Abstract
Soil-air fluxes of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) were determined using a novel application of passive samplers to measure air and soil air, which is air in close proximity and in equilibrium with soil. Existing methods to measure flux of semi-volatile compounds between soil and air require collecting samples from the top soil layer. Yet, the top soil layer is hard to define and oversampling may misrepresent the exchangeable fraction. Alternatively, modified active samplers can measure soil air in situ, but require electricity while deployed. We present a new method to measure time-weighted averages of soil air concentrations in situ using passive sampling and requiring no electricity: a box is placed over low-density polyethylene passive samplers deployed 1 cm above the soil. Passive air samplers were also co-deployed 1.5 m above the soil to measure ambient air concentrations in three U.S. locations: near a former PCB manufacturing facility in Anniston, Alabama; on a former creosoting and the current Wyckoff/Eagle Superfund site near Seattle, Washington; and near the site of a recent oil-train derailment and fire in Mosier, Oregon. Following n-hexane extraction, sampler extracts were analyzed for PAHs with gas chromatography-tandem mass spectrometry and PCBs with dual gas chromatography-electron capture detectors. PAHs were generally depositing at Anniston and Mosier sites, but volatilizing from soil in Wyckoff, the site with historically-contaminated soil. PCBs were detected most frequently at the Anniston site, although levels were lower than previous reports. Variability in concentration measurements was greater among soil air samplers than air samplers, likely due to soil heterogeneity. Environmental conditions under the novel soil air box did not substantially change soil-air partitioning behavior. This method of measuring soil air in situ will allow for understanding of source-sink dynamics at sites with recent and historical contamination, and where conventional sampling is challenging.
Keywords: soil flux, in situ, persistent organic pollutants, fugacity ratio, polycyclic aromatic hydrocarbons, polychlorinated biphenyls
Graphical Abstract
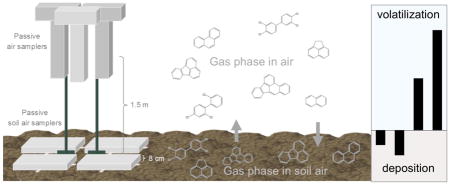
1. Introduction
Soil is an important reservoir of persistent pollutants. Initially, soil can be a sink for hydrophobic chemicals such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), and can remain a lingering source after the point source is removed (Harner et al., 1995). Remediation efforts can be hampered if cleaned soils act as a sink for new contaminants. Effective tools are necessary to understand the direction and magnitude of soil-air partitioning. Previous methods of determining soil-air partitioning involve sampling the soil directly (Bidleman and Leone, 2004; Wang et al., 2014). Soil concentrations are converted to a measure of fugacity with temperature and soil-air partitioning coefficients. Comparing fugacity between ambient air and soil air allows for determination of the magnitude and direction of soil-air partitioning. The top 0.1 to 1 cm of soil is the exchange layer, and it responds quickly to fluctuations in air concentrations (Cabrerizo et al., 2009; Harner et al., 2001). However, this exchange layer is hard to define and can be challenging to collect (Wang et al., 2015). Soil concentrations vary with depth and sampling more than the exchange layer may skew interpretation. For example, a soil sample including not only the soil-air exchangeable layer, but also a portion of the non-exchangeable layer below may misrepresent partitioning between soil and air. Furthermore, levels of compounds extracted with organic solvents exceed the levels that freely exchange with air (Wang et al., 2015).
Alternative in situ techniques have been developed more recently to directly sample the soil air, i.e. air that is in close proximity and equilibrium with the soil (Cabrerizo et al., 2009; Liu et al., 2013b; Meijer et al., 2003; Wang et al., 2017; Wang et al., 2015). In situ techniques are particularly useful in multi-compartment systems, e.g. where compounds may partition between air and ground vegetation (Liu et al., 2013b; Wang et al., 2015). Low-volume active air samplers draw air slowly across the soil surface for nominally 48 hours to ideally ensure that sampled air is in equilibrium with soil. As with other active sampling methods, these devices are bulky, require electricity, care must be taken to ensure sufficient equilibrium time. Alternatively, passive samplers are increasingly used to measure freely-dissolved or gas-phase concentrations of semi-volatile compounds in water and air. Compared to active sampling methods, passive samplers are deployed for weeks at a time, require no electricity, and yield time-weighted averages (Zhang et al., 2011). Previously, passive samplers have been employed to measure in situ flux of hydrophobic organic contaminants between environmental compartments such as air and water (McDonough et al., 2016; Tidwell et al., 2016) and water and sediment porewater (Fernandez et al., 2014; Liu et al., 2013a). The few passive sampling studies that have measured soil air or soil-air partitioning in situ profiled concentration gradients using polyurethane foam passive samplers near the soil surface (Wang et al., 2017; Zhang et al., 2011). We present an alternative method by collecting two concentrations measurements—in ambient air and at the soil surface.
