Abstract
Background
Nonbehavioral methods for identifying cisplatin ototoxicity are important for testing patients with cancer who become too tired or sick to provide a reliable response. The auditory brainstem response (ABR) is a nonbehavioral test that is sensitive to ototoxicity but can be time consuming to implement over a range of frequencies and/or levels. To address this issue, trains of stimuli were developed that offer reliable ABR testing over a range of tone-burst frequencies and levels at a time savings of 77% relative to tone-burst stimuli presented individually. The clinical accuracy of this new method has yet to be determined on a clinical population.
Purpose
This project was designed to determine the test performance of a time-effective ABR methodology aimed at identifying hearing shifts from cisplatin among veterans. A secondary goal was to determine whether improved test performance could be achieved by including our previously developed ototoxicity risk assessment model in the ABR prediction algorithm.
Research Design
A set of discriminant functions were derived using logistic regression to model the risk for cisplatin-induced hearing change. Independent variables were one of several ABR metrics alone and combined with an ototoxicity risk assessment model that includes pre-exposure hearing and cisplatin dose. Receiver operating characteristic curve analysis was used to evaluate the test performance of these discriminant functions.
Study Sample
Twenty-two male veterans treated with cisplatin for various cancers provided data from a total of 71 monitoring appointments.
Data Collection and Analysis
Data were collected prospectively from one ear of each participant as designated below. Hearing shift was determined for frequencies within an octave of each patient’s high-frequency hearing limit, tested in 1/6th-octave steps. ABRs were monitored using a set of two intensity trains from the highest two multiple frequency tone-burst center frequencies (up to 11.3 kHz) that yielded a robust response at baseline. Each intensity train was presented at 65–105 dB peSPL in 10 dB steps. Scorable ABRs were generally limited to the highest two intensities; therefore, analyses concern those levels.
Results
The ABR measurement failure was high, up to 52% for some frequencies and levels. Furthermore, the ABR was not frequently obtained at levels below 85 dB peSPL, consistent with previous studies that suggest a stimulus level of greater than 80 dB peSPL is required to obtain a reliable response to trained stimuli. Using multivariate metrics that included the dose-ototoxicity model, the most accurate scoring function was change in amplitude at lowest half-octave frequency obtained at 105 dB (change in wave V amplitude at frequency 2/105). However, absence of wave V at a monitor patient visit of the ABR response at levels 105 or 95 dB peSPL was deemed the preferred scoring function, because it had lower measurement failure and was within one standard error of the most accurate function.
Conclusions
Because of the large number of responses that could not be measured at baseline, this technique as implemented holds limited value as an ototoxicity-monitoring method.
Keywords: ototoxicity, veterans, cisplatin, auditory brainstem response
INTRODUCTION
Cisplatin, a potent chemotherapeutic agent, results in hearing shift in >50% of patients and new tinnitus in nearly 40% (Dille et al, 2010a, 2012; Reavis et al, 2008). Animal models using rodent species have shown that the primary result of cisplatin ototoxicity is damage to the stria vascularis and destruction of outer hair cells within the cochlea in a base (high-frequency coded) to apex (low-frequency coded) progression with each treatment (Schweitzer, 1993; Laurell et al, 2000; Riedemann et al, 2008; Arora et al, 2009; Hellberg et al, 2009). The action of cisplatin, although largely unknown, is presumed to be an induction of apoptosis likely associated with oxidative stress (Schweitzer, 1993; Alam et al, 2000; Rybak, 2007; Hellberg et al, 2009).
Ototoxic hearing shifts that occur in the speech frequency range can have significant effects on communication and, in the case of children, the development of speech and language. Fausti and colleagues (1999) have shown that monitoring at the high-frequency limit of hearing provides the earliest detection of ototoxicity, consistent with the systematic progression of cochlear damage from base to apex found in animal models. This work led to the development of a screening method called the sensitive range for ototoxicity (SRO), that targets a one-octave, individualized range of frequencies, which when monitored reveals 94% of initial ototoxic shifts among cisplatin-treated patients (Fausti et al, 1999). An advantage of this screening method is that monitoring hearing in the SRO reduces testing time by two thirds in comparison to full-frequency threshold testing. However, ~30% of patients become too ill to provide reliable hearing thresholds because of chemo-therapeutic adverse effects during treatment (Fausti et al, 1991a). Because accurate, early detection of hearing shifts and subsequent changes in the treatment regimen represent the only current means of limiting ototoxic damage, these patients may be at greater risk for disabling hearing loss from cisplatin. Objective techniques such as the auditory brainstem response (ABR) test seem ideally suited for ototoxicity monitoring. However, the challenges in using the ABR for monitoring are threefold. The response must be robust and sensitive to hearing shift, and the procedure must be time efficient.
Standard-click ABRs are robust but best approximate hearing thresholds in the region of 2–4 kHz. Using a 100 dB peSPL click on nine participants with normal hearing in the conventional range of hearing (1.25–8 kHz), De Lauretis et al (1999) compared wave V latency change to behavioral threshold shift at intervals of every two cycles of cisplatin chemotherapy. To relate ABR findings with behavioral threshold shifts > 10 dB, an ABR latency change of ≥ 0.4 msec plus 2 standard deviations from the mean individual response was considered significant. Two participants had significant wave V shifts and prolonged I–V intervals after two cycles of cisplatin (220–240 mg), although hearing in the conventional range did not shift significantly until five to six cisplatin cycles had been administered. The other seven participants had unchanged hearing and no ABR changes. Similarly, Maiese et al (1992) monitored six participants receiving cisplatin for single-sided brain tumors using click ABR adjusted to moderate levels (65 dB SL re: wave V threshold), in the absence of hearing measures. Instead, participants were asked to report a subjective hearing change. One participant with significant wave V prolongation (≥ 0.3 msec) after seven cycles of cisplatin did not report any hearing changes. A second participant who reported hearing change in both ears during treatment had an increase in response threshold as well as wave V prolongation. There were no ABR changes and hearing changes in the other four participants. Both sets of authors concluded that ABR held promise as a monitoring protocol. However, the lack of pattern between ABR changes and hearing changes casts doubt on this conclusion.
The modest success of these findings has fueled other investigations using more sensitive stimuli. Although tone-burst stimuli or a derived-band ABR technique (Coupland et al, 1991) provide better frequency specificity, it is generally done at a cost of less robust responses. Importantly, less robust responses may have negative consequences for those patients entering treatment with significant hearing loss, such as veterans. Fausti and colleagues (Fausti et al, 1991a, 1991b, 1992, 1993; Henry et al, 2000; Mitchell et al, 2004) conducted a series of preliminary studies to establish that frequency-specific stimuli in the extended frequency range (8–14 kHz) could provide robust and reliable ABR responses. Using healthy normal-hearing listeners, they established that tone-burst-and-click ABRs provided similar intrasubject reliability (Fausti et al, 1991a,b). However, they noted that far fewer responses were obtained above 10 kHz. Using 34 participants (61 ears) who were veterans undergoing treatment with ototoxic antibiotics or chemotherapeutic medications, Fausti et al (1992) examined the relationship among behavioral hearing results at each treatment interval, and tone-burst (8, 10, 12, or 14 kHz) and click ABR. An ototoxic-related hearing shift was defined as 1) ≥ 20 dB change at one frequency; 2) ≥ 10 dB change at two or more consecutively-tested frequencies; or 3) loss of response at three consecutively-tested frequencies (ASHA, 1994). A significant ABR shift was established from previous normative research (Fausti et al, 1991a) as 1) wave V latency ≥ 0.3 msec or 2) a morphological change that resulted in an unscorable response. In half of ears (31 ears; 50.8%), a significant shift in hearing occurred during treatment. Of that group, 28 ears had the initial hearing shift at ≥ 8 kHz, whereas only 3 ears had a shift ≤6 kHz. More than 96% (27/28 ears) of these hearing shifts were also identified using tone-burst ABR, whereas only 4% of ears were identified using click ABR. The most frequent change in the tone-burst ABR was a loss of response rather than a criterion shift in latency. The authors concluded that testing at the high-frequency limit of the ABR held considerable promise.
