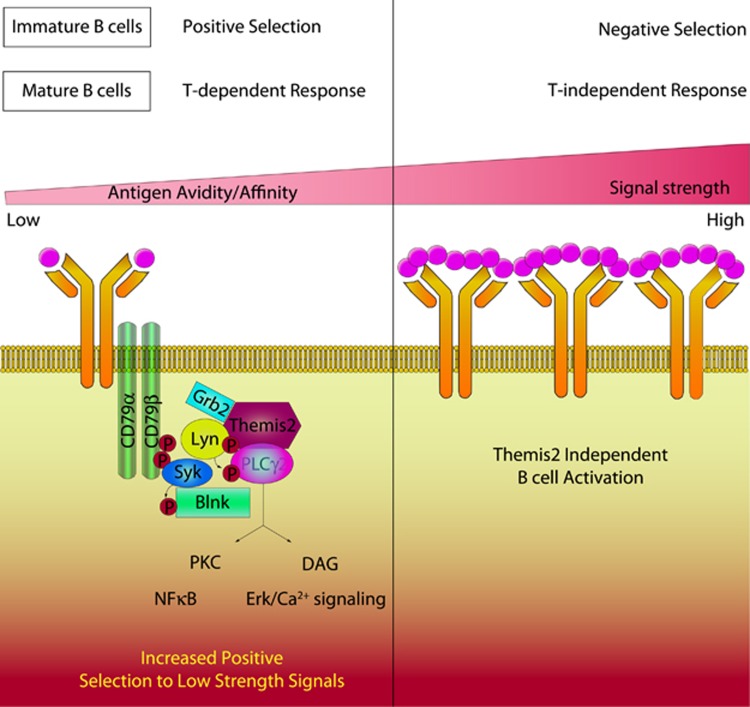
The strength of signal downstream of the B-cell receptor (BCR) determines the positive and negative selection of B cells. Expression of a functional BCR is required for the selection of immature B cells into the peripheral repertoire in the absence of antigen; but selection is also modulated by avidity and affinity-dependent interactions with self and foreign antigens. These antigen-dependent events shape the B-cell repertoire during ontogeny, development and the response to pathogens, and generation of B-cell memory; however, the mechanisms underlying these processes are not very well understood.
During early ontogeny, interactions with intracellular or low abundance self-antigens allow positive selection of innate-like B1 B cells.1 In contrast, the presence of abundant soluble or low-avidity self-antigen leads to B-cell anergy, whereas stronger signals from highly abundant or multivalent antigens trigger receptor editing and/or negative selection of immature B cells through central tolerance.2 These processes must be determined by the state and environment of the B cells, defined in part by the presence of co-stimulation or other signals. Later in the periphery, antigens of similar affinity or avidity elicit B-cell activation, leading to rapid proliferation, plasma cell differentiation and antibody production. During an immune response to pathogens, the majority of B-cell activation involves interactions with lower avidity and/or low-affinity antigens in a soluble, potentially tolerogenic form or coupled via complement or Fc receptors, notably arrayed on the membranes of macrophages, B cells and follicular dendritic cells.3, 4 However, despite the importance of antigen quality and quantity in modulating B-cell responses, very little is understood about the process that defines the signalling threshold between positive and negative selection, allowing B cells to discriminate between antigens of varying strength and form.
In a recent paper published inNature Immunology, Cheng et al.5 show how Themis2, a member of the newly described Themis-family of proteins, modulates the positive selection of B cells in response to self and foreign antigen. Themis2 determines the threshold for activation of B cells by low-affinity and low-avidity ligands via PLCγ2 activation and ERK1/2-dependent pathways (Figure 1). These effects were detected using the immunoglobulin transgenic MD4 mouse model and the cognate antigen ‘hen egg lysozyme’ (HEL). Themis2 has no known catalytic domain and potentially functions as an adaptor protein by strengthening the interactions between PLCγ2 and the upstream kinase Lyn. Loss of Themis2 raises the threshold for the antigen-induced upregulation of CD69, which is required for the retention and proliferation of activated B cells in the lymph node, and reduces the expression of CD86, which is required for receiving ‘co-stimulatory’ T-cell help. Therefore, Themis2 may play an important part in the initial immune response, by increasing the sensitivity of naive B cells to rare and low-affinity antigens.
Figure 1.
Themis2 increases the positive selection of B cells to low-avidity or low-affinity self and foreign antigens during formation of the repertoire and during the immune response.
The Themis-family of proteins is evolutionarily conserved in metazoans from mammals to cnidarians and is characterized by one or more copies of a novel cysteine containing globular CABIT domain.6 Themis2 has two tandem CABIT domains and has two close mammalian homologues: Themis1, expressed primarily in T cells, and Themis3, expressed in the intestine. In addition, mammals express two family members of unknown function, with single copies of the CABIT domain and a proline-rich region, Fam59A (Garem) and FAM59B. Like Themis2, Themis1 is proposed to be an analogue-to-digital convertor, which translates low-affinity signals into a selection event during the positive and negative selection of CD4+CD8+ thymocytes. In the absence of Themis1, T-cell development is blocked at the CD4+CD8lo positive selection point.6, 7, 8, 9 Themis1 is constitutively associated with Grb2 via its proline-rich domain and is rapidly phosphorylated by Lck and ZAP70 and recruited to LAT upon T-cell activation.
