selected one that has serum MPF levels of 50 ng/ml. That mouse line is designed as 01TGZ-N1.
Cell culture and reagents
The 66Cl4 luc tumor cell line was provided by Dr. C. L. Jorcyk (Boise State University). Tumor cells were cultured in IMDM supplemented with L-glutamine, HEPES (Gibco Life Technology) and 10% FBS (HyClone, Thermo Scientific) and 100 U/ml penicillin and 100 μg/ml streptomycin. For 66Cl4-M cells, we added 3 μg/ml puromycin (Glibco Life Technology) to maintain mesothelin expression. All cells were grown and maintained at 37°C with 5% CO2. LMB-100, LMB-100-I and anti-CTLA-4 (clone 9D9, mouse IgG2a isotype) were manufactured by Roche, Penzberg. LMB-100-I is an inactive form of LMB-100 lacking the glutamic acid in position 553; the furin cleavage site (RHRQPRGWEQ) was replaced with a glycine-serine linker (GGGGGSGGGGSGGS) (18). SS1P was manufactured by ABL (Rockville, MD), LMB-2 was manufactured by BDP (NCI-Fredrick, MD) and HA22 was manufactured by MedImmune. Endotoxin levels for all RITs were below 5 EU/mg. Most immunotoxins were diluted in PBS. HA22 was diluted in a 32mM citrate and 0.65% v/v tween 80. Paclitaxel was from TEVA. Trypan blue was from BioWhittaker. Western blot analysis was performed with an anti-mesothelin MORAb-009 antibody (Morphotek) and an anti-GAPDH antibody (Cell Signaling).
In vitro cytotoxicity of RITs
66Cl4-M cells were plated at 5000 cells/well in 96-well plates 24 hours before various RITs were added. Cells were incubated for 3 days, and viability assessed using a WST-8 cell counting kit (Dojindo Molecular Technologies) according to the manufacturer’s instructions.
Mouse experiments
All mouse experiments were approved by the NCI Animal Care and Use Committee. Female mesothelin Tg BALB/c and wild-type (WT) BALB/c mice age 6–12 weeks were used in this study. To produce tumors, we implanted 1x106 66C14-M cells in the right second mouse mammary fat pad. To form a second tumor, we implanted 1x105 66C14-M cells in the left second mammary fat pad. Treatment was initiated when tumors reached 80–100 mm3, typically on days 11 to 13 after tumor implantation. Tumor volume was calculated using the formula 0.4 x length x width2. RIT was injected into tumors in a volume of 30 μl using a 29G needle. Before injection, the tumor surface was sterilized using povidone iodine and alcohol pads. Anti-CTLA-4 was administered i.p. Mice were euthanized when the tumor volume reached 700 mm3. The day of euthanasia was used to calculate survival. Mice reaching complete remission were rechallenged 90 days after the first tumor implantation with 1x106 66C14-M or 66C14 cells. Surviving mice were followed for 6 months following the first tumor implantation.
Depletion of CD8+ T cells
To deplete CD8+ T cells we used anti-mouse CD8 antibody (clone 2.43, Bioproducts for Science [Madison, WI]). The antibody (100–200 μg/dose) was administered i.p. on the same day as the RIT.
Immunohistochemistry of tumor infiltrating CD8+ cells
Tumors were removed, fixed overnight in 10% formalin and submitted to the Pathology/Histotechnology Laboratory (Frederick, MD) for staining with H and E and anti-CD8a antibody (clone 4SM15, e-Bioscience). Automated image processing and analysis software (Toolbox; Leica Biosystems) was used to identify and quantitate the number of CD8+ cells.
Statistics
We used Prism 6 for all statistical calculations and graph fitting. To compare the survival of groups, we used log-rank (Mantel-Cox) test. The Mann–Whitney test was used to analyze the differences in the number of T cells in the tumors. All animal experiments were done twice and often more times. Error bars represent SDs.
Results
Because the targeting moiety of SS1P and LMB-100 is not mouse cross-reactive, we generated a mouse cancer cell line expressing the human mesothelin protein and a transgenic mouse strain expressing human mesothelin and tolerant to the human mesothelin protein to determine whether immune system activation by anti-CTLA-4 can augment the anti-tumor effect of these RITs. We produced BALB/c transgenic mice expressing the full-length human mesothelin gene as described in Materials and Methods. MPF is encoded by the mesothelin gene and released from the pre-protein into the blood by the action of furin (8). To ensure high mesothelin expression, we engineered a plasmid encoding only mature mesothelin fused to the transmembrane domain of the murine transferrin receptor, and used it to establish cell line 66C14-M (Supplementary Figs. S1A and S1B).