However, tone-burst ABR requires testing at multiple frequencies and levels, which is too time consuming for severely ill veterans undergoing cancer treatment. Therefore, Henry et al (2000) compared tone-burst ABR using the traditional latency-intensity series to newly developed trains of 20 tone-burst stimuli composed of multiple frequencies and levels using young, normal-hearing adult participants. The trains contained four conventional test frequencies (1, 2, 4, and 8 kHz) at five levels (96, 86, 76, 66, and 56 dB peSPL). The single tone-burst repetition rate was slow (17.2/sec). The repetition rate of each train was 3.7/sec, although the stimulus presentation rate within each train was rapid (74/sec). To minimize the potential effects of adaptation, the train design featured tone-bursts that were 1) progressively higher in frequency and higher in presentation level, and 2) separated by one octave from the next burst frequency. Further, each train (of 20 stimuli) was followed by 150 msec of silence. Using this innovative testing procedure, they found only two instances of amplitude or latency differences between the two methods. Importantly, they realized a 77% reduction in testing time by using trains over singly presented tone bursts.
To determine if this methodology could be used for high-frequency testing with the same success as testing in the conventional range, Mitchell et al (2004) compared ABR responses obtained from 1) high-frequency clicks (8–14 kHz), 2) conventional clicks, 3) single tone bursts (8, 10, 12, and 14 kHz), and 4) stimulus trains of multiple frequency and level tone bursts (8, 10, 12, and 14 kHz) using young adult participants with normal hearing. The main purpose of this comparison was to find those stimuli that were likely to result in a response. As long as the level of the stimulus exceeded 80 dB peSPL and the frequency was 12 kHz or lower, the manner of stimulus presentation (singly or in trains) did not significantly affect the percentage of scorable ABR responses. Further, they found no false-positive results (i.e., spurious response changes). However, trains of stimuli resulted in systematically longer latency and lower-amplitude ABRs, although test-time efficiency was improved by 40% using a five-stimuli train (five intensities; one frequency) and by 65% using the 15 stimulus train (three intensities; five frequencies) when compared to single tone-burst ABR testing.
The knowledge gained from these developmental studies provides the basis for this report. Building on these projects, test performance of two types of rapidly presented stimulus trains: a frequency train (holding level relatively constant while varying frequency) and an intensity train (holding frequency constant while varying level) was examined. Additionally, we recently developed and validated a cisplatin dose risk assessment model based on two pieces of information obtained at the pretreatment assessment: hearing threshold severity and planned cisplatin dose (Reavis et al, 2008; Dille et al, 2012). When used alone or in combination with information about test-retest changes in distortion-product otoacoustic emissions (DPOAE), these two factors can be used to predict, with relative precision, which patients will experience ototoxic hearing shift and when that shift will likely occur. The purpose of the present study is to report on the accuracy of these ABR-trained stimuli when testing veterans treated with cisplatin for various cancers, and to evaluate improvements in test performance achieved by including our previously developed dose-risk assessment model in the ABR prediction algorithm. The data used for this report were collected as a part of a larger project examining objective measures (ABR and DPOAE) for detection of ototoxicity. The findings from the DPOAE testing were reported previously (Dille et al, 2010b).
METHODS
Multiple-Frequency Tone-Burst Stimulus
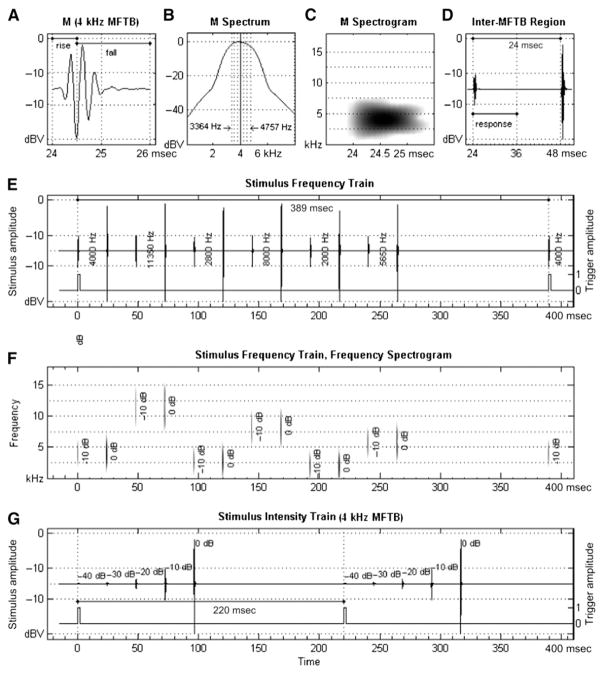
Six individual multiple-frequency tone-burst (MFTB) stimuli varying in frequency and level were created for use in the stimulus trains. Each of the six MFTB stimuli had an overall bandwidth of 1/2 octave and was composed of seven pure-tone frequencies spaced in 1/12 octave steps around the center frequencies of 2, 2.8, 4, 5.6, 8, and 11.3 kHz. The digitally created tones were rapidly gated and phase shifted to precisely control the peak amplitude of the complex. Specific characteristics of an individual MFTB are shown in Figure 1A–D. A time-domain view of the 4 kHz MFTB stimulus, shown in Figure 1A, has a 2 msec period with 0.5 msec rise and 1.5 msec fall times. This asymmetrical shape with a rapid stimulus rise was designed to improve the amplitude of the ABR response while maintaining frequency specificity. Each component of an MFTB was digitally shifted in latency relative to the center frequency so that the peak amplitude of the complex could be predicted and then summed to create the composite waveform. Each composite MFTB was then shifted to the presentation starting point relative to other MFTBs and smoothly gated using a Nuttall-type windowing function (Fig. 1B). (See Ellingson et al, 2008 for additional technical details of MFTB creation algorithm.) The frequency spectrogram of a MFTB as well as the interstimuli interval (24 msec, including response processing time) between individual MFTB when contained in trains of stimuli is shown in Figure 1C–D, respectively. The polarity of the MFTB is specifiable as rarefaction or condensation.
Figure 1.
Schematic view of stimuli and train waveforms. Stimuli are MFTBs each composed of seven pure tones around center frequencies varying from 2–11.3 kHz in 1/2 octave steps. Time-domain waveform (A), and spectrogram (B,C) are displayed for a 4 kHz MFTB played at two levels (95 and 105 dB peSPL).). Interstimulus interval separation of 24 msec including ABR analysis time (12 msec) is also shown (D). The time-domain frequency train (E) and spectrogram (F) and time-domain intensity train (G) are displayed in relative stimulus level and include the intertrain interval for the frequency (389 msec) and intensity (220 msec) trains.