Although research to elucidate the function of Themis1 demonstrates our relatively better understanding of the role of avidity and affinity during T-cell activation and development compared to B cells, there remain contrasting reports about the mode of action of Themis1. In 2013, it was suggested that Themis1 had a role in transmitting T-cell receptor (TCR) signals from low affinity, but not high affinity antigens by attenuating, rather than increasing, signal strength, thus modulating negative selection.10 The authors compared developmentally matched OVA-specific T cells and reported increased TCR-dependent signalling due to low-affinity ligands in the absence of Themis1. From these results, they proposed that in the absence of Themis1, signals generated from weak TCR-ligand interactions are enhanced, partly due to a failure to activate the inhibitory phosphatase SHP1 and/or recruit SHP1 to LAT. In contrast, Choi et al.11 more recently reported in a second Nature Immunologypaper that Themis1-deficient cells have elevated SHP1 activity, and that Themis1 positively regulates T-cell development by a novel mechanism involving the suppression of SHP1 by the Themis2 CABIT domain. Deficiency in SHP1 rescues T-cell development in Themis1-deficient thymocytes. Themis2 is functionally interchangeable with Themis1 in T-cell development, implying that they may have a similar mechanism of action.12 However, cell-specific dissimilarities, including redundancy and a significant difference in the level of expression of phosphatases and Themis1 and 2 in T and B cells may mean this interpretation is too simplistic. Competition between positive and negative regulators linked to Themis-family proteins as well as other binding partners, including inhibitory co-receptors might modulate the differential signalling outcomes of B and T cells.
In reporting the characterization of a novel protein that modulates signal transduction downstream of the BCR, the paper by Cheng et al. raises questions about how B cells discriminate between qualitatively different antigenic signals received via the same BCR and how antigens of different quantity and quality fine-tune the immune response. It also emphasizes the need to adopt new approaches and develop tools to measure these effects experimentally.
Although polyvalent membrane-bound antigens are better able to induce antibody responses and activate B cells,13 it has long been known that soluble, low valency antigens are also able to activate B cells in vitro and in vivo.14 In addition to the maintenance of tolerance and survival by low-affinity interactions and tonic signalling, B-cell responses to antigens of relatively low avidity are likely to be important in most physiological settings. The decision to differentiate into an extrafollicular plasma cell versus becoming a germinal centre B cell during the initial phase of T-dependent antibody responses is likely governed by the nature of the interaction between antigen and BCR.15
However, in vitro and biochemical studies of B-cell activation have almost exclusively used high avidity analogues of T-independent antigens, typically anti-IgM F(ab′)2. These potent forms of antigenic stimulation are likely to miss subtle, but important, aspects of differential BCR signalling. Analogous to the use of antigens of different affinity to assess T-cell responses, similar approaches using cognate antigens of different affinity and avidity, and with a defined BCR specificity, may offer valuable insights into the selection of B cells.
It was reported in a separate study that Themis2-deficiency had no effect on B-cell development or antibody titres following immunization with NP-Ficoll and NP21-CGG or B-cell activation by anti-IgM in vitro, all of which are higher avidity forms of antigen.16 Only when analysis of B-cell responses to lower valency forms of antigen such as NP3-CGG or soluble HEL is carried out, does the role of Themis2 in establishing a signalling threshold and modulating responses to lower avidity antigens become evident.5
Future studies will want to investigate the role of other molecules that allow signal discrimination during situations where antigen is most limiting: for example, during the positive selection of B1 B cells and marginal zone B cells or during the germinal centre response, where B-cell selection must be exquisitely regulated to eliminate self-reactive clones, while allowing progressively enhanced affinity maturation via competition for limiting antigen. A layer of complexity may be added to these studies by employing antigens of variable avidity and affinity, thus better dissecting the processes that fine-tune BCR activation outcomes. Untangling the role of localized cellular signalling and how this is dependent on different environmental cues and developmental stages, as well as on the nature and dose of antigens, will give us a better understanding of how B cells integrate upstream signals and switch between different outcomes.
Footnotes
The authors declare no conflict of interest.
References
- Hardy RR. B-1 B cell development. J Immunol 2006; 177: 2749–2754. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Sprent J, de St Groth BF, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 2005; 435: 590–597. [DOI] [PubMed] [Google Scholar]
- Phan TG, Gray EE, Cyster JG. The microanatomy of B cell activation. Curr Opin Immunol 2009; 21: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol 2009; 9: 15–27. [DOI] [PubMed] [Google Scholar]
- Cheng D, Deobagkar-Lele M, Zvezdova E, Choi S, Uehara S, Baup D et al. Themis2 lowers the threshold for B cell activation during positive selection. Nat Immunol 2017; 18: 205–213. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Aravind L, Shulzhenko N, Morgun A, Choi S-Y, Crockford TL et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol 2009; 10: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourne R, Uehara S, Lee J, Song K-D, Li L, Pinkhasov J et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol 2009; 10: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Vallée S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol 2009; 10: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick MS, Oda H, Hayakawa K, Sato Y, Eshima K, Kirikae T et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. PNAS 2009; 106: 16345–16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Casas J, Rigaud S, Rybakin V, Lambolez F, Brzostek J et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013; 504: 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Warzecha C, Zvezdova E, Lee J, Argenty J, Lesourne R et al. Themis enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol 2017; 18: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourne R, Zvezdova E, Song K-D, El-Khoury D, Uehara S, Barr VA et al. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J Immunol 2012; 189: 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol 1989; 143: 1239–1244. [PubMed] [Google Scholar]
- Avalos AM, Bilate AM, Witte MD, Tai AK, He J, Frushicheva MP et al. Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J Exp Med 2014; 211: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006; 203: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweger H, Schweighoffer E, Davidson S, Peirce MJ, Wack A, Tybulewicz VLJ. Themis2 is not required for B cell development, activation, and antibody responses. J Immunol 2014; 193: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]