Abstract
There is great interest in developing and utilizing non-pharmacological/non-invasive forms of therapy for osteoarthritis (OA) pain including exercise and other physical fitness regimens.
Aims
The present experiments determined the effects of prior wheel running on OA-induced weight asymmetry and trabecular bone microarchitecture.
Main methods
Wheel running included 7 or 21 days of prior voluntary access to wheels followed by OA induction, followed by 21 days post-OA access to wheels. OA was induced with monosodium iodoacetate (MIA), and weight asymmetry was measured using a hind limb weight bearing apparatus. Bone microarchitecture was characterized using ex vivo μCT.
Key findings
Relative to saline controls, MIA (3.2 mg/25 μl) produced significant weight asymmetry measured on post-days (PDs) 3, 7, 14, 21 in sedentary rats. Seven days of prior running failed to alter MIA-induced weight asymmetry. In contrast, 21 days of prior running resulted in complete reversal of MIA-induced weight asymmetry on all days tested. As a comparator, the opioid agonist morphine (3.2– 10 mg/kg) dose-dependently reversed weight asymmetry on PDs 3, 7, 14, but was ineffective in later-stage (PD 21) OA. In runners, Cohen’s d (effect sizes) for OA vs. controls indicated large increases in bone volume fraction, trabecular number, trabecular thickness, and connective density in lateral compartment, and large decreases in the same parameters in medial compartment. In contrast, effect sizes were small to moderate for sedentary OA vs. controls.
Significance
Results indicate that voluntary exercise may protect against OA pain, the effect varies as a function of prior exercise duration, and is associated with distinct trabecular bone modifications.
Keywords: Exercise, Osteoarthritis pain, Trabecular bone
1. Introduction
Osteoarthritis (OA) is a disease of musculoskeletal origin that is associated with debilitating chronic pain, and currently ranks as the most common form of arthritis. Symptoms include chronic pain and decreased mobility due to modification to subchondral bone and degeneration of joint cartilage [1,2]. Pharmacological treatments for OA patients include NSAIDs and/or opioids, which are associated with significant adverse side effects with chronic administration (reviewed in [3]). Further, some patients report OA pain associated with a persistent pain state that is NSAID resistant, referred to as advanced OA pain [4]. These patients often turn to joint replacement therapy that produces alleviation of OA symptoms including joint pain in many patients [5,6]. Alternative and less invasive/expensive methods for treatment are desirable. One potential treatment strategy may be the incorporation of exercise regimens to either reverse or attenuate symptoms of OA pain.
Exercise is the top recommended non-pharmacological treatment for OA patients, and recently there has been an increasing literature base on the protective effects of voluntary exercise in preclinical and clinical pain populations. Voluntary wheel running in rodents has been shown to enhance muscle viability and bone strength [7–9], attenuate allodynia and elevated IL-1β levels in a model of neuropathic pain [10], and increase protective CD206 macrophage production in a model of muscle pain, and decrease pain- and stress-related measures in a model of inflammatory pain [11,12]. Although there is an increasing literature base on exercise-pain interactions [10,11,13,14], less is known about how the behavioral mechanism of wheel running acquisition duration (i.e., prior wheel running) affects the expression of chronic pain-like behavior. Further, it is unknown if prior running + OA pain is associated with distinct modulation to trabecular bone, relative to a sedentary OA pain condition. Toward that end, the present set of studies addressed two issues. First, two voluntary wheel running protocols [15] that differed in prior access to wheels (7 days vs. 21 days) were used to determine if duration of prior wheel running would differentially affect OA-induced pain behaviors, measured by weight asymmetry of the hind-limbs. Second, the present study also characterized the effects of OA on trabecular bone microarchitecture in rats with or without access to running wheels.
In the present set of experiments, OA was chemically induced with monosodium iodoacetate (MIA), a validated preclinical model of OA pain that includes cartilage degradation and sub-chondral bone loss similar to that seen in human and veterinary patients [16–18]. A concentration of MIA (3.2 mg/25 μl) was selected that previously demonstrated tactile allodynia and weight asymmetry [15].
Given the reports on opioid treatment of human OA and MIA-induced OA [19–22], the relative effectiveness of wheel running to reverse OA-induced weight asymmetry was compared to that of the standard prescription mu opioid agonist morphine. Based on our earlier work, we hypothesized that a relatively longer duration of prior wheel running would reduce OA-induced weight asymmetry. Additionally, it was predicted that the magnitude of OA-induced changes to trabecular bone would be distinct in exercised rats compared to sedentary controls.
2. Materials and methods
2.1. Subjects
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN), 200–250 g at the start of the experiment, were used for all studies. Rats were randomly assigned to six separate groups: three different saline-treated groups (saline sedentary/n = 7, saline runner with 7 day acquisition/n = 7, saline runner with 21 day acquisition/n = 7) and three different osteoarthritis MIA-treated groups (MIA sedentary/n= 7, MIA runner with 7 day acquisition/n=8, MIA runner with 21 day acquisition/n = 8). All rats were initially housed in groups of two to three in standard Plexiglas containers with food and water available ad libitum. Following 7 days of acclimation to the animal facility, runners were then moved to individual cages with running wheels attached; sedentary controls rats were also moved to individual cages at the same time. Animals were maintained in a temperature and humidity controlled colony on a 12-h light/dark cycle (lights on at 8:00). All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. The University of New England Institutional Animal Care and Use Committee (IACUC) approved all protocols involving animals.
2.2. Wheel running protocols
Wheel running was measured using an activity wheel monitoring system (Lafayette Instruments, Lafayette, IN). In running wheel experiments, rats were singly housed in a chamber that contained an activity wheel. Each rat had 24 h voluntary access to its own running wheel for the duration of the experiment, and the total distance traveled (m) in the wheels by each rat was recorded. Two separate running wheel protocols were evaluated. Each protocol consisted of three phases: a) an acquisition phase of 7 or 21 days, b) an osteoarthritis induction phase consisting of a single intra-articular injection of either saline or 3.2 mg MIA into the left hind knee, and c) a post-injection observation phase which lasted 21 days [15]. Rats had continuous access to running wheels each day from the beginning of acquisition through the end of post-observation.