The real-time waveform of a frequency train, shown in Figure 1E, was configured with six MFTB stimuli at center frequencies ranging from 2–11.3 kHz in 1/2 octave steps each presented at two levels (95 and 105 dB peSPL). The frequency train played for 288 msec and was repeated every 389 msec for a trigger pulse rate of 2.57/sec. A spectrogram for the frequency train is shown in Figure 1F, which displays the real-time frequency and level distribution of the trained stimuli. The configuration of the frequencies in the frequency train was developed to minimize auditory adaptation.
The real-time waveform of a 4 kHz intensity train is shown in Figure 1G. This train consists of five 4 kHz MFTB stimuli stepped in level from 65–105 dB peSPL in 10 dB increments. Separate intensity trains were created for each of the six center frequencies comprising the frequency train. An intensity train took 120 msec to complete and was repeated every 220 msec, yielding a trigger rate of 4.54/sec.
Instrumentation
All stimuli and stimulus trains were digitally synthesized and controlled using a custom stimulus generation and response acquisition system. The components of the personal-computer–based system consisted of (a) a dynamic signal analyzer (NI4551; National Instruments, Austin, TX); (b) programmable attenuator (PA4; Tucker Davis Technologies [TDT], Alachua, FL); headphone-amplifier buffer (HB4; TDT); biologic amplifier (DB4; TDT); and (c) custom-designed stimulus generation, electroencephalographic acquisition, and ABR scoring software. The dynamic signal analyzer was an internal personal computer card with dual-channel, precision digital-to-analog, and analog-to-digital converters with 16 bit resolution and 48 kHz simultaneous sampling. The signal analyzer operated as a signal-averaging instrument with one output channel to generate the stimulus, and a second channel to provide a synchronization trigger pulse with 1 msec delay relative to the stimulus onset of the biologic amplifier. The programmable attenuator adjusted the overall stimulus level. Stimuli were presented through an earphone (ER4B, Etymotic Research, Elk Grove Village, IL) driven by the headphone buffer. The biologic amplifier had an internal 1 msec processing delay, and the signal analyzer had an internal 33 msec sample delay; both were accounted for in the custom acquisition software application.
The ABR system was acoustically calibrated annually using a commercial coupler (4157; Bruel & Kjaer [B&K]) with a microphone preamplifier (2669; B&K), signal conditioning amplifier (2690; B&K), and sound-level meter (2231; B&K). The broadband noise signal output of another dynamic signal analyzer (SR780; Stan-ford Research Systems, Sunnyvale, CA) was fed to the input of the headphone buffer amplifier, reproduced by the earphone, and recorded on the analyzer. Using this peak acoustical level as a reference, the relative level of each of the 42 MFTB component frequencies making up the six different center frequency MFTB stimuli was determined. The difference between the level of the reference peak on the signal analyzer, and the corresponding relative levels of the pure-tone frequencies at each of the 42 MFTB component frequencies, was stored in the stimulus generation program. The final MFTB stimulus train presentation level was set by establishing a calibrated 1 kHz peak reference level on a digital oscilloscope (TDS420A; Tektronix, Beaverton, OR) using an acoustic calibrator (4230; B&K). Using peak equivalent sound pressure level (peSPL) calibration methods, the attenuator was adjusted until the desired acoustical presentation level was obtained. Level verification of the stimuli occurred twice monthly in the coupler.
Hearing thresholds were acquired for stimuli delivered using the Virtual 320 clinical audiometer (Virtual Corporation) through Koss Pro/4× Plus earphones, modified to improve signal-to-noise ratio for high-frequency testing as described by Fausti et al (1990). Full calibration (ANSI S3.6-2010ANSI S3.6-2010) of the audiometer occurred annually. In addition, intensity verification occurred twice monthly using a platform and silicone rubber coupler to tightly house the measuring condenser microphone (4134; B&K), using procedures described by Fausti et al (1979).
Participants
Participants receiving cisplatin for the treatment of cancer were recruited from the Chemotherapy Unit of the Portland VA Medical Center. A list of patients prescribed chemotherapy was generated daily from the Chemotherapy Unit appointment list; this list was used to identify potential participants. Inclusion criteria were (a) cognitively and physically able to participate; (b) able to provide reliable (±5 dB) behavioral responses at baseline; (c) hearing no worse than 70 dB HL ≤4 kHz; (d) no active or recent history of middle ear disorder, Menière’s disease, or retrocochlear disorder; (e) normal otoscopic and tympanometric findings; and (f) the willingness to participate in the study. All participants were men, were consented to participate in the study following the guidelines of the medical center’s institutional review board, and were compensated for their time.
Testing
All testing, typically during the hydration portion of treatment, was done in a sound suite at the NCRAR by the same experienced audiologist. The baseline evaluation was performed within 24 hr of the initial chemo-therapeutic treatment, typically just before infusion. Monitoring sessions were completed within 24 h of each subsequent treatment and at 1 mo after cessation of treatment. The total number of patient visits (PVs) and intervals between visits varied across participants because treatment regimens depended on cancer type, location and stage, patient health, and other medical factors.
Ototoxic hearing shift was determined by serial pure-tone threshold testing. Participants completed a battery of tests at the baseline session and during each follow-up visit that included otoscopy, tympanometry, and conventional (2–8 kHz) and extended frequency (9–20 kHz) audiometry. Bilateral behavioral thresholds were obtained using the modified Hughson-Westlake technique (Carhart and Jerger, 1959). After completion of threshold testing, the individualized behavioral sensitive range for ototoxicity, SROBEH, was identified for each ear. The upper bound of the SROBEH was defined as the highest frequency at which a threshold of ≤100 dB SPL could be measured. The next adjacent six lower frequencies with thresholds <100 dB SPL, measured in 1/6-octave steps, were then obtained. To determine if thresholds were reliable, all testing was repeated at all frequencies after replacing the earphones. Only the SROBEH was tested at each monitoring visit. If hearing change was noted, then full-frequency (2–20 kHz) testing was done to search for any additional frequencies with hearing change. Behavioral hearing change was assessed relative to thresholds measured at the baseline visit. The determination of a significant hearing change was based on clinical guidelines (ASHA, 1994) and included (a) ≥ 20 dB change at any test frequency, (b) ≥ 10 dB change at any two consecutive test frequencies, or (c) loss of response at three consecutive test frequencies where responses were previously obtained. Tympanometry was considered normal if compliance ranged 0.2–1.8 cm3 and peak pressure ranged within − 150 to +100 daPa.
Out of time considerations, ABR testing was done in one ear only chosen either as the better hearing ear or, in the case of symmetric hearing, by coin toss. Each participant was seated in a comfortable reclining chair within the sound suite. After preparing the skin, disposable electrodes (Norotode 20 Ag/AgCl, Myotronics Inc., Kent, WA) were affixed to the skin surface using the 10–20 system of electrode placement. Electrode impedance of ≤ 3 kΩ were targeted; however, if this impedance was not attainable after three attempts, testing began. Each participant was encouraged to relax and to sleep, if possible. The response was acquired using a two-channel recording with the noninverting electrode at Fz, inverting at each mastoid and ground at Fpz. The contralateral recording was used to verify the presence of waveforms, if necessary. Biologic signals were bandpass filtered at 30–3000 Hz with the biologic amplifier gain adjusted to the highest gain possible (typically 300,000–400,000) with a rejection rate of 10%. Averaging continued until 1000 artifact-free sweeps were obtained. Each response was replicated at each stimulus frequency and intensity condition.