Mouse model
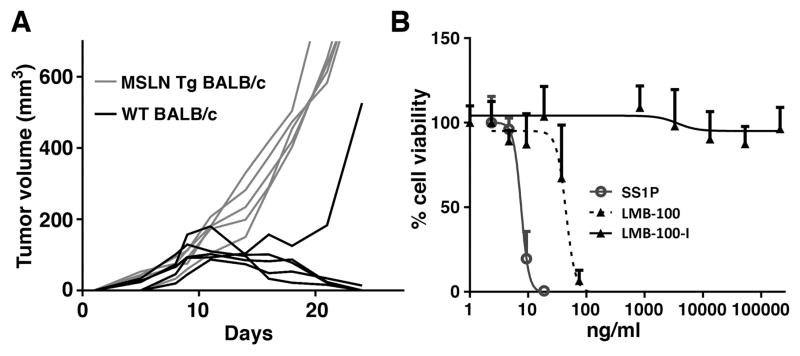
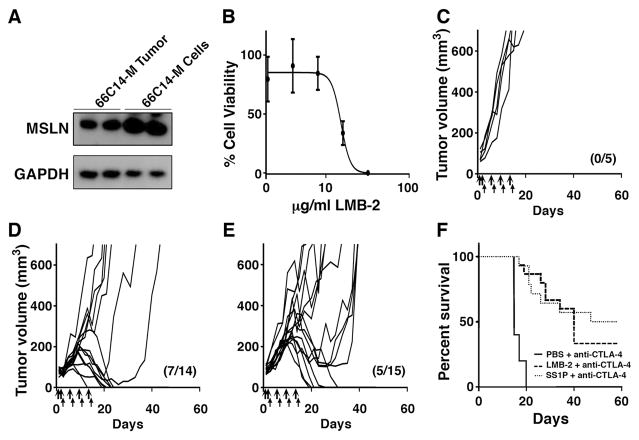
To determine if 66C14-M cells can form tumors, cells were implanted into five WT BALB/c mice and five mesothelin Tg mice. Fig. 1A shows the cells formed tumors in all the transgenic mice, but were rejected in 4/5 normal mice. To determine sensitivity of the 66C14-M cells to the cytotoxic effect of SS1P and LMB-100, a WST-8 cell viability assay was performed. Fig. 1B shows that 66C14-M cells are sensitive to both SS1P and LMB-100. The IC50 of SS1P is 8 ng/ml and that of LMB-100 is 46 ng/ml. We also tested LMB-100-I, mutant immunotoxin with a E553 deletion (see Materials and Methods), and the mutant protein had no effect on cell viability up to 210 μg/ml.
Figure 1. Growth of tumors in mice and activity of immunotoxins on target cells.
(A) Five WT BALB/c and five mesothelinTg BALB/c mice were implanted with 1 x 106 66C14-M cells. Individual tumor growth curves show tumors grow in five mesothelin Tg BALB/c mice but are rejected by 4/5 WT BALB/c mice, with marked tumor delay in the 5th mouse. (B) WST-8 cytotoxicity assays in 66C14-M cells after 3-day incubation with either SS1P, LMB-100 or the inactive LMB-100-I.
The transgenic mice were found to express human mesothelin in the pancreas where mesothelin is not expressed in normal mice or humans (19, 20). We found that some mice died when treated i.v. with 3 doses of 50 μg of LMB-100, whereas non-transgenic mice were not killed by this dose. To avoid this toxicity, we chose to inject LMB-100 or SS1P directly into 66C14-M tumors.
To evaluate how well a locally administered agent would distribute in a tumor, we injected trypan blue, which is visible to the eye, into five (72–117 mm3) 66C14 tumors. One hour after injection, we harvested the tumors and photographed them. We found that trypan blue reached most parts of the tumors (Supplementary Fig. S2). This suggests that direct injection of SS1P or LMB-100 should enable the injected agent to reach the majority of tumor cells.
Combination of local SS1P and systemic anti-CTLA-4 eradicates tumors
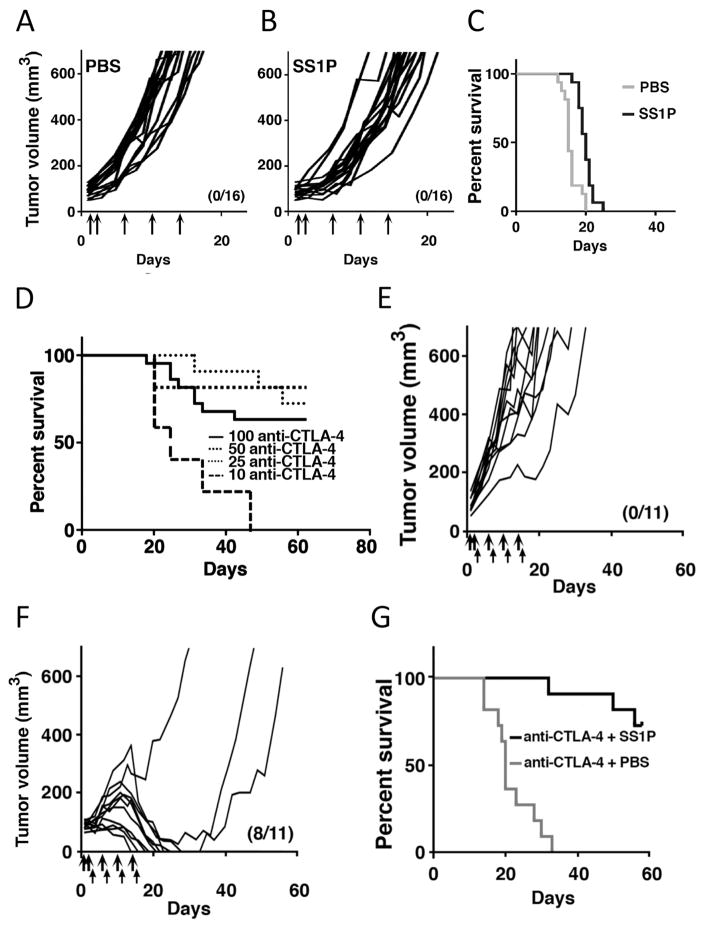
To evaluate the therapeutic effect of SS1P alone, we injected SS1P (5 μg) in PBS or PBS alone into 66C14-M tumors five times, beginning when the tumors reached 80–100 mm3 average size. Mice were injected on days 1, 2, 6, 10 and 14, with day 1 being the day of the first injection. As shown in Fig. 2A, tumors injected with PBS grew rapidly and reached the size of the experimental end point (volume > 700 mm3) at a median time of 15 days. Injecting SS1P into tumors delayed the time to reach 700 mm3 by 5 days, but did not cure any mice (Fig. 2B and C).