2.2.1. Acquisition phase
The length of the acquisition phase (number of days access to wheels before saline or MIA injection) was chosen based on our previous experience with MIA-depressed wheel running protocols [15]. Two different wheel running protocols with distinct acquisition durations were evaluated: a 7-day acquisition period + 21-day post-induction period (protocol 7-21), and a 21-day acquisition period + 21-day post-induction period (protocol 21-21).
2.2.2. Osteoarthritis model
A single intra-articular injection of MIA (3.2 mg/25 μl) into the left hind knee was administered to induce a localized arthritis of the knee joint [15,16]. Control subjects received a single intra-articular injection of saline (25 μl). All i.a. injections occurred between 9:00–9:30 am, and running rats were then returned to the wheels. Sedentary rats were returned to separate cages with no access to wheels.
2.2.3. Post-MIA phase
The post-MIA phase consisted of a 21-day observation phase in which the effects of MIA-induced osteoarthritis or saline on wheel running were recorded daily. On post-days 3, 7, 14 and 21, rats were removed from their activity wheel monitoring system cages and brought into a separate room for testing on the Incapacitance tester [15,23]. After habituation to the testing room and Plexiglas chamber, weight bearing data were recorded as described above. Incapacitance testing was conducted for ~1 h starting at 12:00 pm. Test trials for each rat were approx. 30–60 s.
2.3. Weight bearing assay
An Incapacitance tester (Columbus Instruments, Columbus, OH)was used to determine hind paw weight distribution. Rats were placed in a custom-made, angled Plexiglas chamber so that each hind paw rested on a separate force plate. The change in hind paw weight distribution was automatically calculated by the Incapacitance tester. The apparatus calculates an average weight distribution over the span of 5 s, and three recordings are taken for each rat. All three recordings are then automatically averaged and a mean score is displayed. The primary dependent measure was % weight on ipsilateral hind limb, and represented the percentage of weight on ipsilateral hind paw following a treatment condition (MIA sedentary, MIA sedentary + morphine, MIA + wheel running) subtracted from the percentage of weight on ipsilateral hind paw following the baseline saline condition, and was determined by the following formula:
After habituation to the Plexiglas chamber, baseline recordings were determined. Following baseline determinations, sedentary rats were removed from their home cage and injected with 3.2 mg of intra-articular monosodium iodoacetate (MIA) or intra-articular saline (controls) into the left hind knee, returned to their home cages, and allowed to recover. Similarly, wheel running rats were removed from their running cages and injected with 3.2 mg of i.a. MIA or saline (controls) into the left hind knee, and returned to their wheel cages. The concentration of MIA, and the sequence of weight bearing testing days, were based on earlier publications [15,21,23]. Rats were then tested in the Incapacitance tester on post-injection days 3, 7, 14 and 21. In MIA sedentary rats only, the opioid agonist morphine (3.2–10 mg/kg) was tested for its ability to reverse MIA-induced shifts in hind limb weight bearing. Morphine was delivered subcutaneously (s.c.), with a pretreatment time of 45 min, and testing was accomplished using between-subjects experimental design, such that each dose of morphine was tested in a separate group of subjects across all test days. Testing for all groups was conducted for ~1 h starting at 12 pm (time of day when little to no wheel running was occurring). Test trials for each rat were approx. 30– 60 s. Experimenters were blinded to subject group categories (e.g.: MIA vs. saline; runner vs. sedentary).
2.4. Microcomputed tomography (μCT)
Following wheel running and weight bearing analyses, rats were euthanized and knee joints were dissected and fixed in 10% neutral buffered formalin for 3 days. They were then transferred to 70% EtOH. To characterize exercise-induced changes in joint micro-architecture, the proximal tibias were analyzed with a SCANCO vivaCT 40 scanner (Scanco Medical AG, Bassersdorf, Switzerland). Joints were loaded into 12.3 mm-diameter scanning tubes. Scans were integrated into three-dimensional voxel images (2048 × 2048 pixel matrices for trabecular and 1024 × 1024 pixel matrices for all other individual planar stacks). Rat tibias were scanned at low resolution, energy level of 55 kVp, and intensity of 145 μA. Trabecular bone volume fraction and microarchitecture of the proximal metaphyseal region were evaluated below the growth plate. Approximately 375 consecutive slices were made at 10.5 μm interval at the distal end of the growth plate and extending in a proximal direction, and 250 contiguous slices were selected for analysis. Subchondral trabecular bones were scanned at low resolution, energy level of 55 kVp, and intensity of 145 μA at 10.5 μm. Subchondral trabecular bone for the medial and lateral tibial plateau were analyzed over 50 cross sections. The volume of interest included the subchondral trabecular bone starting below the subchondral plate, extending distally toward the growth plate. The images were segmented using a threshold of 260. The following three-dimensional morphometric parameters were calculated for the medial, the lateral and the total of subchondral trabecular bone. A total of 6 joints per group was analyzed for the subchondral bones. For the metaphysis, some samples were not analyzed due to insufficient area of analysis. Group sizes were saline sedentary/n = 5, MIA-sedentary/n = 5, saline-runner/n = 7, and MIA-runner/n = 7. Bone microarchitecture variables for all groups included lateral and medial compartment for bone volume/total volume fraction (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular separation (TbSp), and bone connective density (Conn Dens).
2.5. Data analysis
The dependent variable for running wheel experiments was total distance traveled in the running wheels (meters) during the dark cycle. The dependent variable of the weight-bearing test was % weight on ipsilateral hind limb. Several different dependent variables were measured in the μCT experiments including BV/TV fraction, trabecular number, thickness, and separation, and connective density. Both medial and lateral subchondral compartments were characterized in all cases. Statistical analysis for wheel running, weight asymmetry, and μCT data were accomplished with two-factor ANOVA. Significant two-way ANOVAs were followed by Duncan post hoc tests. Significance was set a priori at p ≤ 0.05. For μCT compartmental data, Cohen’s d and associated Confidence Intervals (CI) were calculated to determine the magnitude of effect and the precision of that effect size estimate [24,25]. The rationale for using ANOVA in running wheel experiments followed by Cohen’s d in μCT experiments is that the ANOVA tests provided a choice point to make a decision about which group to assess in μCT (21 day or 7 day runners). This use of p values is standard and the subsequent effect size estimate allowed high resolution characterization of effect sizes in the samples of interest [25,45].
