Gamma delta (γδ) T cells are a unique and conserved population of lymphocytes. They are considered ‘unconventional’ T cells because they represent a relatively small subset of T cells and are defined by the expression of heterodimeric T-cell receptors (TCRs) that are composed of γ and δ chains. Data show that γδ T cells function in many types of immune responses and immunopathologies. They provide protective immune responses against a wide range of pathogens and directly eliminate infected or stressed cells.1, 2 Gamma delta T cells produce various cytokines and chemokines that have the capacity to modulate other immune and non-immune cells.3 They can also facilitate the maturation of dendritic cells and modulate the activation of conventional αβ T cells.4, 5 Although γδ T cells can be protective, they can also be deleterious. For instance, the potent cytotoxicity of γδ T cells and their ability to produce IFN-γ are considered protective against cancer, whereas IL-17-producing γδ T cells are associated with tumors.6 Nevertheless, the combination of conventional adaptive functions that are inherent in their TCRs, as well as their rapid innate-like responses and potential to modulate initial immune responses, makes γδ T cells attractive candidates for immunotherapy.7
In contrast to conventional T cells that express αβ TCR and whose development and function are well characterized, the human γδ T-cell lineage remains enigmatic. This is attributed to their poorly defined antigens, the unconventional mechanisms by which they respond to immunological stimuli as well as the difficulty to track them in vivo. Unlike αβ TCRs, the repertoires of human and murine γδ TCRs were thought to be narrow, individual and stable. It is currently unclear if a narrow TCR repertoire is genetically imprinted or whether it is shaped postnatally. Furthermore, it is uncertain whether human γδ T cells undergo clonal proliferation in response to microbial challenge and if they can provide adaptive immunity.
In a recent issue of Nature Immunology, Ravens et al.8 studied human γδ T cells and provided new insight regarding two fundamental questions in γδ T-cell immunobiology. First, how are γδ TCR repertoires shaped in humans? Second, do human γδ T cells undergo clonal proliferation upon activation with their cognate antigen? To address these questions, the authors used RNA-based next-generation sequencing (NGS) to comprehensively analyze the repertoires of both TCRγ and TCRδ chains in γδ T cells purified from patients undergoing transplantation of hematopoietic stem cells. A prospective longitudinal cohort study was performed, and based on previous data, ~20–30% of the patients were expected to experience reactivation of cytomegalovirus (CMV). This scenario enabled the authors to monitor the γδ T-cell pool before and after transplantation and allowed them to determine how human γδ T cells are shaped during ontogeny. Moreover, by tracking γδ TCR repertoires before and after CMV reactivation, the authors were able to examine how γδ T cells respond to a major microbial challenge.
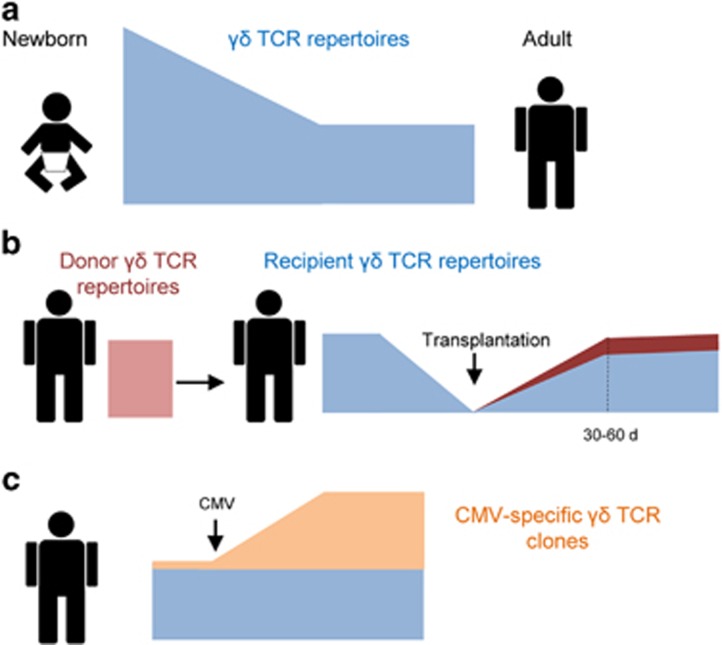
In the first part of the study, the repertoires of steady-state rearranged TCRγ and TCRδ genes (TRG and TRD, respectively) were assessed in patients before transplantation as well as in healthy adults (the control group). Gamma delta T cells purified from cord-blood samples were also analyzed and represent the TCR repertoires at birth. The authors revealed that, at birth, the repertoires of TRG and TRD are highly diverse. Nevertheless, during life, the TCR diversity was reduced due to preferential proliferation of certain γδ T-cell clones (Figure 1a). These adult γδ TCR repertoires were found to be remarkably stable, as they showed no significant alteration over 90 days. It should be noted that this study was the first to perform a NGS analysis of human TRD repertoires, which revealed that the TRD repertoire was consistently more diverse than the TRG repertoire at birth as well as in adults. Furthermore, by comparing the sequence overlap on individual γδ TCR repertoires, the authors found that the human TRG repertoire contained public clones (that is, they were present in many people), whereas the TRD repertoire appeared to be unique to individuals and thus was considered private.