The objective of this work is to demonstrate a new design for a soil air passive sampler that can be used to evaluate volatilization or deposition of hydrophobic organic contaminants. We compare the sensitivity of this novel sampling design among dissimilar sampling locations, both on and near sites of historical contamination, and at a site of recent contamination. Repeatability is measured within and among sampler boxes. Environmental conditions under the soil air sampling boxes are also monitored to ensure the sampling equipment does not substantially alter the environment being measured. Two measures of soil-air partitioning are presented: fugacity ratio and flux.
2. Materials and Methods
2.1 Site descriptions and sampling
Identical sampling schemes were deployed at three locations: Anniston, Wyckoff, and Mosier. We sampled diverse locations that would demonstrate the technology’s ability to measure both volatilization and deposition for multiple chemical classes. The Anniston PCB Superfund site consists of downstream waterways, surrounding residential properties, and a facility that manufactured PCBs from 1929 until 1979 in Anniston, Alabama. Anniston samplers were deployed on adjacent wooded property approximately 0.7 km south of the facility, with the permission of Forever Wild Land Trust. The Wyckoff/Eagle Harbor site on Bainbridge Island, Washington is the location of a former wood treatment facility. Wyckoff samplers were deployed centrally on soil that was historically contaminated with creosote and pentachlorophenol. The third sampling site was in Mosier, Oregon, near the site of a recent oil train derailment, spill, and fire. Cleanup was underway at the time the samplers were deployed in Mosier, three weeks after the accident occurred on 3 June 2016.
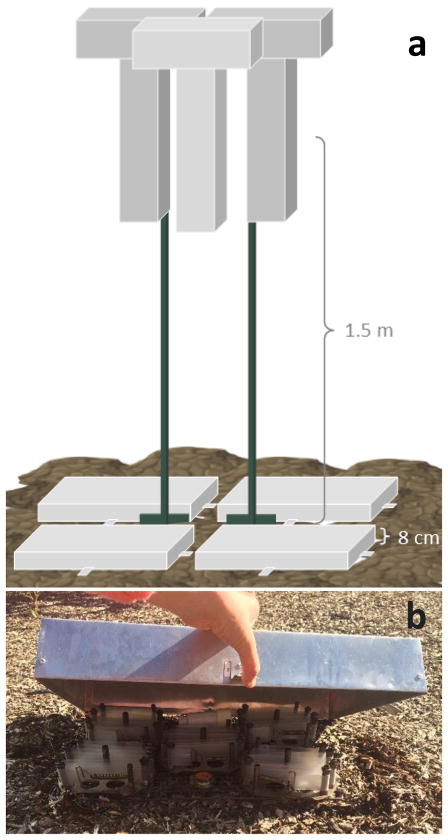
A total of 3 air boxes and 4 soil air boxes were co-deployed at each of the three locations for a duration of 14 days (Figure 1a). Each box contained 5 LDPE passive samplers (Figure 1b). All deployments occurred in May, June, or July of 2016. Five LDPE strips were hung inside metal, T-shaped air sampling boxes that protect from UV radiation but allow airflow, as used previously (Paulik et al., 2016). The LDPE strips are contained within the upright portion (55 × 14 × 9 cm) positioned under the top portion (5 × 25 × 9 cm). Air boxes were hung on trees in Anniston and Mosier, and on cleaned metal fence posts at Wyckoff, approximately 1.5 m above the soil at a height used commonly in studies using high-volume active air samplers (Cabrerizo et al., 2009).
Figure 1.
Schematic of air and soil air sampling design (a) and arrangement of LDPE passive sampling strips under the soil air box (b).
Four soil air sampling boxes were deployed on soil immediately adjacent to the air boxes. Five LDPE strips were strung on carriers and placed on a grate immediately above the soil (Figure 1b). The soil air sampling box was placed over the LDPE passive samplers. The soil air box (50 × 30 × 8 cm) is open on the soil side, but impermeable on the other surfaces to reduce free exchange with ambient air. Air diffuses into the soil air box by permeating through the soil near where the lip is placed on the soil surface; it is assumed this air has reached equilibrium with soil air. Cabrerizo et al. (2009) report that the soil air reaches equilibrium with PAHs and PCBs in soil within 4 minutes. Care was taken at all sites to deploy in shaded locations. Temperature and relative humidity (RH) loggers were placed inside one air box and one soil air sampling box at each site and recorded at thirty minute intervals for the duration of deployment. A soil sample (top 15 cm) was taken at each site from outside a soil air box at time of deployment. Soil samples were analyzed for texture, organic matter, and moisture content at the Central Analytical Laboratory at Oregon State University.