Figure 2. SS1P and anti-CTLA-4 effect on tumor growth in mice.
(A–B) Individual tumor growth curves of 66C14-M tumors after treatment with intratumoral PBS (n=16) (A) or 5 μg/dose SS1P (n=16) (B). Arrows mark the days of injection. A–B contains data from three experiments. (C) Long-term survival of mice described in (A–B); P < 0.0001 (D) Mice bearing 66C14-M tumors were treated with SS1P (5 μg/dose) and 10–100 μg/doses of anti-CTLA-4, according to our standard regimen. No difference in response rate was noted among groups treated at a 25 (n=11), 50 (n=6) or 100 (n=16) μg dose level. Treatment with a 10 μg dose of anti-CTLA-4 (n=6) and SS1P yielded a significantly lower survival rate than treating with 25 μg anti-CTLA-4 (P < 0.01). D containes data from four experiments. (E) Individual growth curves of 66C14-M tumors treated with PBS (thick arrows) and anti-CTLA-4 (thin arrows) or (F) 5 μg SS1P (thick arrows) and anti-CTLA-4 (thin arrows). E-F data was pooled from two experiments. (G) Long-term survival of mice treated with PBS and anti-CTLA-4 (25 μg) or SS1P (5 μg) and anti-CTLA-4 (25 μg) (P < 0.0001) as described in (D–F). The number of mice in complete remission and total mice per group are shown in parentheses.
To increase the anti-tumor effect of SS1P, we administered anti-CTLA-4 i.p. (days 3, 7, 11 and 15). Fig. 2D shows experiments in which a constant dose of SS1P (5 μg) was combined with increasing doses of anti-CTLA-4, ranging from 10 μg to 100 μg. We found that therapeutic effect of the combination did not improve above a dose of 25 μg, and therefore used this dose in further experiments. Figs. 2E–G shows an experiment in which 11 mice were treated with anti-CTLA-4 (25 μg) and PBS or SS1P (5 μg). We observed that there were no tumor regressions in mice treated with anti-CTLA-4 and intra-tumoral PBS, but in mice treated with anti-CTLA-4 and intra-tumoral SS1P 8/11 tumors disappeared (Fig. 2F); two became much smaller but eventually grew back. Fig. 2G shows that there is a significant survival benefit when treating with SS1P and anti-CTLA-4 (P < 0.0001). Mouse weights were stable during therapy, indicating that the combination therapy is well tolerated (Supplementry Fig. S3A).
Studies with LMB-100
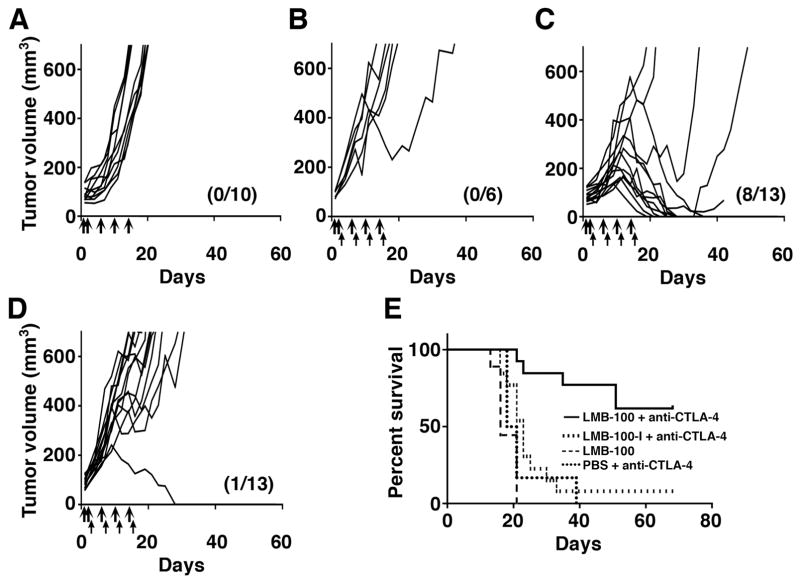
LMB-100 is an improved, less immunogenic, form of SS1P that is currently being evaluated in phase I/II clinical trials. To determine if LMB-100 could also induce tumor regressions, we treated 66C14-M tumor-bearing mice with LMB-100 and anti-CTLA-4. Because LMB-100 is 6-fold less potent than SS1P on the 66C14-M cell line (Fig. 1B), we used 30 μg of LMB-100 for anti-tumor experiments. All tumors treated with LMB-100 alone continue to grow (Fig. 3A). When anti-CTLA-4 was combined with PBS, transient tumor shrinkage was observed in 1/6 mice and no complete remissions were obtained (Fig. 3B). However, combining anti-CTLA-4 and LMB-100 resulted in tumor size reduction of 11/13 mice, and complete tumor elimination in 8/13 mice (61%) (Fig. 3C). To determine whether cell killing by the immunotoxin is required for the anti-tumor effect, we tested LMB-100-I, an inactive mutant form of LMB-100. Of 13 mice treated with 30 μg LMB-100-I and anti-CTLA-4, only one achieved a complete remission (Fig. 3D). Survival was comparable to that of mice treated with anti-CTLA-4 and PBS, and significantly lower than the survival of mice treated with anti-CTLA-4 and LMB-100 (P < 0.001, Fig. 3E). Mice treated with LMB-100 and anti-CTLA-4 did not lose weight during therapy, again implying lack of major toxicity (Supplementary Fig. S3B)
Figure 3. Tumor eradication requires an immunotoxin with cytotoxic activity and anti-CTLA-4.