3. Results
3.1. Seven-day acquisition of wheel running produces MIA-depressed wheel running
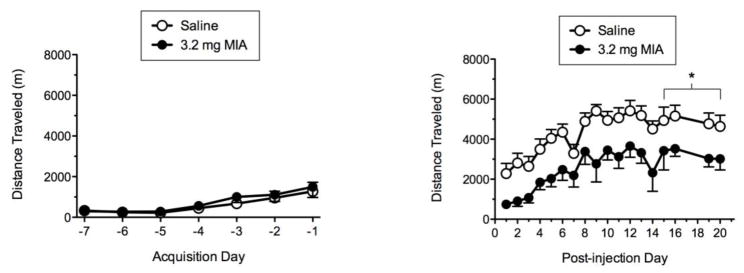
Fig. 1 shows total distance traveled during acquisition and post-monosodium iodoacetate (MIA) phases for the 7-21 wheel running protocol. The left panel indicates that all rats showed an increase in wheel running across the 7-day acquisition phase. The right panel shows that relative to saline, administration of 3.2 mg MIA produced significant decreases in total distance traveled relative to saline controls on PDs 16–17, and PDs 20–21 (p ≤ 0.05).
Fig. 1.
Acquisition (left panel) and post-MIA (right panel)wheel running for the 7-21 protocol. * indicates distance traveled for MIA runner significantly less than saline runner (p ≤ 0.05).
3.2. Twenty-one day acquisition of wheel running does not produce MIA-depressed wheel running
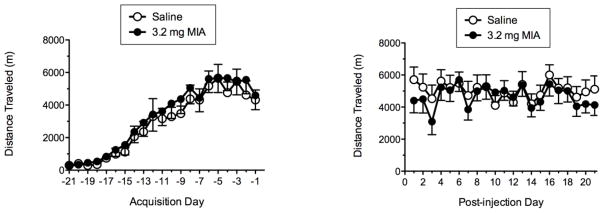
Fig. 2 shows total distance traveled during acquisition and post-MIA observation phases for the 21-21wheel running protocol. The left panel indicates that all rats showed a steady increase in wheel running across the 21-day acquisition phase with running distance stabilizing across the final week of the acquisition period. The right panel shows that there were no differences between saline and MIA groups in total distance traveled, up to 21 days post-injection.
Fig. 2.
Acquisition (left panel) and post-MIA (right panel) wheel running for the 21-21 protocol. No significant differences were noted between groups.
3.3. MIA-induced weight asymmetry
During all baseline weight bearing recordings, rats distributed ~50% of their weight on each hind limb, yielding a balanced posture. For sedentary rats, intra-articular administration of MIA into the left hind knee produced a weight shift onto the uninjured right hind limb within 3 days. On post-day 3, MIA alone produced a 29% shift in weight off the injured hind limb and onto the uninjured hind limb. This weight asymmetry was maintained during post-days 7, 14 and 21 with a 20% shift in weight being present on the final post-day 21. Fig. 3 show % shift from baseline (i.e. weight asymmetry) following MIA administration for post-days 3, 7, 14 and 21.
Fig. 3.
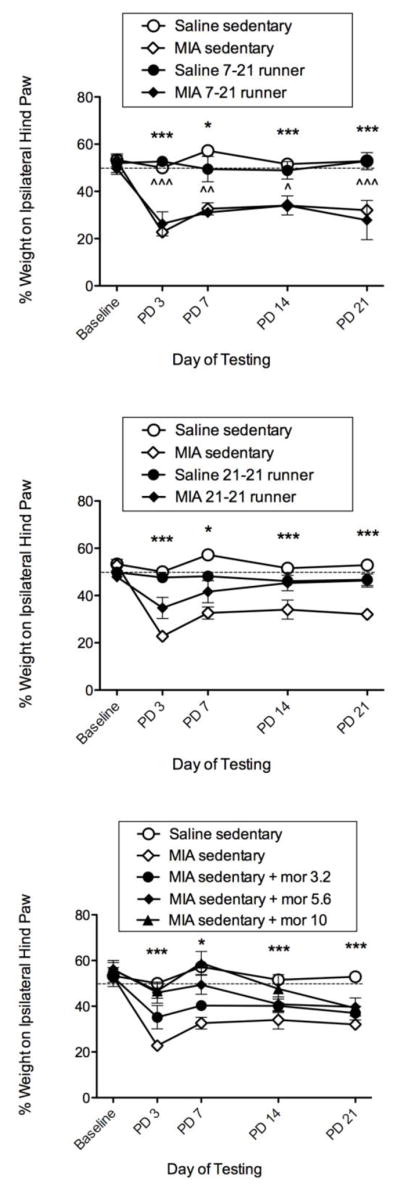
Effects of the 7-21 running protocol, the 21-21 running protocol, and acute morphine (3.2–10 mg/kg, s.c.) on MIA-induced weight asymmetry. The top panel shows sedentary (hollow symbols) compared to the 7-21 running wheel (RW) protocol (filled symbols). For sedentary rats, MIA produced significant weight asymmetry compared to saline across all days measured. For 7-21 RW rats, MIA also produced significant weight asymmetry compared to saline across all days measured. The middle panel shows that the 21-21 RW protocol reversed MIA-induced weight asymmetry at all time points as there were no significant differences between MIA and saline runners (filled symbols). Sedentary data same as in top panel. The bottom panel shows that morphine produced a dose-dependent reversal of MIA-induced weight asymmetry at PDs 3, 7, and 14, but was ineffective at PD 21. *, *** indicate saline sedentary significantly different from MIA sedentary (p ≤ 0.05, 0.001, respectively). ^, ^^, ^^^ indicate 7-21 saline runner significantly different from 7-21 MIA runner (p ≤ 0.05, 0.01, 0.001, respectively).
3.4. Seven days prior wheel running has no effect on MIA-induced weight asymmetry
Fig. 3 (top panel) shows the effects of 7-day acquisition duration in saline-treated and MIA-treated runners (closed symbols) relative to saline-treated and MIA-treated sedentary controls (open symbols). For sedentary controls, there were significant differences between saline sedentary (open circles) and MIA sedentary (open diamonds) across all post-days tested: PDs 3, 7, 14, and 21 (F = 12.97, df = 7, p ≤ 0.0001). The 7-21 runner protocol did not block MIA-induced weight asymmetry, and runners on the 7-21 wheel running protocol also showed significant differences between saline runners (closed circles) and MIA runners (closed diamonds) across all post-days tested: PDs 3, 7, 14 and 21 (F = 9.51, df= 7, p = 0.002).