2.2 Standards, solvents, and materials
Native PAH and PCB compounds of purity 97% or greater were purchased from Accustandard (New Haven, Conn., USA). Complete lists of target PAH and PCB compounds are given in Table 1; CAS numbers and physicochemical properties are given in Tables S1 and S2. Deuterium-labeled compounds used as performance reference compounds (PRCs, Tables S1 and S2) and extraction surrogates and internal standards (Table S3) were purchased from Cambridge Isotope Laboratories (Tewksbury, Mass., USA) and C/D/N Isotopes (Pointe-Claire, Quebec, Canada). Extraction solvent n-hexane and solvents used for rinsing, isopropanol, hexanes, and acetone, were Optima™ grade or better (Fischer Chemical, USA). Passive samplers were transported in polytetrafluoroethylene (PTFE) bags with Clip N Seal closures purchased from Welch Fluorocarbon, Inc. (Dover, New Hampshire, USA). Temperature and RH data loggers were purchased from Onset Computer Corporation (Bourne, Mass., USA). Passive samplers were constructed from LDPE lay-flat tubing purchased from Brentwood Plastics, Inc. (St. Louis, Missouri, USA). Average membrane thickness is 75–95 μm, average width of tubing is 2.7 cm, and average transient polymer cavity size is 10 Å (Anderson et al., 2008).
Table 1.
Target analytes. PAHs are listed in order of GC retention time. CAS numbers and physicochemical properties are provided in Table S1 and S2.
| PAHs | naphthalene, 2-methylnaphthalene, 1-methylnaphthalene, 2-ethylnaphthalene, 2,6-dimethylnaphthalene, 1,6-dimethylnaphthalene, 1,4-dimethylnaphthalene, 1,5-dimethylnaphthalene, 1,2-dimethylnaphthalene, 1,8-dimethylnaphthalene, 2,6-diethylnaphthalene, acenaphthylene, acenaphthene, fluorene, dibenzothiophene, phenanthrene, anthracene, 2-methylphenanthrene, 2-methylanthracene, 1-methylphenanthrene, 9-methylanthracene, 3,6-dimethylphenanthrene, 2,3-dimethylanthracene, fluoranthene, 9,10-dimethylanthracene, pyrene, retene, benzo[a]fluorene, benzo[b]fluorene, benzo[c]fluorene, 1-methylpyrene, benz[a]anthracene, cyclopenta[cd]pyrene, triphenylene, chrysene, 6-methylchrysene, 5-methylchrysene, benzo[b]fluoranthene, 7,12-dimethylbenz[a]anthracene, benzo[k]fluoranthene, benzo[j]fluoranthene, benz[j]aceanthrylene & benz[e]aceanthrylene, benzo[e]pyrene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[a,h]anthracene, benzo[a]chrysene, benzo[ghi]perylene, anthanthrene, naphtho[1,2-b]fluoranthene, naphtho[2,3-j]fluoranthene, dibenzo[a,e]fluoranthene, dibenzo[a,l]pyrene, naphtho[2,3-k]fluoranthene, naphtho[2,3-e]pyrene, dibenzo[a,e]pyrene, coronene, dibenzo[e,l]pyrene, naphtho[2,3-a]pyrene, benzo[b]perylene, dibenzo[a,i]pyrene, dibenzo[a,h]pyrene |
| PCB congeners | 1, 4, 5, 8, 10, 11, 16, 17, 18, 21, 28, 31, 33, 37, 44, 49, 50, 52, 60, 66, 70, 74, 77, 81, 82, 87, 99, 101, 104, 105, 110, 114, 118, 123, 126, 128, 138, 145, 153, 156, 157, 158, 166, 167, 169, 170, 179, 180, 183, 187, 189, 204 |
2.3 Sample preparation
Each passive sampling strip was constructed from 1.1 m lengths of lay-flat LDPE tubing after Anderson et al. (2008). Strips were cleaned with three successive 24-hour washes in hexanes to remove potential chemical interferences. Once dry, one end was heat-sealed, a 50 μL PRC solution in isooctane was added, and the other end was heat-sealed. This method of infusion and heat-sealing was chosen because it requires less solvent than other equilibration techniques. Strips were immediately placed in individual PTFE bags with airtight closures for transport to and from sampling locations and up to 3 weeks’ storage at −20°C. We do not expect PAH degradation following previous work showing concentrations of representative PAHs sequestered in LDPE passive samplers are stable out to 14 days at 35°C (Donald et al., 2016). Each strip was infused with nominally 2 μg fluorene-d10, 1 μg pyrene-d10, 1 μg benzo[b]fluoranthene-d12, 0.2 μg PCB-116-d5, and 0.2 μg PCB-65-d5. The average of three blank infused LDPE strips was used to determine initial t=0 PRC concentrations (Table S4).
Following field deployment and upon receipt in the laboratory, all samplers were cleaned briefly in two washes of isopropanol to remove particulate matter and superficial fouling. Five LDPE replicates from one soil air box from each site were analyzed initially to ensure PAHs were sufficiently above detection limits. Remaining Anniston samples were composited and extracted by combining the five LDPE strips within each sampling box to achieve greater sensitivity, while remaining Wyckoff and Mosier samplers were extracted as individual strips. For extraction, samplers were placed in two successive solutions of 50 mL hexane containing extraction surrogate standards. Dialysates were combined and reduced to 0.5 mL. Exposure to ambient light was minimized during all laboratory steps.