Tumor growth curves of individual mice treated with (A) LMB-100 alone, (B) PBS (thick arrows) and anti-CTLA-4 (thin arrows), (C) Mice treated with 30 μg LMB-100 (thick arrows) and anti-CTLA-4 (thin arrows), (D) 30 μg LMB-100-I (thick arrows) and anti-CTLA-4 (thin arrows). The number of mice in complete remission and total mice per group is shown in parentheses. A, C and D shows pooled data from two repeats experiments. (E) Long-term survival of different treatment groups. Only mice in group C showed a significant increase in survival (P < 0.001).
Role of CD8+ T cells
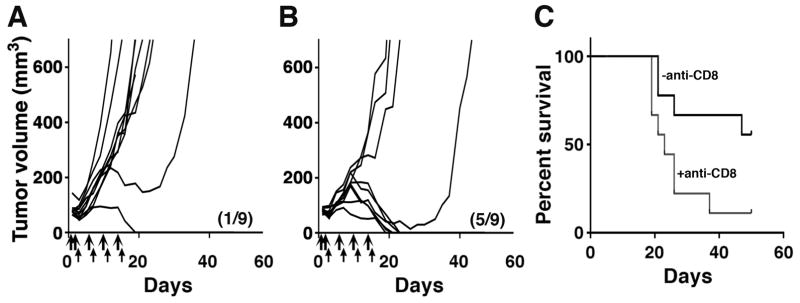
To determine whether CD8+ T cells were required for the therapeutic effect, we treated 66C14-M tumor bearing mice with anti-CD8 antibody (100 μg), intra-tumoral SS1P (5 μg) and anti-CTLA-4 (25 μg). Anti-CD8 antibodies were given on the same schedule as SS1P (days 1, 2, 6, 10 and 14). Figs. 4A and 4B show that anti-CD8 reduced the number of complete remissions in mice treated with SS1P and anti-CTLA-4 from 5/9 (55%) to 1/9 (11%) and significantly decreased survival (P < 0.05) (Fig. 4C). Two additional CD8 depletion experiments were done using LMB-100 (30 μg), anti-CTLA-4 (100 μg), and anti-CD8 antibody (100–200 μg) (Supplementary Fig. S4). In one experiment, a single mouse out of six (16%) treated with LMB-100, anti-CTLA-4, and anti-CD8 reached complete remission, whereas in the other experiment none out of six mice (0%) were cured. In mice treated with the combination drugs without CD8 depletion, complete remission was achieved in 10/14 mice (71%).
Figure 4. Anti-tumor effect of anti-CTLA-4 and SS1P depends on CD8+ T cells.
(A) Individual growth curves of 66C14-M tumors treated with anti-CTLA-4 (thin arrows) and SS1P (thick arrows) and 100 μg anti-CD8 antibody given i.p on days 1, 2, 6, 10 and 14. (B) Growth curves of tumors treated with SS1P and anti-CTLA-4. (C) Long-term survival of mice. The number of mice in complete remission and total mice per group is shown in parentheses. P < 0.05.
Tumor immunity
Anti-CTLA-4 induces anti-tumor immunity through the adaptive immune system (21). To evaluate whether the combination of SS1P or LMB-100 with anti-CTLA-4 produced long-term anti-tumor immunity, we injected 66C14 cells into 38 mice or 66C14-M cells into 6 mice about 45 days after the mice achieved complete remissions. We found that in 37/38 mice, the 66C14 cells were unable to form tumors. Also no tumors formed in 6/6 mice receiving 66C14-M cells (Supplementary Table S1).