Rarefaction and condensation standard clicks were used at the baseline session to determine the polarity that resulted in a response that had the clearest waveform morphology and highest amplitude wave V response. This “best” polarity was then used for all subsequent testing. The frequency train incorporating six MFTB stimuli was used at the baseline session to determine the highest octave (in 1/2 octave steps) that resulted in a reproducible wave V response, at 105 dB peSPL and at least one additional lower intensity level, typically 95 dB. The top frequency (F1) was chosen if the response was reliably obtained at both intensity levels across two independent runs. The only exception was in the instance when hearing was very poor. In this instance, if a reliable wave V was obtained at only one level, typically 105 dB peSPL, at 2.8 kHz and a reliably wave V response was obtained at the lowest available tone-burst frequency, 2 kHz, at more than one level, both frequencies were monitored. Intensity trains at both highest half-octave frequencies were then used to collect the ABR at five intensities (65–105 dB peSPL) in 10 dB steps. Each frequency and intensity train was replicated and summed resulting in grand averages of 2000 ABR runs at each frequency and level. Subsequent (monitoring) testing was done at each treatment interval and included only intensity trains from the two highest frequencies (F1 and F2) established at baseline.
The same experienced audiologist scored all available response waveforms for latency and amplitude. Other waves (I, III) were also scored, if present. Amplitude was measured from the peak of the response to the following trough in nanovolts. Because the stimulus artifact was present at the highest intensity level, latency was measured from the peak stimulus artifact to the peak response of wave V. At lower intensity levels (< 105 dB peSPL) without stimulus artifact, the wave V was scored relative to the wave V response established at the highest level. Peak wave V latency measured from the peak of the stimulus artifact ranged from 7–10 msec, depending on test frequency. Each participant acted as his own control such that latency and amplitude measures obtained at each treatment interval were compared for change with the measures obtained at baseline. Scorable ABRs were generally limited to the highest two intensities tested (105, 95 dB peSPL); therefore, most of the analyses presented concern those levels.
Data Analysis
Briefly, a set of discriminant (scoring) functions were derived using logistic regression to model the risk for cisplatin-induced hearing change within the SROBEH. Independent variables were one of several ABR metrics alone each combined with a dose ototoxicity risk assessment model. Receiver operating characteristic curve analysis was used to evaluate and compare the test performance of these scoring functions. Details of the data analyses are described below.
Following our previous work, a scoring function was developed, denoted Rij for the ith PV on the jth patient, that best distinguishes PVs with a behavioral hearing change from those without a hearing change. In this analysis, the scoring function was defined as
| (1) |
where Mij is an ABR measurement such as, change in latency, taken on the jth participant during the ith PV, and WM is the weight assigned to the metric. DOij (dose ototoxicity) is the log odds on hearing change of the jth participant at the ith PV conditional on pretreatment hearing and cumulative dose of cisplatin, and WDO is the weight assigned to that effect. DO was defined as
| (2) |
Bj is the standardized, pretreatment SROBEH average pure-tone threshold for the jth participant, and L is the standardized log cumulative cisplatin dose in milli- grams. A univariate scoring function has WDO = 0 and WM = 1 so that identification of hearing change lay solely with the metric M under consideration. This contrasts with a multivariate scoring function where both the Dose Ototoxicity component and the metric under consideration contribute to the scoring function so that WDO ≠ 0 and WM ≠ 0.
Recall that ABRs were collected at each treatment interval using a pair of intensity trains at the two highest MFTB frequencies tested that generated a robust response at baseline. Candidate metrics were indexed by frequency (F1, F2) and level (95, 105 in dBSPL). Frequency 1 (range, 11.3–2 kHz) refers to an intensity train with the highest relative center frequency; Frequency 2 (range, 8–2 kHz) was the train corresponding to the lower relative center frequency. For each frequency by level combination, change in latency (CLat), change in amplitude (CAmp), and loss of ABR response (LoR) were considered. Also considered were summary metrics that involved changes at any stimulus frequency and level combination tested. These were any change in latency > 0.3 msec (LatInc) and any loss of ABR response (AnyLoss). The change measures were defined as the monitoring value minus the baseline value. The change in amplitude measures were multiplied by −1 so that larger values (as opposed to smaller values) on all metrics would be associated with hearing change (Dille et al, 2010b). Both univariate and multivariate scoring functions were defined for each candidate metric. Throughout the analysis, the weights (W) were established using logistic regression.
Scoring functions were compared using the area under the receiver operating characteristic curve (AUC). The AUC is an estimate of the average true positive rate over the domain of false positive rates (Pepe, 2003). A higher AUC is associated with a more accurate method that correctly identifies hearing change with relatively few false positives. The AUC was estimated using an analog to the Wilcoxon-Mann-Whitney U-statistic (Hanley and McNeil, 1982; Obuchowski, 1997). We anticipated that the ABR measurements taken on a participant over the course of ototoxicity monitoring would be correlated. Estimates of the AUC under these circumstances that are based on the U-statistic are correct, but the standard error of the estimated AUC is incorrect, motivating the use of the nonparametric estimator suggested by Obuchowski (1997). AUC and standard errors for each candidate metric were estimated using Leave-One-Out Cross Validation, with participants (as opposed to PVs) constituting the held-out unit. This methodology mirrors our earlier work on DPOAEs in the same sample (Dille et al, 2010b).
For a variety of reasons, the ABR measurement was prone to missing data. It was important to describe how these affected the evaluation of ABR methods for ototoxicity monitoring and, therefore, how missing data can affect clinical usefulness. A clinically useful ototoxicity monitoring procedure has to be accurate with relatively few inconclusive results. Accuracy, measured using the AUC, was defined above. Inconclusive results occur when measurement attempts fail. Failed measurements decrease the clinical usefulness of an ototoxicity monitoring method because they yield no relevant information.
In the current study, ABR measurement failure rate occurred primarily because of a tester-related problem (e.g., equipment issue) or poor ABR waveform morphology yielding an unscorable response. Furthermore, at times, participants could not be tested (CNT) because of extenuating circumstances such as chemotherapeutic-related illness. A session resulting in CNT was always a measurement failure because the participant was unable to be tested despite the best efforts of the tester. However, those missed sessions in which no measurement (DNT) was obtained were not counted as a measurement failure because, in theory, the session could have been completed. The final reason for a failed measurement was an absent ABR metric. In this circumstance, ABR metrics could not be extracted for analysis. These instances were scored as NR (no response). Whether NR was a measurement failure depended on the metric under consideration and the session in which the NR occurred. During baseline visits, NR was always a measurement failure because it did not permit evaluation of change in latency or amplitude at that frequency by level combination at subsequent monitor visits. During a monitoring visit, NR also failed to provide certain metrics from which to compare the response obtained at baseline. However, in this instance, NR was not a measurement failure but, rather, a true loss of the ABR response. Using this rationale to proceed, measurement failure rates for each metric were computed.