2.4 Instrumental analysis
Analysis for 62 PAHs was performed with an Agilent 7890A gas chromatograph (GC) with an Agilent 7000 GS/MS-MS mass spectrometer (Anderson et al., 2015). Select samples were also analyzed for 52 PCBs on a dual-column Agilent 6890N GC equipped with dual electron capture detectors. Instrument parameters are detailed in Table S3. Detection and quantitation limits for all compounds at the instrument and in both air and soil air are included in Tables S1 and S2. Instrument concentrations were quantitatively corrected for loss during laboratory processing steps using extraction surrogate compound recoveries. Average extraction surrogate recovery was 73% (range 29–114) where recoveries were generally lower for relatively more volatile compounds, e.g. naphthalene-d8.
2.5 Environmental concentrations
Time-weighted average gas-phase air and soil air concentrations were determined using an empirical uptake model. Sampling rates were derived using performance reference compounds (PRCs) as in situ calibration standards. Sampler-air partition coefficients are adjusted using the average temperature while deployed. Detailed equations are given in supplemental information (SI).
2.6 Fugacity ratio and flux calculations
The unitless fugacity ratio (fratio) indicates the net direction of exchange of a compound between the air and soil surface (Cabrerizo et al., 2009; Degrendele et al., 2016; Harner et al., 2001; McDonough et al., 2016; Wang et al., 2014; Wang et al., 2015):
| Eq. 1 |
where fratio > 1 indicates volatilization out of the soil and fratio < 1 indicates deposition. Fugacity of soil air (fsoil air, atm) and air (fair, atm) are calculated using the same equation:
| Eq. 2 |
where C(soil)air (ng m−3) is the concentration of analyte in soi) is the concentrat3 atm K−1 mol−1) is the gas constant, T (K) is the temperature measured within soil air or air box, MW (g mol−1) is molecular weight of the analyte, and 1015 is a unit conversion factor (Cabrerizo et al., 2009; Degrendele et al., 2016; Wang et al., 2015). Alternatively, Eq. 1 might be represented more simply as the ratio of two concentrations, but we present fugacity ratios to allow incorporation of small temperature differences. Uncertainty of fratio was estimated at 45% after incorporating all error ranges in air concentrations, soil air concentrations, and log Koa (Harner et al., 2001; Wang et al., 2016); more details are provided in SI. This range of uncertainty is similar to previous reports measuring soil-air partitioning: 43% (Degrendele et al., 2016) and 30–40% (Liu et al., 2016). In this work, values of fratio outside 0.55—1.45 (log10 fratio: −0.26—0.19) indicate significant deviations from equilibrium (SI).
Flux was calculated following Fick’s law of diffusion (Davie-Martin et al., 2013; Eek et al., 2010; Fernandez et al., 2014):
| Eq. 3 |
where positive values of flux indicate volatilization from soil to air (ng m−2 h−1). Csoil air and Cair are concentrations in soil air and air (ng m−3). The boundary layer (δL, m), is set at 0.001, the value used in the Pesticide Leaching Model (PELMO) simulation (Davie-Martin et al., 2013; Ferrari et al., 2003). DT (m2 h−1) is the temperature-corrected diffusivity in air using DT of pyrene at 298 K as a reference (Davie-Martin et al., 2013; Gustafson and Dickhut, 1994; Schwarzenbach et al., 2003). Calculations and values for DT for target compounds are given in SI. Values of flux >1 indicate volatilization, and flux <1 indicates deposition from air to soil. Uncertainty for flux measurements using propagation of error are detailed in SI (Liu et al., 2016; Minick and Anderson, 2017).
2.7 Statistical analysis
Mean temperature and RH in air and soil air sampling boxes were compared using two-sided t-tests with serial correlation corrections. Mean air and soil air concentrations were compared using two-sided t-tests assuming unequal variance. Paired t-tests were used to compare relative standard deviation (RSD) of air and soil air measurements to assess differences in within- and between-box variability. Significance for all tests was set at α = 5 %. Average PAH concentrations, fugacity ratio, and flux calculations were only calculated at each location for compounds that were above quantitation limit in all replicates in both air and soil air. Environmental concentrations of PCBs were calculated for all analyzed samples when detected, but fugacity and flux were not determined for PCBs because of low detection frequencies. Statistical analyses were performed with JMP Pro 12.0.1 and Microsoft Excel 2016.
2.8 Quality control
Quality control samples compromised 21% of all samples. Blank LDPE passive samplers that served as procedural blanks were prepared, sent without opening to and from the field sites, cleaned following deployment, and extracted. These procedural blanks (n = 3) were below limit of quantitation for all compounds, except 9 PAHs (Table S5). Average instrument concentrations for these 9 compounds were subtracted from all samples before environmental concentrations were calculated. The sum of these background-subtracted compounds compromised an average of 2% (range 2–23%) of the sum instrument concentrations of 62 PAHs. Blank solvent runs were included in each analytical batch of 20 samples. Continuing calibration verifications were included in each analytical batch to ensure a minimum of 80% of compounds were within ± 25% of true value for PAHs and ± 40% of true value for PCBs.