Figure 1.
Dynamics of γδ T-cell receptor (TCR) repertoires. (a) After birth, the TCR repertoires of γδ T cells present high diversity. This changes during life as the γδ TCR repertoires become more focused and narrow, likely due to environmental and genetic factors. The adult γδ TCR repertoires remain remarkably stable in healthy adults. (b) In individuals undergoing transplantation of hematopoietic stem cells, γδ T cells are rapidly reconstituted in the host and present γδ TCR repertoires typical to adults. This newly generated repertoire differs greatly from the γδ TCR repertoires of both the donor and recipient before the transplantation, suggesting that γδ T cells differentiate de novo in the recipient’s thymus. Nevertheless, donor-derived γδ T cells also contribute directly to the reconstituted γδ T-cell pools, probably via homeostatic proliferation. (c) Cytomegalovirus (CMV) infection or reactivation represents an intense challenge to the immune system. CMV-reactive γδ T-cell clones exist in the γδ TCR repertoires of stem cell recipients and, upon activation, massively proliferate in an antigen-specific manner, leading to a significant increase in frequency among total γδ TCR repertoires.
Next, the impact of hematopoietic stem cell transplantation on γδ TCR repertoires was examined. The authors discovered that γδ T cells were reconstituted rapidly within 30–60 days and that their repertoires remained stable over 180 days. Although the reconstituted repertoires resembled adult TRG and TRD repertoires, they were fundamentally different from the original repertoires of the patients prior to transplantation. Moreover, the reconstituted γδ TCR repertoires also differed from the donor repertoires (Figure 1b). The authors concluded that γδ T cells were generated de novo in the adult thymus of the transplanted patients. Nevertheless, it was also evident that some of the donor γδ T clones contributed directly to the recipient γδ T cells, most likely via homeostatic proliferation.
In the last part of the study, the authors focused on patients experiencing CMV reactivation after transplantation. Upon initial reconstitution of new γδ TCR repertoires, reactivation induces major perturbations in the already rearranged TRG and TRD repertoires. The authors identified individual γδ T-cell clones that massively proliferated after the reactivation (Figure 1c). A single-cell analysis of the TRG and TRD sequences further confirmed that the proliferating γδ T-cell clones are indeed CMV-reactive.
While the work by Ravens et al. addresses fundamental issues in γδ T-cell immunobiology, it also highlights several questions that require further examination. First, γδ T cells are located in peripheral tissues, in which they play a critical immunological role. The present work examined γδ T cells in the blood; it is still unclear how reconstitution after transplantation affects the γδ TCR repertoires of tissue-resident γδ T cells. Second, a large portion of γδ T cells develop during embryonic development; these cells are likely to encounter a unique signaling environment during their differentiation that might not exist in the adult thymus after transplantation. Thus, although the γδ TCR repertoires of reconstituted γδ T cells resemble ‘normal’ adult repertoires, it is uncertain whether these cells will function as embryonic γδ T cells, migrate to the peripheral tissues and have the capacity to regulate local immunity. Third, with regard to the robust expansion of virus-specific γδ T-cell clones, further investigation is required to dissect their immunological functions, including their ability to protect the host and become memory cells similar to conventional αβ T cells.
In conclusion, the study by Ravens et al. provides compelling evidence demonstrating that (i) γδ TCR repertoires become oligoclonal during adulthood but remain highly diverse and stable; (ii) upon transplantation of hematopoietic stem cells, γδ T cells reconstitute rapidly, and their γδ TCR repertoires retain the overall complexity and proportion typical of adult γδ TCR repertoires; and (iii) CMV reactivation induces robust proliferation of CMV-reactive γδ T-cell clones, suggesting that γδ T cells might be capable of generating anti-viral adaptive immune responses. These findings should have direct clinical implications, since the rapidly differentiating γδ T cells are considered to have beneficial properties after transplantation. Thus, this work has important translational and prognostic implications; in the future, γδ T cells might be considered during hematopoietic stem cell transplantation.
Footnotes
The author declares no conflict of interest.
References
- Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol 2012; 42: 2221–2231. [DOI] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Paul S, Singh AK, Shilpi, Lal G. Phenotypic and functional plasticity of gamma-delta (gammadelta) T cells in inflammation and tolerance. Int Rev Immunol 2014; 33: 537–558. [DOI] [PubMed] [Google Scholar]
- Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol 2005; 174: 252–260. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005; 309: 264–268. [DOI] [PubMed] [Google Scholar]
- Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol 2015; 15: 683–691. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 2013; 13: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdörfer L et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol 2017; 18: 393–401. [DOI] [PubMed] [Google Scholar]