The analytic goal was to select the ABR metric that (1) achieved the highest accuracy for correctly identifying hearing as changed or not changed (based on the cross-validated AUC), and (2) provided the most data with the smallest measurement failure rate. To this end, the AUC was plotted against measurement failure rate. The optimal ABR ototoxicity-monitoring method was defined as the scoring function with the smallest measurement failure rate that was within one standard error of the AUC obtained from the most accurate metric. This application of the “one standard error rule” selects the method with smallest risk for inconclusive results and test performance that is statistically similar to the most accurate method (Hastie et al, 2009).
RESULTS
Table 1 shows characteristics of the 22 participants included in this study and the number of visits that were included in the analysis. The participants provided a total of 71 monitoring appointments during treatment with cisplatin. The average number of monitoring visits was 3.2 (range, 1–13 visits). Thirty-one (43.7%) of these appointments resulted in an ASHA-criterion hearing change. Mean age of the participants was 62.4 yr (age range, 51–79 yr). Most participants had head and neck cancers (n = 14; 63.6%) followed by lung cancer (n = 6; 27.3%).
Table 1.
Characteristics of Participants and Test Sessions
| Characteristic | Statistic | Result | % |
|---|---|---|---|
| Total Number of Participants | N | 22 | |
| Monitoring Visits | N | 71 | |
| Mean | 3.2 | ||
| Min | 1 | ||
| Max | 13 | ||
| Visits with Hearing Change | N | 31 | 43.7 |
| Age (yr) | Mean | 62.4 | |
| Min | 51 | ||
| Max | 79 | ||
| Cancer Location | |||
| Bladder | N | 1 | 4.5 |
| Head/Neck | N | 14 | 63.6 |
| Lung | N | 6 | 27.3 |
| Skin | N | 1 | 4.5 |
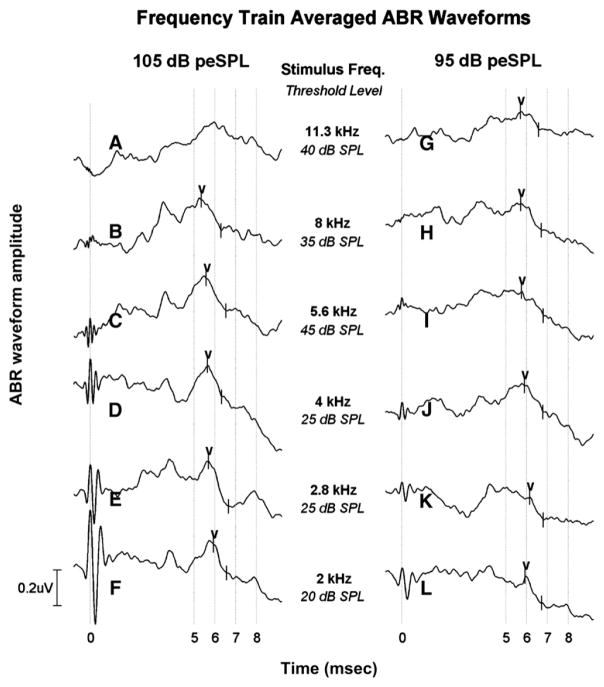
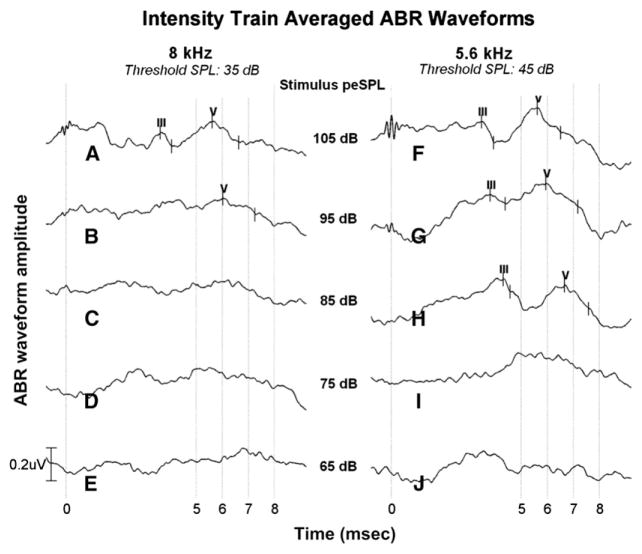
Figures 2-3 show a representative example of results obtained from an enrolled participant tested during chemotherapeutic treatment at the baseline (initial) visit. Figure 2 shows the ABR response obtained from a frequency train in which MFTB stimuli vary in their center frequency (11.3–2 kHz) and were presented at 105 and 95 dB peSPL. Amplitude (in microvolts) is shown as a function of time (in milliseconds) with frequency as the parameter. Shown under each frequency label is the behavioral hearing threshold (in dB SPL) at the same or near the center frequency of the MFTB obtained in the same testing session. For this participant, the top two MFTB frequencies at which an ABR response was reliably obtained at both intensity levels were 8 and 5.6 kHz. Note also the progression of latency increase as center frequency decreased. Figure 3 shows the ABR results obtained in the same testing session using intensity trains chosen from the top two frequencies identified from the frequency train results. For this participant, wave V was reliably obtained at the highest two intensities using the 8 kHz intensity train, whereas both waves III and V were apparent at the top three levels using the 5.6 kHz intensity train. As can be seen, with each reduction in stimulus level, the response latency migrated out in time as would be expected.
Figure 2.
Frequency train plots A–L display the grand averaged (n = 2000 responses) and scored ABR waveform amplitude (in micro-volts) as a function of time (milliseconds). Wave V responses were obtained during the baseline test session just before cisplatin administration. Behavioral hearing thresholds (dB SPL) corresponding to MFTB center frequency used to obtain the ABR are shown in the center area. For this participant, the highest two frequencies with reliable responses at both intensity levels (105 and 95 dB peSPL) were determined to be 8 and 5.6 kHz.
Figure 3.
Plots A–J display grand averaged and scored ABR waveforms obtained to 8 and 5.6 kHz intensity trains presented at five levels: 105, 95, 85, 75, and 65 dB peSPL. Behavioral thresholds at each frequency are provided at the top of the figure. Hearing thresholds and ABRs were obtained during the baseline session on the same participant shown in Figure 2. At 8 kHz, only the highest two levels (105 and 95 dB peSPL) resulted in reproducible ABR responses (plots A and B). At 5.6 kHz, ABR responses were obtained at three levels (105, 95, and 85 dB peSPL). ABR waves III and V are shown, although only wave V was used in the analysis. Amplitudes (in microvolts) are shown as a function of time (milliseconds).