3. Results
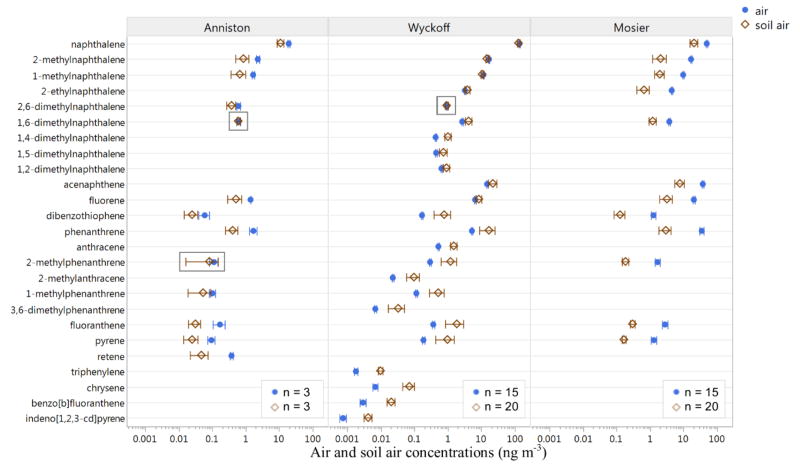
Estimates of the soil-air partitioning, measured with the novel passive sampling design, suggest that PAHs are partitioning from air to soil in Anniston and Mosier, but the majority of PAHs were volatilizing from soil at Wyckoff. Mean air and soil air levels were different in all but three instances (Figure 2 and Table S6, two-sample t-test assuming unequal variance, α = 0.05). When statistically different, concentrations of detected PAHs at Anniston and Mosier were greater in air than soil air. All but four PAHs at Wyckoff had greater concentrations in soil air: naphthalene, 2-methylnaphthalene, 1-methylnaphthalene, and 2-ethylnaphthalene. Soil-air partitioning for PCBs was not calculated because PCBs were not detected consistently at levels above quantitation limits. Four PCB congeners were detected in Anniston samples: PCB 4, 17, 77, and 118; and PCB 4 and 17 were also detected in some Wyckoff samples (Table 2). PCBs were below detection limit in all samples from Mosier. Complete PCB results for all analyzed samples are given in Table S7.
Figure 2.
Air and soil air PAH concentrations at three sampling locations. Data are omitted for a site if below quantitation limit in any air or soil air replicate. The 25 PAHs shown were above quantitation limit for all replicates at one or more sites. Error bars represent 95% confidence interval. Boxes indicate instances where levels were not different between air and soil air (two-sample t-test assuming unequal variance, α = 0.05). Anniston samples were composited before analysis and thus have smaller n.
Table 2.
Mean concentrations of PCBs in air and soil air for data above limits of detection.
| Sampling location | Air | Soil air | |||||
|---|---|---|---|---|---|---|---|
| Mean (ng/m3) | RSDa | Number of detections (n=3) | Mean (ng/m3) | RSDa | Number of detections (n=3) | ||
| Anniston | PCB 4 | 0.31 | 45% | 3 | 0.49 | 26% | 3 |
| PCB 17 | 0.034 | 2% | 3 | 0.046 | 114% | 3 | |
| PCB 77 | 0.0033 | 8% | 2 | 0.010 | 28% | 2 | |
| PCB 118 | 0.0038 | c | 1 | b | c | 0 | |
| Wyckoff | PCB 17 | 0.0079 | c | 1 | 0.029 | 9% | 2 |
| Mosier | no PCBs detected | b | c | 0 | b | c | 0 |
relative standard deviation
not detected
not calculated because of low detection frequency
3.1 Temperature and relative humidity
Conditions within the air and soil air boxes were measured to evaluate if the sampling equipment was altering the in situ environment. No significant differences in mean temperature were found between the soil air boxes and air boxes at each site (Table 3). Mean RH was greater in the soil air box for all sites. Generally, diurnal fluctuations for temperature and RH were muted inside the soil air box (SI). Environmental conditions recorded at local weather stations agreed with temperature and RH measurements from within air boxes, indicating the micro-environment inside the air boxes is similar to ambient air.
Table 3.