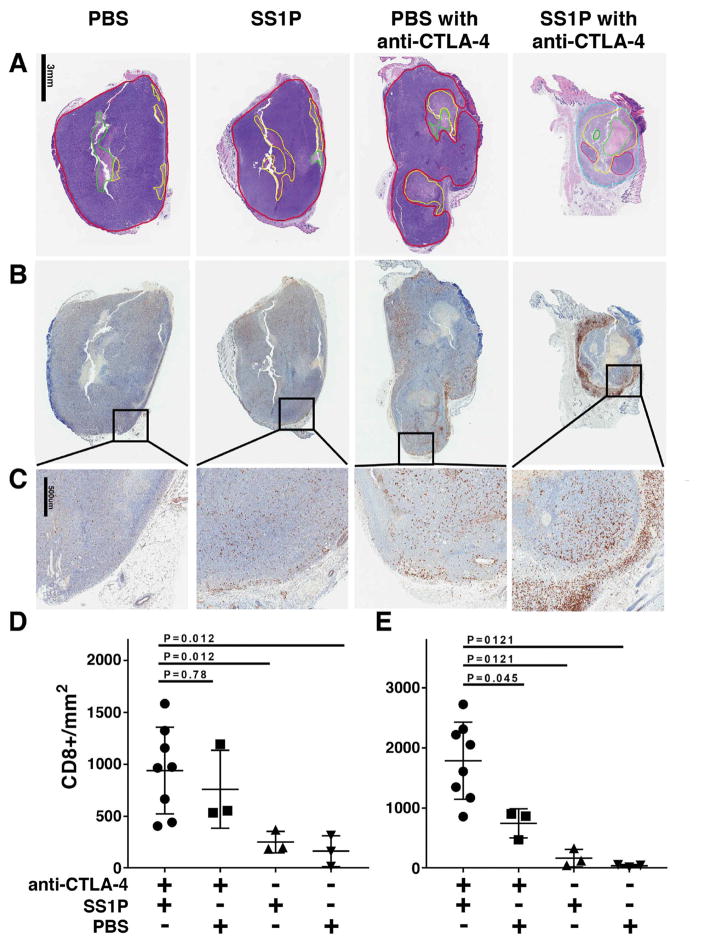
We examined tumor sections by H and E staining and used immunohistochemistry to determine the number of CD8+ cells infiltrating the tumors of mice responding to treatment. Mice with 66C14-M tumors were treated with SS1P (5 μg) and anti-CTLA-4 (25 μg). When the tumor size was reduced by 20–60% from maximum the tumors were harvested. Also harvested were tumors from non-responding mice treated with PBS alone, SS1P alone, or anti-CTLA-4 and PBS. Fig. 5 shows representative sections stained with H and E and for CD8+ cells for all four treatment groups. The areas outlined in red are viable tumor cells; the areas outlined in green and stained pink are necrotic and the areas outlined in yellow are micro-abscesses with necrotic material and admixed/degenerative granulocytes.
Figure 5. Analysis of tumors treated with PBS, SS1P, PBS and anti-CTLA-4 or SS1P and anti-CTLA-4.
(A) Sections of tumors stained with H and E. Areas outlined in green are necrotic, areas out-lined in yellow show micro-abscesses, and areas with viable tumor cells are outlined in red. The area outlined in blue in the tumor treated with SS1P and anti-CTLA-4 is a collar containing inflammatory cells. (B) Sections of tumors stained with anti-CD8 at low magnification (1X). (C) Sections of tumors stained with anti-CD8 at high magnification (5X). Tumors from the PBS, SS1P and PBS with anti-CTLA-4 groups were harvested when the tumors reached about 300 mm3 in size. Tumors from the combination treated group were harvested when the tumor volume was reduced by 20–60% from the maximum (8 mice). (D) Number of CD8+ T cells present in the tumor mass. (E) Number of CD8+ T cells in the collar surrounding the tumor.
In tumors injected with either PBS (n=3) or SS1P (n=3) or PBS and anti-CTLA-4 (n=3), there were small areas of necrosis in the center of the tumors, which may have been caused by the injection procedure or could represent central necrosis seen in many rapidly growing tumors. In 7/8 tumors treated with SS1P and anti-CTLA-4, the central necrosis was very pronounced (Fig. 5A) with few viable tumor cells present and these were admixed with PMNs. These tumors usually contain a collar of admixed eosinophils and mononuclear cells, making up to 30% of the total “mass”. Whether this collar is part of the tumor and contains dying tumor cells or is just close to the tumor has not yet been established.
Figures 5B and C show representative sections stained for CD8+ T cells. A very large number of CD8+ cells were observed in the inflammatory collar of tumors treated with SS1P and anti-CTLA-4. To quantify these differences, the number of CD8+ cells in the entire tumor and the number present only in the inflammatory collar were measured. Fig. 5D shows that there are more CD8+ T cells in the tumors of mice treated with anti-CTLA-4 with SS1P or without SS1P than in mice treated with SS1P alone or PBS alone. Fig. 5E shows that there were more CD8+ cells in the inflammation collar of mice treated with anti-CTLA-4 and SS1P than in the other mice (P < 0.05).