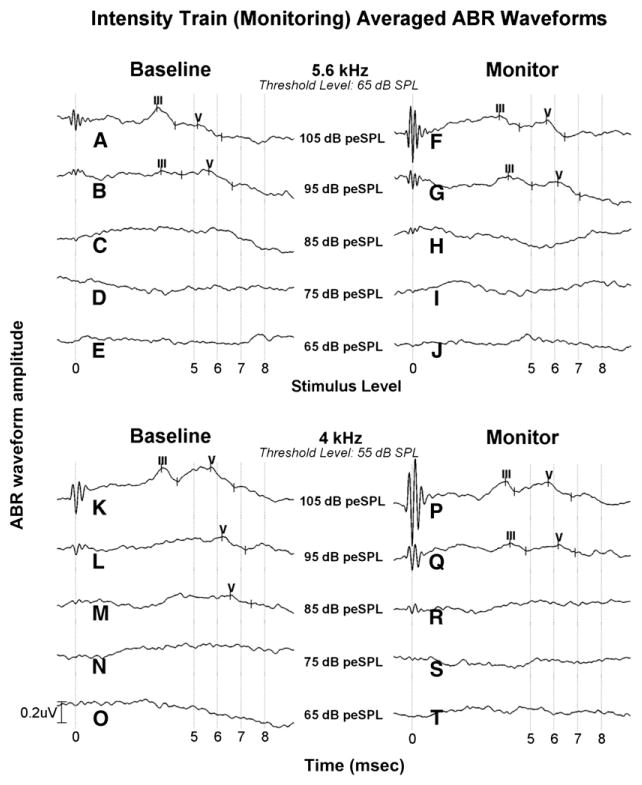
Figure 4 illustrates change in the ABR response on a participant who experienced ototoxicity during treatment. Using intensity trains at each treatment interval, the ABR response was compared to the baseline test result for change in latency, amplitude, and the presence of the response. At the top frequency (5.6 kHz), wave V latency increased by 0.5 msec at both intensity levels compared to the baseline findings. Wave V at 4 kHz during the monitor visit was present with stable latency at 95 and 105 dB peSPL but was absent to at the next lower stimulus level (85 dB peSPL). This participant’s hearing had changed since baseline. An ASHA-significant hearing shift was found in the test ear of +10 dB at 9, 10, and 11.3 kHz at a cumulative cisplatin dose of 190 mg. No further testing was done because cisplatin chemotherapy treatment was suspended.
Figure 4.
ABR test results and behavioral thresholds at 5.6 and 4 kHz on a participant seen at baseline (left column) and during a monitor (right column) visit using intensity train stimuli are shown. Comparing wave V latency at both intensity levels (105 and 95 dB peSPL) from baseline to monitor visit at 5.6 kHz, latency increased by 0.5 msec. At 4 kHz, wave V was absent at 85 dB peSPL at the monitoring visit, although latency did not significantly change (0.04–0.05 msec) at the highest two levels (95 and 105 dB). This participant also experienced a 10–15 dB ototoxicity hearing decrease at 8–11.2 kHz. Chemotherapeutic treatment was halted. Amplitude (in microvolts) is shown as a function of time (milliseconds).
Table 2 shows each of the candidate ABR wave V metrics [e.g., 95 change in latency 1(CLat1/95)] considered in this analysis, along with mean values for PVs with and without an ASHA-criterion hearing change. Other potential waves (waves I and III) were not present often enough to be included in the analysis. The final column of Table 2 shows the measurement failure rate for each test. High failure rates occurred in more than half of the candidate metrics and were exceptionally high at Frequency 1/level = 95 dB peSPL, the highest relative frequency. Furthermore, obtaining a response at the top two frequencies at both levels (105 and 95 dB peSPL) using a frequency train did not ensure that the response would also be present using the intensity train. A test that is inconclusive at more than half the time is not clinically useful, so failure rates ≥ 50% were not considered in the remaining analyses.
Table 2.
Results Obtained for FrEquation 1 (Higher) and 2 (Lower) Relative Frequencies, Stimulus Levels, and ABR Metrics Obtained at Each Treatment Interval for Participants with or without ASHA-Criterion Hearing Shift
| No ASHA Significant Hearing Change
|
ASHA Significant Change
|
Measurement Failure Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| Freq (kHz) | Stimulus Level (dB peSPL) | Metric | N (%) | Mean [lat (msec)or amp (nV)] | N (%) | Mean [lat (msec) or amp (nV)] | |
| 1 | 95 | Change in Latency (CLat1/95) | 9 | −0.05 | 12 | 0.12 | 52 |
| Change in Amplitude (CAmp1/95) | 9 | 25.86 | 12 | −13.61 | 52 | ||
| Loss of Response (LoR1/95) | 12 | 0.25 | 18 | 0.33 | 20 | ||
| 105 | Change in Latency (CLat1/105) | 27 | −0.26 | 23 | −0.05 | 18 | |
| Change in Amplitude (CAmp1/105) | 27 | 1.40 | 23 | 8.43 | 18 | ||
| Loss of Response (LoR1/105) | 29 | 0.07 | 25 | 0.08 | 14 | ||
| 2 | 95 | Change in Latency (CLat2/95) | 18 | 0.12 | 20 | 0.29 | 32 |
| Change in Amplitude (CAmp2/95) | 18 | 20.01 | 20 | 20.64 | 32 | ||
| Loss of Response (LoR2/95) | 24 | 0.25 | 23 | 0.13 | 19 | ||
| 105 | Change in Latency (CLat2/105) | 13 | 0.06 | 24 | −0.01 | 26 | |
| Change in Amplitude (Camp2/105) | 13 | 10.01 | 24 | 57.55 | 26 | ||
| Loss of Response (LoR2/105) | 17 | 0.24 | 24 | 0.00 | 22 | ||
| Latency Increase ≥ 0.3 msec (Lat Inc) | 31 | 0.03 | 26 | 0.27 | 11 | ||
| Any Loss of Response (Any Loss) | 33 | 0.33 | 26 | 0.27 | 10 | ||
Note: ABR measurement failure rates are also noted (stimulus level in dB peSPL; latency in milliseconds, and amplitude in nanovolts).
A potentially accurate ABR metric shows a high degree of separation between PVs with and without hearing change. Table 2 presents the candidate variables for both frequencies and all metrics of those frequencies. Note that the number of metrics increases as the test frequency changes from high (F1) to low (F2). Recall that at the baseline visit, F1 (highest center frequency) was selected only if a response was obtained at two intensity levels, typically 105 and 95 dB peSPL. Table 2 suggests that good candidates may be amplitude CAmp at F1/level = 105 dB peSPL (mean among PVs with hearing change = 8.43 nV; mean among PVs without hearing change = 1.40 nV) and CAmp at F2/level = 105 dB peSPL (mean with hearing change = 57.55 nV; mean without change = 10.01 nV). Also, the percentage of PVs with any latency increase > 0.3 msec was higher among PVs with hearing change (27%) than those without (3%). Table 2 also indicates a large difference in the loss of response metric for F2/level = 105 dB peSPL. None of the PVs (0.0%) with a hearing change had a loss of response at this stimulus compared to 24% among PVs without an ASHA hearing change. This finding indicates a backward effect that ears without ASHA-criteria hearing change are more likely to lose ABR response at this stimulus. We believe that this is idiosyncratic because the results were generated by two participants during two monitoring appointments where they had no hearing change. Accordingly, this metric was ignored in subsequent analyses.