Estimated soil characteristics and environmental parameters. Mean temperature and relative humidity (RH) were compared with t-test adjusted for serial correlation. Asterisks denote significant differences between air and soil air boxes.
| Sampling location | Soil type | foc (%) | Moisture content (%) | Temp. (°C) | RH (%) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | p-value | Mean | p-value | |||||
| Anniston | sandy loam | 8 | 20 | Air | 22.2 | 0.429 | 70.0 | 0.002* |
| Soil air | 20.4 | 97.3 | ||||||
| Wyckoff | sandy loam | 5 | 2 | Air | 16.9 | 0.841 | 67.5 | 0.026* |
| Soil air | 16.5 | 84.0 | ||||||
| Mosier | organic | 30 | 50 | Air | 21.0 | 0.336 | 59.0 | <0.001* |
| Soil air | 19.0 | 94.7 | ||||||
3.2 Replicate analysis
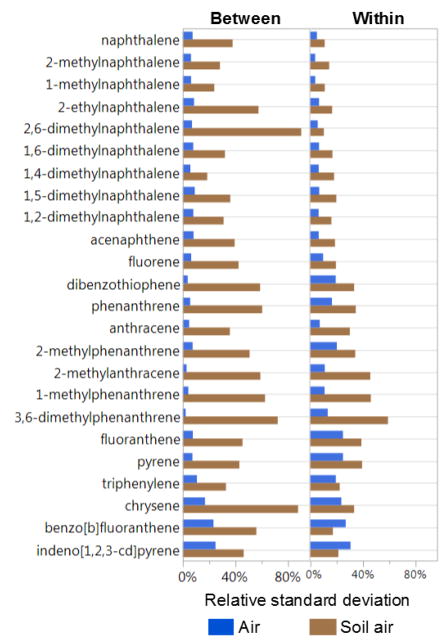
Variability of PAH levels, measured by relative standard deviation (RSD), was greater in soil air than air (Figure 4). Between-box variability for PAH compounds was significantly greater in soil air than air (paired t-test, two-sided p-value < 0.001). Average within-box variability was also assessed by analyzing five separate LDPE strips within each air or soil air box. Average within-box variability for PAHs was also significantly greater for soil air than air across all detected PAHs at Wyckoff and Mosier (paired t-test, two-sided p-value < 0.001). The majority of Anniston samples were analyzed as composites, and thus, within-box variability was not assessed for this site.
Figure 4.
Average relative standard deviation for between-box and within-box air and soil air samplers. Between-box variability for PAH compounds was significantly greater in soil air (average 40%) than air (average 8%; paired t-test, two-sided p-value < 0.001). Average within-box variability for PAHs was also greater for soil air (average 23%) than air (average 13%) across all detected PAHs at Wyckoff and Mosier (paired t-test, two-sided p-value < 0.001).
3.3 Fugacity ratio (fratio) and flux
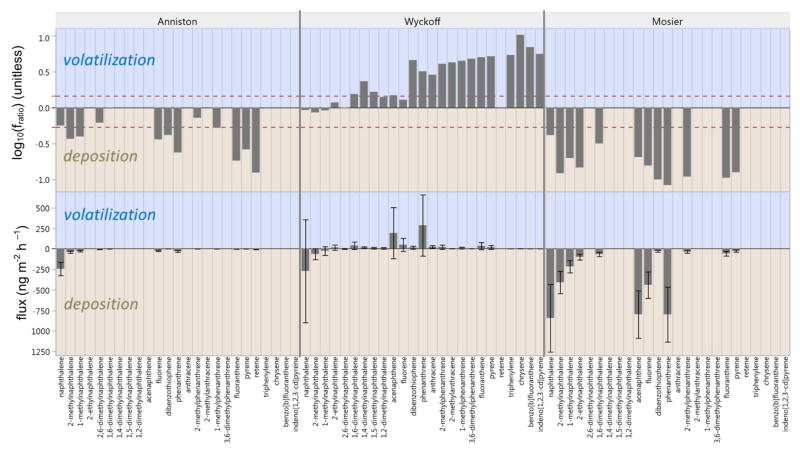
Numerous PAHs were in a state of deposition at both Anniston and Mosier, and none were observed to be volatilizing (Figure 3). The magnitude of deposition, measured by flux, was an average of 10 times greater in Mosier than in Anniston for all PAHs detected. The highest degree of flux, in either direction, was measured for naphthalene at −844 ng m−2 h−1 in Mosier, the site of the oil train fire in close proximity to a major highway. Our analysis also suggests volatilization from soil to air for many detected PAHs at Wyckoff, the site with substantial historical soil contamination, however fratio and flux data indicate different levels of significance. By both metrics, lower-molecular weight PAHs are in equilibrium between air and soil air.
Figure 3.
Soil-air partitioning at three sampling locations represented by fugacity ratio (fratio) and flux. Bars in the positi volatilization, and bars in the negative direction indicate deposition. Bars (fratio) outside the red dashed line indicate sign equilibrium between soil and air. Error bars for flux measurements show propagation of error (SI). Data are omitted for limit in any air or soil air replicate. PAHs are only shown if above quantitation limit for all replicates at one or more site.
3.4 Soil moisture and organic content
Soil samples were collected from the top 15 cm and analyzed for descriptive qualities (Table 3). Moisture and organic carbon content are estimated, because the soil samples included approximately 1–5 cm duff that was excluded in analysis. Fraction of organic (foc) was estimated by dividing fraction of organic matter by 2 (Schwarzenbach et al., 2003). Moisture content ranged between 2–50%, and foc was between 5–30%.