Specificity of targeting mesothelin in tumors
66C14-M cells are sensitive to the cytotoxic effect of SS1P with an IC50 of 8 ng/ml (Fig. 1B). Nonetheless, we needed to inject 5 μg of SS1P directly into the tumor mass to achieve complete remissions. No cures were obtained in six mice treated with 0.5 μg SS1P and anti-CTLA-4 (Supplementary Fig. S5).
To determine if the tumor cells growing in mice lost mesothelin expression, we did a western blot with an anti-mesothelin antibody. Mesothelin levels in the tumor sample (Fig. 6A) are about 50% lower than in cultured cells, which is expected because about 50% of the tumor is made up of mouse stromal and inflammatory cells. Thus, loss of mesothelin does not explain the need for high dose anti-mesothelin RIT. To further examine how much of the therapeutic effect is mesothelin-mediated, we used LMB-2 (anti-TacFv-PE38). LMB-2 has the same toxin moiety as SS1P, but targets human CD25, an antigen not expressed on mouse 66C14-M cells. Using a WST-8 viability assay we found that LMB-2 has an IC50 of 14 μg/ml (Fig. 6B), which is 1700-fold higher than the IC50 of SS1P (Fig. 1B).
Figure 6. Comparison of the effect of SS1P with an immunotoxin targeting human CD25.
(A) Western blot comparing mesothelin expression in 66C14-M tumor with 66C14-M cultured cells. (B) Three day WST-8 cytotoxicity assay with 66C14-M cells showed that a high concentration of 5 μg LMB-2 is required for cytotoxic effect. C–E. Individual growth curves of 66C14-M tumors treated with either anti-CTLA-4 and intratumoral PBS (C), anti-CTLA-4 and 5 μg dose SS1P (D), or anti-CTLA-4 and 5 μg dose LMB-2 (E). Pooled data from two experiments is presented. The number of mice in complete remission and total mice per group is shown in parentheses. Thick arrows mark the days of intatumoral injections, Thin arrows mark the days of i.p. injections. (F) Survival of mice treated with anti-CTLA-4 and either SS1P or LMB-2 was significantly longer than that achieved with PBS and anti CTLA-4 (P < 0.0001).
We then treated 66C14-M tumor bearing mice with SS1P (5 μg) or LMB-2 (5 μg) and anti-CTLA-4 using our standard protocol. As shown in Fig. 6C, tumors continued to grow in all mice that were treated with intratumoral PBS and anti-CTLA-4, whereas 50% of the mice (7/14) treated with SS1P and anti-CTLA-4 achieved complete remissions (Fig. 6D) and 5/15 mice treated with LMB-2 and anti-CTLA-4 achieved complete remissions (Fig. 6E). The survival of the SS1P and LMB-2 groups was greater than that of mice treated with PBS and anti-CTLA-4 (P < 0.0001), but the difference in survival between mice treated with SS1P and LMB-2 was not significant (Fig. 6F).
To determine if other immunotoxins not targeted to mesothelin were also effective in producing an anti-tumor effect, we evalutaed HA22, an immunotoxin that targets human CD22, in combination therapy with anti-CTLA-4. We found that in 66C14-M tumor-bearing mice, no complete remissions were achived by vehicle, HA22 alone or vehicle and anti-CTLA-4, whereas combining anti-CTLA-4 with HA22 produced complete remissions in 6/10 mice (Supplementary Fig. S6A). Survival benefit was found in mice treated with HA22 and anti-CTLA-4 compared to control groups (P < 0.0001, Supplementary Fig. S6A). We also tested HA22 and anti-CTLA-4 in 66C14 tumors not expressing mesothelin and observed that 4/7 tumors underwent a complete remission.
To determine the effect of anti-mesothelin immunotoxin on cells that do not express the target, we examined the effect of intra-tumoral SS1P (10 μg) combined with anti-CTLA-4 on parental non-mesothelin expressing 66C14 tumors. Supplementry Fig. S6B shows that 10/10 66C14-M tumor bearing mice underwent complete regressions, while 8/10 mice with 66C14 parental tumors also reached complete remissions. We also evaluated the activity of native PE in combination with anti-CTLA-4 but found it was very toxic to the mice so that we could not give more than 100 ng. At this dose, the anti-tumor effect was minimal.
To detemine if another kind of cytotoxic agent could recapitulate the synergy, we injected tumors with paclitaxel and anti-CTLA-4 and found no increase in rate of complete remissions or survival benefit (Supplementry Fig. S7).