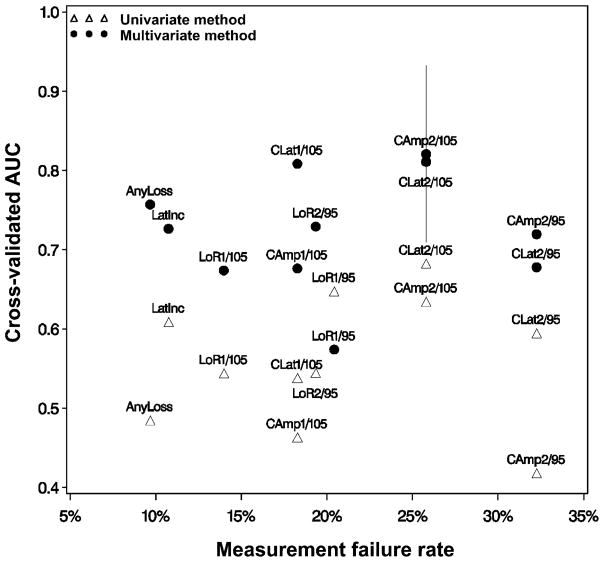
Although Table 2 hints at the clinical usefulness of univariate ABR metrics, it does not indicate how multivariate scoring functions might compare. To maximize the clinical usefulness of an ABR-based ototoxicity monitoring method, the most accurate method that has the smallest measurement failure rate is required. To achieve this objective, the cross-validated AUC is plotted against the sample measurement failure rates in Figure 5. The scoring function with the lowest measurement failure rate that was ± 1 standard error of the best performing model was selected as the optimal ABR-based scoring function. Filled circles show multivariate scoring function results while open triangles show univariate scoring function results. With the exception the multivariate LoR at F1/level = 95 dB peSPL and univariate CLat at F2/level = 105 dB peSPL, the multivariate methods provide uniformly greater accuracy than the univariate results.
Figure 5.
Cross-validated AUC curve as a function of measurement failure rate. The univariate method (open triangles) is shown compared to the multivariate method (filled circles). The multivariate method of scoring (ABR metrics + dose-ototoxicity model) has greater accuracy overall than the univariate method (ABR metrics alone). The preferred scoring method associated with the lowest measurement failure is “AnyLoss” of the ABR wave V response + dose-ototoxicity model.
The method with the highest overall accuracy was the multivariate scoring function using CAmp at F2/level = 105 dB peSPL (cross-validated AUC = 0.82). This method also has a relatively high measurement failure rate of 26%. The AUC ± 1 standard error is indicated by the vertical bar at the multivariate CAmp at F2/level = 105 dB peSPL point. According to the “one standard error rule,” the most clinically useful scoring function is the one with the smallest measurement failure rate that is within the limits of the standard error bar. Thus, the preferred method is the multivariate scoring function of “AnyLoss” of response, which is statistically similar to the most accurate method but with considerably lower measurement failure rates (10%). This method is the optimal ABR ototoxicity monitoring method determined for this sample.
DISCUSSION
Detection of ototoxicity using objective measures such as the ABR is necessary because many patients become incapacitated at some point during treatment and cannot undergo the rigors of a hearing test. Having a test available that can act as a proxy for a hearing test and can be submitted to comfortably is an important tool in an audiologist’s armamentarium because ototoxic hearing changes can be considered dose limiting. To be clinically useful, however, ototoxicity monitoring procedures must be robust, sensitive to hearing shift, and time efficient. This report examines the test performance of our previously developed trains of stimuli that offer rapid, reliable ABR testing of a range of tone-burst frequencies and levels. Unlike prior reports, here, we use statistical approaches rooted in clinical decision theory to assess the accuracy with which rapid ABR testing could determine whether hearing had changed, taking into account measurement failure rate (the inability to obtain a scorable ABR when testing was attempted, not including loss of response at a treatment visit).
We found that including our previously developed and validated cisplatin ototoxicity risk assessment model on the basis of pretreatment hearing thresholds and cumulative cisplatin dose (Reavis et al, 2008; Dille et al, 2012) improved prediction accuracy. Multivariate (ABR plus dose ototoxicity model) metrics accurately identified hearing shifts when scorable ABRs could be obtained, with best performing metrics achieving AUC values of > 80%. This performance would be considered excellent for a clinical test that rivals the performance of DPOAE testing (Reavis et al, 2008; Dille et al, 2010b). The clinical usefulness of ABR is somewhat more hampered than that of DPOAEs by a relatively high measurement failure rate. Depending on stimulus frequency and level and on the metric selected (amplitude, latency, and presence), the ABR measurement failure rate ranged from 10–52%. This finding was foreshadowed by others. Mitchell et al (2004), using normal-hearing participants, measured 7–29% fewer ABR responses to trains of tone-burst stimuli when compared to singly presented tone bursts at the same frequency. Henry et al (2000) found that the ABR responses were always lower in amplitude when presented in trains. Both studies reported the potential for auditory adaptation apparent in the latency increases noted for trained stimuli when compared to singly presented stimuli. Consistent with our findings, Mitchell et al (2004) also obtained consistently fewer responses at low intensity levels (< 80 dB peSPL). In comparison, only ~10% of ears had to be excluded for a lack of DPOAEs at baseline among veterans participating in two prospective trials investigating the use of DPOAEs for detecting ototoxic-induced hearing shifts (Reavis et al, 2008; Reavis et al, 2011). DPOAEs rarely were unscorable, perhaps because averaging was variable and continued until the noise floor was low. A similar approach is used in some automated ABR programs (Iwasaki et al, 2003); however, increased averaging time would slow testing.
Because a test sensitive to early detection should monitor at regions within the cochlea that are likely to change first, ABR testing was done near the high-frequency limit of the response. This trend implies that the ABR may be somewhat elusive at the highest frequency (F1). In fact, we did find that the failure rate at this frequency was much higher than at F2, but only at a lower level (95 dB peSPL). At a higher level (105 dB peSPL), the metrics were equivalent. Regardless, the measurement failure rate was unacceptably high at almost all levels and frequencies. It is hard to imagine that clinical decisions regarding change in chemotherapy regimen could be made using a metric that failed to provide a definitive indication of hearing shift at 20% or more of the time. Therefore, the DPOAE method may be preferable to the ABR method. On the other hand, it is possible that other methods for obtaining auditory brainstem measures quickly might perform better than our MTBF trains. For example, the auditory steady-state response has been used to obtain responses at multiple frequencies simultaneously (Hatton and Stapells, 2011). High modulation rates produce auditory steady-state responses from the brainstem with favorable signal-to-noise ratios obtained more quickly than for analogous measures using tone-burst stimuli presented individually (McNerney and Burkard, 2010, 2012).
Acknowledgments
This work was supported by the VA RR&D Service (VA RR&D grants C4183R and C7113N awarded to M.F.D. and D.K.-M.).
Abbreviations
- ABR
auditory brainstem response
- ABR metric
latency and/or amplitude wave V measurement
- AnyLoss
absence of wave V at a monitor patient visit
- ASHA
American Speech-Language-Hearing Association
- AUC
area under the receiver operating characteristic curve
- Camp
change in amplitude
- CAmp1
Change in wave V amplitude at frequency 1
- CAmp2
change in wave V amplitude at frequency 2
- CLat
change in latency
- CLat1
change in wave V latency at frequency 1
- CLat2
change in wave V latency at frequency 2
- CNT
could not test because of subject unavailability (i.e., measurement failure)
- DO
dose-ototoxicity model, DNT, did not test due to equipment or tester problem
- DPOAE
distortion-product otoacoustic emission
- F1
highest 1/2 octave center frequency at which wave V was obtained
- F2
lowest 1/2 octave center frequency at which wave V was obtained
- kΩ
kilo-ohms
- LatInc
latency increase of the wave V (>0.3 msec)
- LoR
loss of wave V ABR response (during monitor test session)
- MFTB
multiple frequency tone burst
- NR
no wave V ABR response
- PV
patient visit
- SPL
sound pressure level
- SRO
sensitive range for ototoxicity
- SROBEH
sensitive range for ototoxicity using behavioral threshold testing
Footnotes
Portions of this article were presented at the 2010 American Auditory Society Meeting in Scottsdale, AZ.