3.5. Twenty-one days prior wheel running blocks MIA-induced weight asymmetry
Fig. 3 (middle panel) shows the effects of 21-day acquisition duration in saline-treated and MIA-treated runners (closed symbols) relative to saline-treated and MIA-treated sedentary controls (open symbols, and same controls used above). In contrast to rats on the 7-21 wheel running protocol, rats on the 21-21 wheel running protocol showed significant reversal of MIA-induced weight asymmetry at all time points manifested as no significant difference between saline runners (filled circles) and MIA runners (filled diamonds) on any post-day tested: PD 3, PD 7, PD 14 and PD 21 (F = 6.04, df= 7, p = 0.162), indicating blockade of MIA-induced weight asymmetry. In contrast, there were significant differences across all post-days tested between sedentary saline and sedentary MIA controls (same controls used to compare against 7-21 runners above: F=12.97, df=7, p ≤ 0.0001), indicating MIA-induced weight asymmetry.
3.6. Morphine dose-dependently blocks MIA-induced weight asymmetry
Fig. 3 (bottom panel) shows the effects of MIA + morphine (3.2– 10 mg/kg) on hind limb weight bearing in sedentary rats. Acute administration of s.c. morphine produced a significant dose-dependent reversal of MIA-induced weight asymmetry on post-day 3 (F = 4.038, df = 7, p = 0.021) PD 7 (F = 6.280, df = 7, p = 0.003) and PD 14 (F=3.708, df=7, p=0.028). However, morphine was not effective in reversing MIA-induced shifts on post-day 21. Post-hoc analyses indicate that the 10 mg/kg dose was significantly lower than vehicle at all time-points tested (*p < 0.05; ***p < 0.001 vs. control (MIA 3.2)).
3.7. Comparison of running protocols and morphine on post-day 21
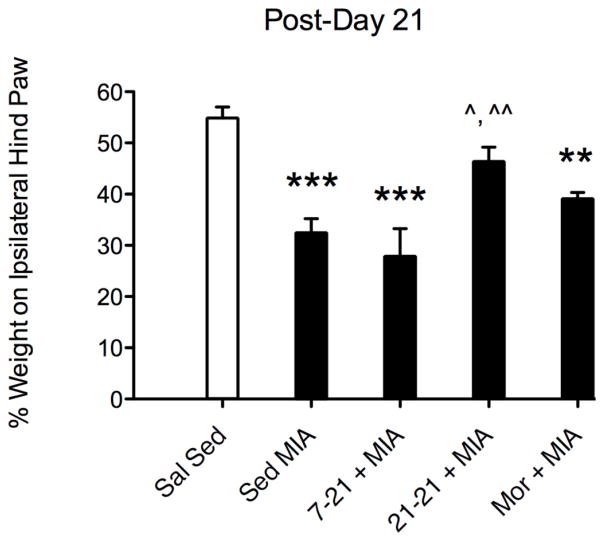
Fig. 4 shows comparisons among saline sedentary controls (white left-most bar) to sedentary MIA, 7-21 runners + MIA, 21-21 runners + MIA, and sedentary morphine (10 mg/kg, s.c.) + MIA in the weight bearing apparatus on post-day 21 only. Sedentary MIA, 7-21 MIA runners, and morphine groups showed weight asymmetry, defined as significantly less weight on ipsilateral hind limb relative to sedentary saline controls that had no arthritis (p ≤ 0.001; p ≤ 0.01, respectively). In addition, sedentary MIA and 7-21 MIA runners had significant weight asymmetry relative to 21-21 MIA runners (p ≤ 0.01; p ≤ 0.05, respectively). There were no significant differences between the saline sedentary controls and the 21-21 MIA runners on post-day 21.
Fig. 4.
Comparison of sedentary saline controls to sedentary MIA, 7-21 RW + MIA, 21-21 RW + MIA, and sedentary morphine (10 mg/kg, s.c.)+ MIA in weight bearing apparatus on post-day 21 only. **, *** indicate significantly different from saline sedentary controls (p ≤ 0.01, 0.001, respectively). ^, ^^ indicate 21-21 RW + MIA significantly greater (i.e., less weight asymmetry) than sedentary MIA and 7-21 RW + MIA (p ≤ 0.05, 0.01, respectively). There were no significant differences between saline sedentary controls and MIA 21-21 runners.
3.8. μCT images show MIA-induced changes to cartilage and subchondral bone
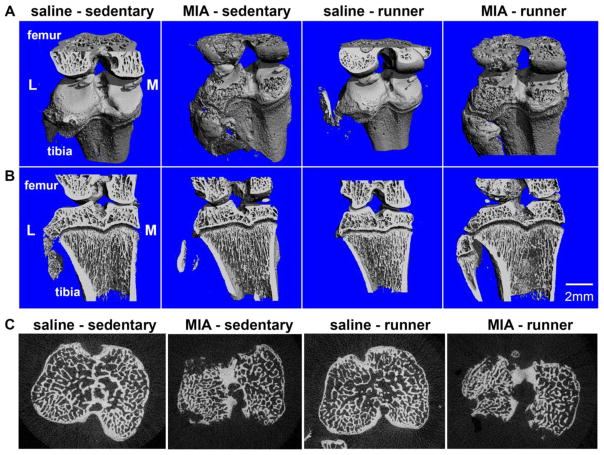
Fig. 5 shows three-dimensional full scans (top row A) and half scans (middle row B) of distal femur and proximal tibia, and cross sections (bottom row C) for saline- and MIA-treated sedentary, and saline- and MIA-treated 21-21 runners. Lateral sections are shown on left-most portion of scan and medial sections are shown on right-most portion of scans. Saline-sedentary and saline-runners show healthy cartilage and bone, whereas MIA-sedentary and MIA-runners show cartilage erosion, exposure of subchondral bone, and pitting on articular surfaces. MIA-sedentary and MIA-runners show distinct changes to medial vs. lateral compartments.
Fig. 5.
Full (panel A), half (panel B), and cross-section (panel C) μCT scans showing lateral and medial compartments for saline-sedentary, MIA-sedentary, saline-runner, and MIA-runner.