4. Discussion
4.1 Sensitivity of passive fugacity sampler at three unique locations
Direction of soil-air partitioning of semi-volatile organic contaminants can be represented both by fugacity and flux. Regardless of the metric used, partitioning measured with the novel sampling equipment design suggests that the direction and magnitude of soil-air partitioning varies by site. PAHs were either volatilizing or at equilibrium at the historically contaminated soil at the Wyckoff Superfund, while PAHs were in deposition near the Anniston Superfund site and the site of a recent oil train fire in Mosier.
The objective of this work was to demonstrate a novel sampling device for measuring soil to air flux. While our aim was not to characterize or monitor the sites, comparisons of contaminant profiles among the three sites provide interesting insights. Diagnostic ratios are often used to assess potential sources of PAHs (Stogiannidis and Laane, 2015). A discussion of numerous sourcing ratios in included in SI, with inconclusive results between pyrogenic/petrogenic sources as well as contributions of creosote, paving materials, and tire dust.
4.1.1 Anniston
Of the three selected sites, PCBs were expected to be detected at Anniston near the site of historical PCB contamination. Anniston also had the lowest measured PAH concentrations. This site was in a wooded recreation area with trails, approximately 0.7 km slightly uphill from the main facility and 200 m from the on-site south landfill. Large machinery could be heard, but not seen from the deployment location. Average sum of 52 PCBs at the Anniston site in the present work was 0.35 and 0.55 ng/m3 for air and soil air, respectively. For the two PCBs that were detected in all analyzed samples at this site, PCB 4 and PCB 17, levels were not different between soil air and air (p = 0.15 and 0.24 respectively, t-test assuming unequal variance). These results suggest PCB equilibrium between air and soil air, or more likely, that more data is needed to conclude the direction of soil-air flux. Previous research at the Anniston site suggests that the source of atmospheric gas-phase PCBs is material buried in the landfill, while volatilization from surface soil is a minor contributor (Hermanson et al., 2003). Hermanson et al. (2003) report sum air concentrations of 120 PCB congeners between ~5 and 12 ng m−3. A more recent report found sum PCB concentrations between 3 and 19 ng m−3 (Agency for Toxic Substances and Disease Registry, 2015). Sampling in an undisturbed, wooded area and/or analyzing for fewer target PCBs may have led to lower sum PCBs levels in this work than previous reports.
4.1.2 Wyckoff
The samplers were placed in a location within the Superfund site with the known highest contaminations based on historical data (pers. comm. Helen Bottcher 2016). Samples from this site also had the highest PAH levels compared to Mosier and Anniston, as well as the heaviest, least-volatile PAHs up to the 6-ring PAH indeno[1,2,3-cd]pyrene. Adjacent to the samplers was an active remediation well that pumps contaminated groundwater to the surface before treatment at the on-site plant. A storage tank by the well head contains product with an obvious odor that may have impacted measured PAH concentrations in air. The Wyckoff site is not close to any major roads, but is adjacent to the ferry route that runs > 20 times daily between Seattle and Bainbridge Island. Regardless of potential current PAH sources in the area that might lead to deposition, many detected PAHs were volatilizing from the soil, while none were significantly depositing. The major PAH constituents of the creosote NAPL (non-aqueous phase liquid) at the Wyckoff site are, in order, phenanthrene, fluoranthene, pyrene, acenaphthalene, and naphthalene (Brenner et al., 2002). These PAHs were found in all Wyckoff samples, although not in the same ratios likely because of weathering and differential volatilization.
4.1.3 Mosier
This site was anticipated to have the most diverse PAH sources. A railroad and a large highway run parallel to the Columbia River through the town of Mosier. Samplers were intentionally placed in a shaded, calm location in close proximity to the site of oil train derailment and fire. The sampling location was approximately 60 m north of the railroad, and 60 m south of the highway. Charred brush could be seen from the deployment location. Post-spill clean-up operations included repaving a short section of road approximately 100 m south of the samplers. Finally, a brush fire covering >2800 acres occurred 25 km east of Mosier while samplers were in place. The dominant wind direction during summer months and this deployment is from the west, so this brush fire would be expected to have only small, if any, effects on measured PAH concentrations in air. Numerous diagnostic ratios yielded conflicting evidence of pyrogenic or petrogenic sources that may reflect the diverse PAH sources, including most notably crude oil (petrogenic) and the fire resulting from the derailment (pyrogenic) (SI). More information is needed to understand the relative contribution of these and other likely sources including exhaust and tire dust from the adjacent highway.