References
- Alam SA, Ikeda K, Oshima T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141(1–2):28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI) American National Standard Specifications for Audiometers. ANSI S3.6-2010. New York: ANSI; 2010. [Google Scholar]
- American Speech-Language-Hearing Association (ASHA) Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines] ASHA. 1994;36:11–19. Available from: www.asha.org/policy. [Google Scholar]
- Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, Seam RK. Cisplatin-based chemotherapy: Add high-frequency audiometry in the regimen. Indian J Cancer. 2009;46(4):311–317. doi: 10.4103/0019-509X.55551. [DOI] [PubMed] [Google Scholar]
- Carhart R, Jerger J. Preferred method for clinical determination of pure-tone thresholds. J Sp Hear Dis. 1959;24:330–345. [Google Scholar]
- Coupland SG, Ponton CW, Eggermont JJ, Bowen TJ, Grant RM. Assessment of cisplatin-induced ototoxicity using derived-band ABRs. Int J Pediatr Otorhinolaryngol. 1991;22(3):237–248. doi: 10.1016/0165-5876(91)90078-p. [DOI] [PubMed] [Google Scholar]
- De Lauretis A, De Capua B, Barbieri MT, Bellussi L, Passàli D. ABR evaluation of ototoxicity in cancer patients receiving cisplatin or carboplatin. Scand Audiol. 1999;28(3):139–143. doi: 10.1080/010503999424707. [DOI] [PubMed] [Google Scholar]
- Dille MF, Konrad-Martin D, Gallun F, et al. Tinnitus onset rates from chemotherapeutic agents and ototoxic antibiotics: results of a large prospective study. J Am Acad Audiol. 2010a;21(6):409–417. doi: 10.3766/jaaa.21.6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dille MF, McMillan GP, Reavis KM, Jacobs PG, Fausti SA, Konrad-Martin D. Ototoxicity risk assessment combining distortion product otoacoustic emissions with a cisplatin dose model. J Acoust Soc Am. 2010b;128(3):1163–1174. doi: 10.1121/1.3473693. [DOI] [PubMed] [Google Scholar]
- Dille MF, Wilmington D, McMillan GP, Helt WJ, Fausti SA, Konrad-Martin D. Development and validation of a cisplatin dose-ototoxicity model. J Amer Acad Audiol. 2012;23(7):510–521. doi: 10.3766/jaaa.23.7.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson R, Dille MF, Leek MR, Fausti SA. On the synthesis of multiple frequency tone burst stimuli for efficient high frequency auditory brainstem response. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:4158–61. doi: 10.1109/IEMBS.2008.4650125. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Gray PS, Frey RH, Mitchell CR. Rise time and center-frequency effects on auditory brainstem responses to high-frequency tone bursts. J Am Acad Audiol. 1991b;2(1):24–31. [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Henry JA, Knutsen JL, Olson DJ. Reliability and validity of high-frequency (8–20 kHz) thresholds obtained on a computer-based audiometer as compared to a documented laboratory system. J Am Acad Audiol. 1990;1(3):162–170. [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Henry JA, Olson DJ, Schaffer HI. Early detection of ototoxicity using high-frequency, tone-burst-evoked auditory brainstem responses. J Am Acad Audiol. 1992;3(6):397–404. [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Erickson DA, Rappaport BZ, Cleary EJ, Brummett RE. A system for evaluating auditory function from 8000–20 000 Hz. J Acoust Soc Am. 1979;66(6):1713–1718. doi: 10.1121/1.383643. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Rappaport BZ, Frey RH, et al. Reliability of evoked responses to high-frequency (8–14 kHz) tone bursts. J Am Acad Audiol. 1991a;2(2):105–114. [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20(6):497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Olson DJ, Frey RH, Henry JA, Schaffer HI, Phillips DS. High-frequency toneburst-evoked ABR latency-intensity functions in sensorineural hearing-impaired humans. Scand Audiol. 1993;24:19–25. doi: 10.3109/01050399509042205. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference and Prediction. New York: Springer Press; 2009. p. 244. [Google Scholar]
- Hatton J, Stapells DR. The efficiency of the single- versus multiple-stimulus auditory steady state responses in infants. Ear Hear. 2011;32(3):349–357. doi: 10.1097/AUD.0b013e3181ff352c. [DOI] [PubMed] [Google Scholar]
- Hellberg V, Wallin I, Eriksson S, et al. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst. 2009;101(1):37–47. doi: 10.1093/jnci/djn418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA, Fausti SA, Kempton JB, Trune DR, Mitchell CR. Twenty-stimulus train for rapid acquisition of auditory brainstem responses in humans. J Am Acad Audiol. 2000;11(2):103–113. [PubMed] [Google Scholar]
- Iwasaki S, Hayashi Y, Seki A, et al. A model of two-stage newborn hearing screening with automated auditory brainstem response. Int J Pediatr Otorhinolaryngol. 2003;67(10):1099–1104. doi: 10.1016/s0165-5876(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Laurell G, Viberg A, Teixeira M, Sterkers O, Ferrary E. Blood-perilymph barrier and ototoxicity: an in vivo study in the rat. Acta Otolaryngol. 2000;120(7):796–803. doi: 10.1080/000164800750061624. [DOI] [PubMed] [Google Scholar]
- Maiese K, Walker RW, Gargan R, Victor JD. Intra-arterial cisplatin—associated optic and otic toxicity. Arch Neurol. 1992;49(1):83–86. doi: 10.1001/archneur.1992.00530250087021. [DOI] [PubMed] [Google Scholar]
- McNerney KM, Burkard RF. The effects of a second stimulus on the auditory steady state response (ASSR) from the inferior colliculus of the chinchilla. Int J Audiol. 2010;49(8):561–573. doi: 10.3109/14992020903473449. [DOI] [PubMed] [Google Scholar]
- McNerney KM, Burkard RF. The auditory steady state response: far-field recordings from the chinchilla. Int J Audiol. 2012;51(3):200–209. doi: 10.3109/14992027.2011.631589. [DOI] [PubMed] [Google Scholar]
- Mitchell CR, Ellingson RM, Henry JA, Fausti SA. Use of auditory brainstem responses for the early detection of ototoxicity from aminoglycosides or chemotherapeutic drugs. J Rehabil Res Dev. 2004;41(3A):373–382. doi: 10.1682/jrrd.2003.05.0089. [DOI] [PubMed] [Google Scholar]
- Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53(2):567–578. [PubMed] [Google Scholar]
- Pepe M. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford, UK: Oxford University Press; 2003. p. 28. [Google Scholar]
- Reavis KM, McMillan GP, Austin D, et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011;32(1):61–74. doi: 10.1097/AUD.0b013e3181e8b6a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavis KM, Phillips DS, Fausti SA, et al. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008;29(6):875–893. doi: 10.1097/AUD.0b013e318181ad99. [DOI] [PubMed] [Google Scholar]
- Riedemann L, Lanvers C, Deuster D, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8(1):23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Mechanisms of cisplatin ototoxicity and progress inotoprotection. Curr OpinOtolaryngol HeadNeck Surg. 2007;15(5):364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- Schweitzer VG. Cisplatin-induced ototoxicity: the effect of pigmentation and inhibitory agents. Laryngoscope. 1993;103(4 Pt 2):1–52. [PubMed] [Google Scholar]