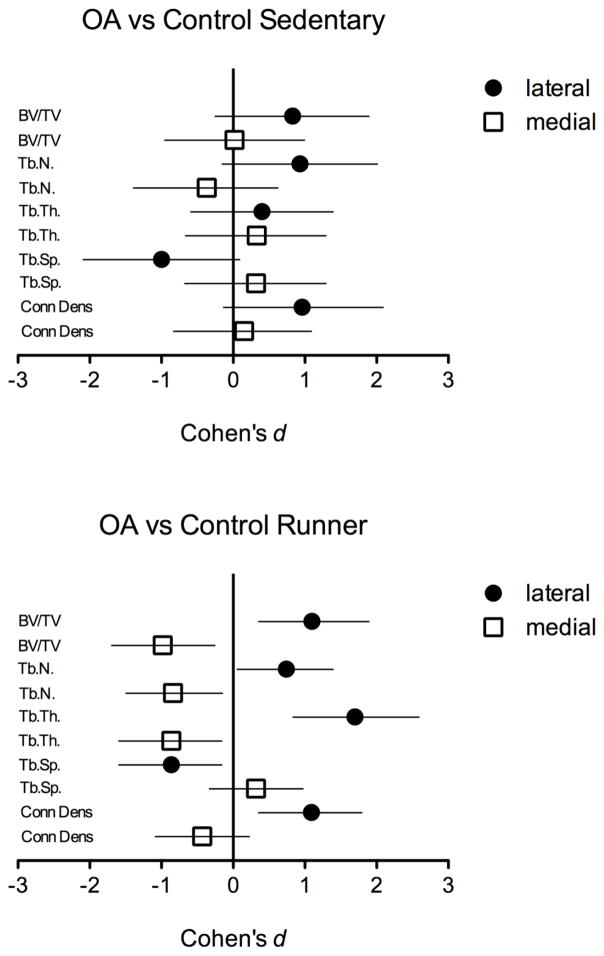
3.9. μCT quantitative analysis of subchondral bone in OA vs. controls varies as a function of running
Table 1 shows Effect Sizes (Cohen’s d statistic) for OA vs. control condition in sedentary (left-most columns) and exercise (right-most columns) conditions. Trabecular bone measures are listed in the left column and include bone volume fraction (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular spacing (TbSp), and connective density (Conn Dens).
Table 1.
Effect sizes (Cohen’s d) with confidence intervals for bone microarchitecture endpoints for sedentary (control vs. OA) and runners (controls vs. OA). Bold = d ≥ 0.8, and CI do not overlap with zero.
| Sal sed (±SD) | OA sed (±SD) | % Change | ES (CI) | Sal run (±SD) | OA run (±SD) | %Change | ES (CI) | |
|---|---|---|---|---|---|---|---|---|
| BV/TV | ||||||||
| Lateral | 0.38 ± 0.059 | 0.43 ± 0.074 | 15 | 0.83 (−0.26 to 1.9) | 0.38 ± 0.059 | 0.46 ± 0.077 | 20 | 1.1 (0.35 to 1.9) |
| Medial | 0.41 ± 0.068 | 0.41 ± 0.054 | 0.32 | 0.02 (−0.96 to 1) | 0.42 ± 0.047 | 0.38 ± 0.015 | −8.2 | −0.98 (−1.7 to −0.25) |
| TbN | ||||||||
| Lateral | 5.3 ± 0.66 | 6.1 ± 1.1 | 16 | 0.93 (−0.16 to 2.02) | 5.4 ± 1.0 | 6.3 ± 1.4 | 16 | 0.74 (0.05 to 1.4) |
| Medial | 5.2 ± 1.0 | 4.9 ± 0.56 | −5.9 | −0.37 (−1.4 to 0.63) | 5.2 ± 0.56 | 4.8 ± 3.9 | −7.7 | −0.84 (−1.5 to −0.14) |
| TbTh | ||||||||
| Lateral | 0.096 ± 0.006 | 0.098 ± 0.006 | 2.5 | 0.40 (−0.6 to 1.4) | 0.097 ± 0.003 | 0.10 ± 0.005 | 7.6 | 1.7 (0.83 to 2.6) |
| Medial | 0.11 ± 0.009 | 0.11 ± 0.012 | 3.1 | 0.33 (−0.67 to 1.3) | 0.11 ± 0.007 | 0.11 ± 0.004 | −4.2 | −0.86 (−1.6 to −0.15) |
| TbSp | ||||||||
| Lateral | 0.20 ± 0.021 | 0.17 ± 0.034 | −15 | −1.0 (−2.1 to 0.1) | 0.19 ± 0.034 | 0.16 ± 0.036 | −15 | −0.86 (−1.6 to −0.15) |
| Medial | 0.20 ± 0.038 | 0.21 ± 0.012 | 4.6 | 0.32 (−0.68 to 1.3) | 0.20 ± 0.021 | 0.21 ± 0.019 | 3.2 | 0.32 (−0.34 to 0.98) |
| Conn Dens | ||||||||
| Lateral | 95 ± 19 | 119 ± 27 | 24 | 0.96 (−0.14 to 2.1) | 105 ± 29 | 139 ± 34 | 33 | 1.09 (0.35 to 1.8) |
| Medial | 76 ± 16 | 78 ± 12 | 2.8 | 0.15 (−0.84 to 1.1) | 90 ± 16 | 84 ± 12 | −6.3 | −0.43 (−1.09 to 0.23) |
Data indicate large effect sizes (defined as d ≥ 0.8 and CI that do not overlap with zero) for exercise condition, manifested as OA-induced increases in BV/TV, TbN, TbTh, and Conn Dens in lateral compartment, and OA-induced decreases in the same parameters. In contrast, effect sizes for sedentary condition are small to large, and confidence intervals overlap substantially with zero.
Fig. 6 shows a graphic illustration of Table 1 data. The top panel shows effect sizes for OA vs. controls for the sedentary condition, and the bottom panel shows effect sizes for OA vs. controls for the exercise/ runner condition. The magnitudes of effect for lateral (increases) and medial (decreases) compartments of BV/TV, TbN, TbTh, TbSp, and Conn Dens are greater for runners (bottom panel) compared to sedentary rats (top panel), as are the precision of those estimates, indicated by greater magnitude of Cohen’s d value, and confidence intervals that do not overlap with zero, respectively.
Fig. 6.
Magnitudes of effect (effect sizes and confidence intervals) for MIA vs. controls in sedentary (top panel) and runners (bottom panel).