Combining local SS1P with anti-CTLA-4 eradicates distant uninjected tumors
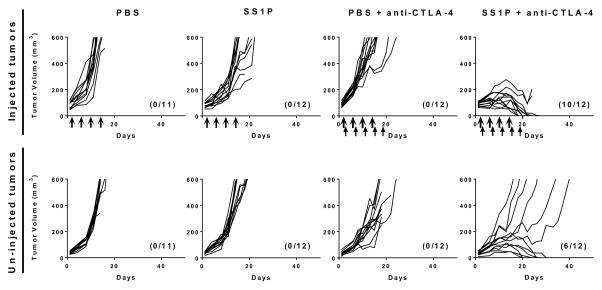
The ability of anti-CTLA-4 and intra-tumoral SS1P to promote anti-tumor immunity as indicated by rejection of a second tumor challenge, lead us to hypothesize that this therapy can also control metastatic tumor growth. To determine if uninjected tumors would respond, we implanted 66C14-M cells into both right and left mammary fat pads. We inoculated 106 cells in the right side and 105 cells on the left side. SS1P (25 μg) was injected directly into one tumor and anti-CTLA-4 (25 μg) given i.p. Fig. 7 shows a representative experiment in which mice were treated with SS1P (25 μg) on days 1, 5, 9 and 13, and with anti-CTLA-4 (25 μg) on days 2, 6, 10, 14 and 18. Tumor regressions occurred in 10 out of 12 SS1P injected tumors (83%) and in 6 of 12 uninjected contralateral tumors (50%). No tumor regressions occurred with drugs given separately. In two repeat experiments 91% and 83% of the mice treated with anti-CTLA-4 and SS1P achieved complete remission in the injected tumors (11/12 and 5/6 mice) and in 58% and 50% of uninjected tumors (7/12 mice and 3/6 mice).
Figure 7. Anti-CTLA-4 and SS1P eradicate injected tumors and uninjected tumors in a bilateral tumor model.
Individual growth curves of 66C14-M tumors implanted in two locations on the mice and treated with PBS (thick arrows) or 25 μg SS1P (thick arrows) alone or in combination with anti-CTLA-4 (thin arrows). The injected tumors are represented in the upper panel and the uninjected tumors are in the lower panel. The number of mice in complete remission and total mice per group are shown in parentheses.
Discussion
We report here that locally delivered RITs combined with systemic anti-CTLA-4 causes eradication of injected as well as uninjected tumors. When given alone, each agent has very little activity. Our data indicate that immunotoxin-mediated cell death is immunogenic and leads to induction of a T cell response against the tumor that contributes to tumor regression. This also results in long-term immune memory and protection against subsequent rechallenge with the tumor.
To carry out these studies we produced a mouse breast cancer cell line that expresses human mesothelin. Because the cells are rejected by normal mice, we developed a mouse line that expresses human mesothelin so that the mice are tolerant to human mesothelin. Because human mesothelin is expressed in the pancreas of these mice, we could not administer the immunotoxin i.v. and instead administered it directly into the tumors. This route avoided systemic exposure responsible for the on-target toxicity caused by atypical tissue expression in the transgenic animals.
The difference in mesothelin expression patterns between transgenic mice and humans makes it difficult to predict toxicity. Nevertheless, we show that in this model, in which mesothelin is expressed by essential organs, combining anti-CTLA-4 with localized immunotoxin is well tolerated. Another concern arising from combining immunotoxin with anti-CTLA-4 is increasing the immunogenicity of the immunotoxin. Patients given SS1P i.v. establish neutralizing antibodies against the drug after 2–3 weeks. The concentration of anti-drug antibodies may increase when an immunotoxin is combined with an immune checkpoint inhibitor. However, given the high concentration of SS1P injected into the tumors (5–30 μg in 30 μl for a mouse and potentially 1000 μg/ml for humans), we believe that the levels of antibody against the immunotoxin would not be high enough to neutralize the RIT in the local tumor microenvironment.
The tolerance of the transgenic mice to human mesothelin may account for the lack of requirement of mesothelin as an antigen for rejection of the tumor, and thus for the cross-protection against rechallenge with the parental tumor cell line lacking mesothelin. This indicates that the anti-tumor immunity develops against tumor neoantigens rather then human mesothelin.
We found that we needed to inject a high dose of SS1P (5 μg) into tumors to achieve a therapeutic response even though the cells are sensitive to low doses of SS1P in cell culture. In addition, combining SS1P, or non-mesothelin-specfic LMB-2 or HA22, with anti-CTLA-4 yielded a similar therapeutic effect when tested on 66C14-M tumors or even on 66C14 parental tumors not expressing mesothelin. Altogether, these findings indicate that the immunotoxin does more than target specific cells in this model of localized tumor injection. We confirmed that mesothelin, the target of the immunotoxins, continued to be expressed in 66C14-M tumors. Further work is needed to understand the mechanism underlying the target independency, but after intratumoral injection much of the RIT uptake may be mediated by endocytosis independent of anti-mesothelin targeting.
The current experiments were stimulated by our clinical observation that several patients with mesothelioma who received both immunotoxin therapy and immuno-modulatory drugs pentostatin and cyclophosphamide had tumor responses weeks to months after the immunotoxin therapy (12). Because the mouse tumors we employed grew rapidly, we needed to begin treatment about 10 days after tumor implantation and finish treatment within a two-week period, so the time scale is compressed compared to the human studies.
We observed that the major anti-tumor response began 12–15 days after administration of anti-CTLA-4. The presence of a lag period indicates several days are needed for an adaptive immune response to develop (22). Tumor regressions were accompanied by inflammation and CD8+ cell infiltration, most prominently found in the tumor margins. Our observations are consistent with previous studies showing that anti-CTLA-4 therapy increases the percentage of tumor infiltrating CD8+ T cells and depends on this cell subset (23,24).