4. Discussion
This manuscript compared the effectiveness of two protocols of voluntary wheel running to reduce/prevent osteoarthritis (OA)-induced pain-related behavior. The two main findings were (1) that twenty one days (but not seven days) of prior wheel running completely prevented osteoarthritis-induced pain-related weight asymmetry at later time-points that were insensitive to pain alleviating effects of morphine, and (2) that twenty one days of prior running had distinct effects on bone microarchitecture in arthritis rats compared to sedentary rats, with larger OA-associated increases in bone volume, trabecular number, trabecular thickness, and connective density in lateral compartments and larger OA-associated decreases in the same parameters in medial compartments. These pain/weight asymmetry data and μCT data extend an earlier report that OA produced robust depression of wheel running in rats with seven days of acquisition [15].
One potential strategy toward attenuating OA pain may lie with utilization of exercise-based regimens either alone or in combination with other modes of therapy. We thus tested the ability of 2 different voluntary running wheel protocols [15] to reverse monosodium iodoacetate (MIA), OA-induced weight asymmetry. The protocol with 7 days of acquisition was ineffective in reversing weight asymmetry. In contrast, the protocol with 21 days of acquisition was fully effective in preventing pain-related behavior at all time points tested. The two running protocols not only had divergent effects on MIA-induced pain-related weight asymmetry, but also had divergent effects on total distance traveled relative to saline controls. For example, 7 days of prior running produced robust MIA-induced suppression of wheel running relative to controls, whereas 21 days of prior running did not significantly alter wheel running relative to saline controls. Several interpretations for the absence of pain-depressed wheel running with 21 days of acquisition include distinct modifications to trabecular lateral and medial compartments (see below; [26]), upregulated endogenous pain inhibitory pathways [27–29], and/or diverted attention from OA pain [30]. Finally, the dichotomous effects of the two protocols on post-injury wheel running replicates earlier work from our group [15] and further suggests that wheel running is an objective and relevant marker for quantifying pain-related behavior [13].
The finding that voluntary wheel running was protective against OA-induced pain is consistent with an emerging preclinical literature showing the beneficial effects of voluntary exercise against pain-like states in rodents. For example, 6 weeks of prior wheel running was shown to attenuate mechanical allodynia and normalize neuroimmune signaling in a CCI model of neuropathic pain [10], while 8 weeks of prior wheel running enhanced the protective immune status by elevation of CD206 macrophage production in a model of chronic muscular pain [11]. Non-voluntary, forced exercise routines have also been shown to be protective against pain-like states. For example, treadmill exercise was shown to be effective in blocking OA-induced changes to cartilage and subchondral bone in a DMM model of OA [31]. Recent clinical reports have also indicated that moderate and high intensity exercise has beneficial effects by reducing pain scores in men and women with moderate to severe knee OA [32–34]. For example, a 5-year clinical study on Intensive Diet and Exercise for Arthritis (IDEA) showed that an intensive exercise regimen that included aerobic walking, strength training, a secondary aerobic session reduced joint loading and decreased plasma levels of the inflammatory biomarker IL-6 [34].
The finding that acute administration of morphine was ineffective at blocking pain in sedentary rats at PD 21 is interesting in light of evidence that at this time point in MIA-induced OA, progression of cartilage and/or bone destruction is greater than at earlier time points [16,23]. One interpretation of the lack of morphine efficacy 21 days post-injury, is that the efficacy requirement at PD 21 may be too high for the dose range of morphine tested under the specific parametric conditions. However, it is unlikely that higher doses would have been effective given that the 5.6 mg/kg dose is an effective analgesic dose [35,36]. A second interpretation is the potential change in the chronic pain state at later time points. For example, anti-inflammatory drugs have been shown to be effective at early but not late time points, and drugs used to treat neuropathic pain such as SNRIs, and gabapentin are more effective at late time-points [21,37,38], suggesting a shift from inflammatory to neuropathic features [39,40]. A third interpretation is that wheel running-induced increases in endogenous opioid signaling resulted in cross-tolerance to the effects of morphine on PD 21 [41].
The present study also characterized the effects of OA on trabecular bone microarchitecture in rats with access to running wheels compared to sedentary condition. Qualitative analysis of μCT images indicated that MIA-treated sedentary controls and MIA-treated runners both showed cartilage and subchondral bone deterioration relative to saline-treated sedentary controls and saline runners, consistent with previous reports [42–44]. Quantitative effect size analysis indicated that running altered OA-induced bone remodeling, manifested as a greater increase in BV/TV, TbN, TbTh, and Conn Dens in lateral compartment, and a greater decrease of the same parameters in the medial compartment, compared to sedentary condition. Running + arthritis thus produced a greater magnitude of change than sedentary + arthritis. For these parameters, effect sizes for OA vs. control in the running condition were >0.86 (mean of exercise group at 81st percentile of sedentary group with ~51% non-overlap between groups) and were as high as 1.7 (mean of exercise group at 96th percentile of sedentary group with ~75% non-overlap between groups). In contrast, effect sizes for OA vs. control in the sedentary condition were associated with smaller magnitudes of effect ranging as low as 0.02 and including larger effect sizes of 1 that contained significant CI overlap with zero. This is consistent with standard interpretation of Cohen’s effect sizes of 0.2 small effect, 0.5 medium effect, ≥0.8 large effect, and ≥1.2 extra-large effect, and precision of that estimate manifested as no overlap with zero [25,45].
The clinical human OA literature is challenging to interpret as there are reports of discordance between pain and radiograph severity. For example, OA knee pain is typically confirmed according to magnitude of joint degeneration scored on a K/L (Kellgren/Lawrence) scale, but there is no agreement on the relationship between pain intensity and radiographic severity [46–49]. Second, knee pain has been reported to be a better predictor of decreased function and quality of life, than radiographic damage [50]. And, bone pain is associated with severity of bone marrow lesions [22], and with joint space narrowing and presence of osteophytes [51]. A recent MRI study [52] analyzed medial and lateral parameters of subchondral bone in women with OA. Interestingly, as OA severity increased (defined as decrease in cartilage), BV/TV and TbTh increased on the medial side perhaps due to increased loading on the medial space. In contrast, increased OA severity was correlated with decreases in BV/TV, TbTh, TbN on the lateral side perhaps due to decreased loading on the lateral space. Although cartilage integrity was not measured in our rodent study, our data show decreases in medial and increases in lateral parameters, that were exacerbated by exercise. Thus, although pain scores were attenuated, the compensatory subchondral changes were exaggerated by exercise, and it is unknown if wheel running enhanced or blocked OA development.