The generation of immunity to cancer is a cyclical process that can be self-propagating, leading to an accumulation of immune-stimulatory factors that in principle should amplify and broaden T-cell responses. The cycle is also characterized by inhibitory factors that lead to immune regulatory feedback mechanisms, which can halt the development or limit the immunity. For the cancer-immunity cycle to be efficient, release of antigens from the cancer cell must occur and these antigens must be processed and presented by APCs in order to prime and activate effector T cells that need to infiltrate into the tumor site (25). Immunotoxin killing, as shown, can promote the cancer-immunity cycle by killing tumor cells locally, thereby releasing tumor antigens and allowing for their presentation by professional APCs.
Here we show that locally delivered RITs combined with anti-CTLA-4 promoted regression of a distant tumor. Unlike other anti-cancer modalities, immunotherapy does not directly kill cancer cells, but reshapes the conditions in which the immune system targets cancer. Thus, a local immune activation can spread and eliminate distant tumor sites. Our findings with regression of untreated distant or contralateral tumors suggest that locally delivered treatment can be used to treat systemic cancers.
Other therapies that induce tumor cell death, including chemotherapy and radiotherapy, promote anti-tumor immunity (26). One example is abscopal phenomena in which local radiotherapy results in regression of a distant tumor foci. Similar to our results, combining check point blockade with local radiotherapy improves the therapeutic effect (27).
Rechallenging mice that had undergone complete tumor regressions with tumor cells 45 days after the regressions occurred demonstrated that anti-tumor immunity was present. The mice rejected tumors expressing mesothelin, but also rejected 66C14 tumors not expressing mesothelin. Because this long-term immunity does not require mesothelin expression it is probably due to responses to some of the mutant proteins present in the 66C14 cells.
The finding of T cells within tumors treated with PBS alone suggests a mild immune response may be present in untreated mice that is enhanced by the combination of anti-CTLA-4 and immunotoxin. Our finding of occasional partial responses in mice treated with anti-CTLA-4 and PBS or inactive-immunotoxin is probably due to enhancement of low-grade anti-tumor immunity. Because the toxin is of bacterial origin, we were concerned it might be activating receptors for pathogen-associated molecular pattern. This possibility was eliminated, because an immunotoxin (LMB-100-I) with mutations that abrogate cytotoxic activity in cell culture, showed no synergy with anti-CTLA-4 in the mice.
Clinical studies showed that intra-tumoral injection of TP-38 targeting the EGF receptor or an immunotoxin scFv(FRP5)-ETA targeting ErbB2 induced regressions of breast, melanoma and brain tumors. (13, 14, 28). We speculate that anti-CTLA-4 can potentiate the therapeutic effect of these RITs as well.
Altogether, this study demonstrates the anti-tumor effect of local immunotoxin therapy combined with systemic injection of anti-CTLA-4. We believe this combination therapy has potential for clinical use.
Supplementary Material
Acknowledgments
Financial Support: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and with a Cooperative Research Development Agreement (#2791) between the NCI and Roche Pharmaceuticals.
We thank Dr. Diana Haines for assistance with pathology and Donna Butcher for assistance with immunostaining.
Footnotes
Conflict of Interest: I.P. is an inventor on several patents on immunotoxins that have all been assigned to the NIH and has a Cooperative Research and Development Agreement (#2791) with Roche Pharmaceuticals.
Author Contributions:
Conception and design: I. Pastan, Y. Leshem
Development of methodology: Y. Leshem, X. Liu, T. Bera
Acquisition of data; Y. Leshem, X. Liu, T. Bera, J. O’Brien
Analysis and interpretation of data: All authors
Writing, review, and/or revision of manuscript: All authors
Study supervision: I. Pastan
References
- 1.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 3.Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–90. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6:133–46. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–12. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci U S A. 1975;72:2284–8. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 11.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson JH, Reardon DA, Friedman AH, Friedman HS, Coleman RE, McLendon RE, et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol. 2005;7:90–6. doi: 10.1215/S1152851703000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 15.Alewine C, Xiang L, Yamori T, Niederfellner G, Bosslet K, Pastan I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–61. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollevoet K, Mason-Osann E, Liu XF, Imhof-Jung S, Niederfellner G, Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–9. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolin C, Sutherland C, Tawara K, Moselhy J, Jorcyk CL. Novel mouse mammary cell lines for in vivo bioluminescence imaging (BLI) of bone metastasis. Biol Proced Online. 2012;14:6. doi: 10.1186/1480-9222-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas CM, Collier RJ. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987;169:4967–71. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–81. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 20.Zervos E, Agle S, Freistaedter AG, Jones GJ, Roper RL. Murine mesothelin: characterization, expression, and inhibition of tumor growth in a murine model of pancreatic cancer. J Exp Clin Cancer Res. 2016;35:39. doi: 10.1186/s13046-016-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–73. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 22.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9:741–7. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 23.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–9. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 27.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 28.Azemar M, Djahansouzi S, Jager E, Solbach C, Schmidt M, Maurer AB, et al. Regression of cutaneous tumor lesions in patients intratumorally injected with a recombinant single-chain antibody-toxin targeted to ErbB2/HER2. Breast Cancer Res Treat. 2003;82:155–64. doi: 10.1023/b:brea.0000004371.48757.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.