The preclinical literature contains several studies that have characterized the effects of OA on trabecular bone in qualitative and quantitative dimensions in rodents with low-dose 0.2 mg [44], 1 mg MIA [53], and intermediate dose 2 mg MIA [42,43], compared to our higher tested dose of 3.2 mg MIA. Another notable difference is that with the exception of the low dose group in the Mohan study, previous reports did not distinguish between lateral and medial compartments. Vermeirsch and colleagues reported slight correlations between bone parameters and weight bearing, whereby bone volume was slightly negatively correlated with weight asymmetry, consistent with our sedentary bone volume fraction findings in lateral compartment. Kalff and colleagues [43] showed MIA-induced decreases in bone volume fraction and trabecular number whereas our results with a higher dose (3.2 mg) showed slight decreases in medial compartment and slight increases in lateral compartment in sedentary rats.
The mechanism behind the finding that a longer 21-day acquisition (but not a shorter 7-day acquisition) was protective against OA pain is unknown. The preclinical literature contains reports on exercise-bone interactions. For example, Boudenot and colleagues [54] showed that a 10-week exercise regimen in rats with MIA-induced OA did not differentially affect trabecular parameters compared to non-exercised MIA controls, although exercise did increase osteocyte surface and occupancy in groups with or without OA. Iijima and colleagues [31] assessed the effects of treadmill exercise and discovered exercise-induced suppression of osteocyte cell death, and significant subchondral bone thickening was found in the exercise group, and Yu and colleagues [55] demonstrated that inhibition of bone lesions blocked OA pain measured by weight asymmetry, but joint swelling and synovial reaction were unaffected. Long duration running may be preventative against MIA-induced cartilage and/or osteocyte death. For example, voluntary exercise was shown to be protective against OVX-induced osteocyte death in rats, and enhanced muscle mass in exercised but not sedentary rats [7]. Finally, it may be possible that the longer prior running regimen simply resulted in rats that were more physically fit and thus more able to sustain the MIA chronic pain manipulation [56–58].
Although there are several recent and comprehensive reports that utilize a variety of different wheel running acquisition durations (in days) and different session frequencies (1 h, 12 h, 23 h) in rodents [10,11,13,59], it is likely too early to state the definitive relationship between these behavioral mechanisms and subsequent expression of pain. Of special interest was the finding that 21 days of prior wheel running was the only manipulation to reverse OA weight asymmetry on PD 21. Acute administration of the opioid agonist morphine, and the 7 days prior wheel running were ineffective at this stage of OA progression.
5. Conclusion
In conclusion, the present study indicates that voluntary exercise can attenuate behavioral pain scores, and that the magnitude of pain varies as a function of exercise acquisition duration. The exercise-induced blockade of pain was associated with increased bone volume fraction, trabecular number and thickness in lateral compartment with corresponding decreases in medial compartment. These results suggest that there is more work to be done to ascertain the interactions among behavioral, biological, and structural mechanisms that are driving the beneficial effects of exercise on pain expression.
Acknowledgments
This research was supported by a NASA undergraduate research grant to J.C., a NIH COBRE grant (P20GM103643) that supports an animal behavior core facility, and a National Institutes of Health (NIAMS) R15 AREA grant AR054975 to G.W.S. Bone microcomputed tomography was performed at Maine Medical Center Research Institute in a core facility supported by NIH grant 8P30GM103392 (R. Friesel, PI).
References
- 1.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis – an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 2.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Krashin D, Sullivan M, Ballantyne J. What are we treating with chronic opioid therapy? Curr Rheumatol Rep. 2013;15:311. doi: 10.1007/s11926-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 4.Hawker GA, Davis AM, French MR, Cibere J, Jordan JM, March L, Suarez-Almazor M, Katz JN, Dieppe P. Development and preliminary psychometric testing of a new OA pain measure – an OARSI/OMERACT intiative. Osteoarthr Cartil. 2008;16(4):409–414. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gee AO, Lee GC. Alternative bearings in total knee arthroplasty. Am J Orthop. 2012;41(6):280–283. [PubMed] [Google Scholar]
- 6.Berend ME, Berend KR, Lombardi AV. Advances in pain management: game changers in knee arthroplasty. Bone Joint J. 2014;96-B(11 Suppl A):7–9. doi: 10.1302/0301-620X.96B11.34514. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca H, Moreira-Goncalves D, Esteves JLS, Viriato N, Vaz M, Mota MP, Duarte JA. Voluntary exercise has long-term in vivo protective effects on osteocyte viability and bone strength following ovariectomy. Calcif Tissue Int. 2011;88:443–454. doi: 10.1007/s00223-011-9476-2. [DOI] [PubMed] [Google Scholar]
- 8.Manabe Y, Gollisch KSC, Holton L, Kim Y-B, Brandauer J, Fujii NL, Hirshman MF, Goodyear LJ. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013;280(3):916–926. doi: 10.1111/febs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvey SM, Russ DW, Skelding MB, Dugle JE, Edens NK. Molecular and metabolomics effects of voluntary running wheel activity on skeletal muscle in late middle-aged rats. Phys Rep. 2015;3(2):1–17. doi: 10.14814/phy2.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson NA, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung A, Gregory NS, Allen L-AH, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157:70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell MC. Modest amounts of voluntary exercise reduce pain- and stress-related outcomes in a rat model of persistent hind limb inflammation. J Pain. 2017 doi: 10.1016/j.jpain.2017.01.006. http://dx.doi.org/10.1016/j.jpain.2017.01.006 (Feb 6, in press) [DOI] [PMC free article] [PubMed]
- 13.Kandasamy R, Calsbeek JJ, Morgan MM. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods. 2016;263:115–122. doi: 10.1016/j.jneumeth.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157(2):387–398. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98(1):35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. Monoiodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–1679. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 17.Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370:236–240. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Likar R, Schafer M, Paulak F, Sittl R, Pipam W, Schalk H, Geissler D, Bernatzky B. Intraarticular morphine analgesia in chronic pain patients with osteoarthritis. Anesth Analg. 1997;84:1313–1317. doi: 10.1097/00000539-199706000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell JR, Rapoport RJ, Davis JC, Offenberg HL, Marker HW, Roth SH, Yuan W, Eliot L, Babul J, Lynch PM. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results froma randomized, placebo-controlled, double-blind trial and open-label extension trial. J Pain Symptom Manag. 2002;23(4):278–291. doi: 10.1016/s0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 21.Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, Walker K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114:339–346. doi: 10.1016/j.pain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Kidd BL, Langford RM, Wodehouse T. Current approaches in the treatment of arthritic pain. Arthritis Res Ther. 2007;9:1–7. doi: 10.1186/ar2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata, and effect size calculator. 2004 Retrieved, Feb 24, 2017, from http://mason.gmu.edu/~dwilsonb/ma.html.
- 25.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 26.Galois L, Etienne S, Grossin L, Watrin-Pinzano A, Coumil-Henrionnet C, Loeuille D, Netter P, Mainard D, Gillet P. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthr Cartil. 2004;12:779–786. doi: 10.1016/j.joca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobinski F, Ferreira TA, Cordova MM, Dombrowski PA, da Cunha C, Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treede RD. Gain control mechanisms in the nociceptive system. Pain. 2016;157(6):1199–1204. doi: 10.1097/j.pain.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 30.Elomaa MM, de Williams C, AC, Kalso EA. Attention management as a treatment for chronic pain. Eur J Pain. 2009 Nov;13(10):1062–1067. doi: 10.1016/j.ejpain.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Iijima H, Aoyama T, Ito A, Yamaguchi S, Nagai M, Tajino J, Zhang X. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthr Cartil. 2015;23:1563–1574. doi: 10.1016/j.joca.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Messier SP. Effects of exercise interventions in older adults with knee osteoarthritis. HSS J. 2012;8(1):49–50. doi: 10.1007/s11420-011-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messier SP, Mihalko LS, Beavers DP, Nicklas BJ, DeVita P, Carr JJ, Hunter DJ, Williamson JD, Bennell KL, Guermazi A, Lyles M, Loeser RF. Strength training for arthritis trial (START): design and rationale. BMC Musculoskelet Disord. 2013;14:208. doi: 10.1186/1471-2474-14-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF. Effects of Intensive Diet and Exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan D, Picker MJ. Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behav Pharmacol. 1996;6:261–275. [PubMed] [Google Scholar]
- 36.Morgan D, Cook CD, Picker MJ. Sensitivity to the discriminative stimulus and antinociceptive effects of u opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the u opioid receptor. J Pharmacol Exp Ther. 1999;289:965–975. [PubMed] [Google Scholar]
- 37.Ivanavicius SP, Ball AD, Heapy CG, Wetwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterization. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T. Central sensitization and neuropathic features of ongoing pain in a rat model of advanced osteoarthritis. J Pain. 2016;17(3):374–382. doi: 10.1016/j.jpain.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. PLoS One. 2012;7(3):e33730. doi: 10.1371/journal.pone.0033730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10(6):374–380. doi: 10.1038/nrrheum.2014.47. [DOI] [PubMed] [Google Scholar]
- 41.Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav. 2001;74:245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- 42.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349–359. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Kalff K-M, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010;641:108–113. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Mohan G, Perilli E, Kuliwaba JS, Humphries JM, Parkinson IH, Fazzalari NL. Application of in vivomicro-computed tomography in the temporal characterization of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 2011;13:R210. doi: 10.1186/ar3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan GM, Feinn R. Using effect size – or why the p value is not enough. J Grad Med Educ. 2012;9:279–281. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 47.Duncan R, Peat G, Thomas E, Hay E, McCall I, Croft P. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. How do pain and function vary with compartmental distribution and severity of radiographic knee osteoarthritis? Rheumatology. 2008;47:1704–1707. doi: 10.1093/rheumatology/ken339. [DOI] [PubMed] [Google Scholar]
- 49.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:166. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheum. 2000;39:490–496. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 51.Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, Coleman RE. Association of bone scintigraphic abnormalities with knee alignment and pain. Ann Rheum Dis. 2009;68(11):1673–1679. doi: 10.1136/ard.2008.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiba K, Uetani M, Kido Y, Ito M, Okazaki N, Taguchi K, Shindo H. Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: a 3-T MRI study. Osteoporos Int. 2012;23:589–597. doi: 10.1007/s00198-011-1585-2. [DOI] [PubMed] [Google Scholar]
- 53.Lee JH, Chun KJ, Kim HS, Kim SH, Han P, Jun Y, Lim D. Alteration patterns of trabecular bone microarchitectural characteristics induced by osteoarthritis over time. Clin Interv Aging. 2012;7:303–312. doi: 10.2147/CIA.S32513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boudenot A, Presle N, Uzbekov R, Toumi H, Pallu S, Lespessailles E. Effect of interval-training exercise on subchondral bone in a chemically-induced osteoarthritis model. Osteoarthr Cartil. 2014;22:1176–1185. doi: 10.1016/j.joca.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Yu D, Liu F, Liu M, Zhao X, Wang X, Li Y, Mao Y, Zhu Z. The inhibition of subchondral bone lesions significantly reversed the weight-bearing deficit and the overexpression of CGRP in DRG neurons, GFAP and Iba-1 in the spinal dorsal horn in the monosodium iodoacetate induced model of osteoarthritis pain. PLoS One. 2014;8(10):e77824. doi: 10.1371/journal.pone.0077824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng B, Karamizrak O, Noakes TD, Dennis SC, Lambert EV. Time course of the effects of a high-fat diet and voluntary exercise on muscle enzyme activity in Long-Evans rats. Physiol Behav. 1997;61(5):701–705. doi: 10.1016/s0031-9384(96)00522-7. [DOI] [PubMed] [Google Scholar]
- 57.Lemieux AM, Diehl CJ, Sloniger JA, Henriksen EJ. Voluntary exercise training enhances glucose transport but not insulin signaling capacity in muscle of hypertensive TG(mREN2)27 rats. J Appl Physiol. 2005;99(1):357–362. doi: 10.1152/japplphysiol.00100.2005. [DOI] [PubMed] [Google Scholar]
- 58.Smith HK, Merry TL. Voluntary resistance wheel exercise during post-natal growth in rats enhances skeletal muscle satellite cell and myonuclear content in adulthood. Acta Physiol. 2012;204(3):393–402. doi: 10.1111/j.1748-1716.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 59.Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2015;271:279–290. doi: 10.1016/j.expneurol.2015.05.017. [DOI] [PubMed] [Google Scholar]