4.2 Previous reports of PAH soil-air partitioning
In the Northeastern region of the United States, total flux for ten PAHs was estimated to be −82 ng m−2 h−1 (Simonich and Hites, 1994). Approximately 10% of flux rate for these PAHs, −8.2 ng m−2 h−1, is directly partitioning to soil, while the majority is sequestered in vegetation before falling and decaying. For this subset of ten PAHs in the present work, average soil-air flux in Anniston and Mosier was −12 and −290 ng m−2 h−1 respectively. At Wyckoff, these PAHs were volatilizing at an average of 52 ng m−2 h−1. Flux of PAHs in Anniston is consistent with Simonich and Hites (1994), and Anniston is likely most representative of a regional average because it is undeveloped but in proximity to a city. Flux at Mosier may be greater than a regional average because of the recent oil train spill and fire, as well as its proximity to a major highway and railway. Finally, flux at the Wyckoff Superfund site has substantial contamination and represents a highly contaminated site.
Previous researchers have reported volatilization or deposition trends based on physicochemical properties. Wang et al. (2014) found that low-molecular weight PAHs were volatilizing, and high-molecular weight PAHs were depositing to soil on “pristine” pastureland of the Tibetan Plateau. In contrast, Degrendele et al. (2016) measured numerous semivolatile contaminants at background sites in Hungary, and found that the more volatile, less-chlorinated PCB congeners were depositing, while the heavier, less-volatile PCBs were volatilizing. Similarly, our data suggest that the most-volatile, lightweight compounds were either depositing or were at equilibrium during our study period. General trends regarding the direction of soil-air partitioning are not expected to be dependent on chemical volatility alone, as trends are the result of multiple site parameters including, for example, age and type of contamination, climate, and soil characteristics.
4.3 Replicate analysis
Variability in PAH levels, measured by relative standard deviation (RSD), was greater in soil air than air (Figure 4). The differences between the two sampled matrices are likely attributable to the nonhomogenous nature of soil, while air is comparatively more mixed. These results suggest that multiple air boxes may serve as replicates, but adjacent soil air samplers are pseudo-replicates that also incorporate the soil heterogeneity. Such heterogeneity is important to measure in future applications for accurate site characterization. Even within sampling boxes, we found more variability with soil air samplers. This result suggests that air diffusion is slow under the soil air box and that individual passive sampling strips are strongly influenced by the soil directly over which they are deployed.
Cabrerizo et al. (2009) assessed repeatability of soil fugacity measurements by collecting samples on consecutive days, and found agreement within 10% in similar weather conditions. In comparison to the active sampling methods of Cabrerizo et al. (2009), passive samplers like those described in the present work are deployed for weeks at a time. Day-to-day variability is incorporated into the resulting time-weighted averages and it cannot be quantified for a direct comparison between active and passive sampling. Instead of repeating measurements in time, we repeated samples in close proximity and found higher variability in samples that incorporate the heterogeneity of the soil.
One set of within-box replicate (n=5) soil air samples from each site were analyzed initially to check for instrument sensitivity. The least-volatile PAH detected in these individual samples from Anniston was triphenylene (log Kow = 5.49, log Koa = 10.69). Remaining Anniston soil air samplers were composited, and PAHs up to benzo[b]fluoranthene (log Kow = 5.78, log Koa = 10.35) were then above detection limit. Resulting environmental concentrations of individual and composited sampled were nominally the same. In this case, compositing multiple passive sampling strips allowed for the detection of less volatile compounds that were previously below detection limits. The mass of passive sampling material per analyzed sample can be adjusted in future applications to optimize sensitivity.
4.4 Environmental conditions
The design of the soil air box affects Ksoil-air by lowering the temperature and increasing RH, relative to the air cage. The effects of temperature and RH offset each other, which is a result of the soil air box design providing shade but limiting the exchange of water vapor with ambient air. Together, these effects can change the environment slightly during sampling. Notably, active air sampling methods also change the soil air microenvironment when drawing air slowly across soil surface. Further, both active and passive in situ sampling methods change the environmental conditions less than ex situ soil sample collection methods.
Even small changes in temperature and RH can correspond to large changes in partitioning behavior between air, the passive sampling material (Huckins et al., 1990), and soil (Davie-Martin et al., 2015). Field measurements of temperature are incorporated into partition coefficients between air and the sampling material (Ksample-air) using a modified van ‘t Hoff equation (SI). Soil-air partition coefficients, Ksoil-air, are also affected, and artificially high temperature or high humidity can increase partitioning from soil to air. In previous work that samples soil directly rather than with active or passive sampling, Ksoil-air is incorporated in estimating fsoil air from the concentration in soil (Csoil) (Cabrerizo et al., 2009; Wang et al., 2015):
| Eq. 4 |
Ksoil-air is an additional term in the denominator in this expression that is analogous to Eq. 2. Following Eq. 4 and Eq. 1 above, a change in Ksoil-air is inversely related to both fsoil air and fratio.
Davie-Martin et al. (2015) developed an equation that predicts Ksoil-air of individual compounds using 22 pesticide compounds in varying conditions:
| Eq. 5 |
where log Koa is the logarithm of the octanol-air partition coefficient, T (K) is the mean environment temperature during deployment, RH (%) is relative humidity, and log foc (%) is the logarithm of soil organic carbon content. The pesticides used in the Davie-Martin model have similar physicochemical properties (log Koa range 6.4–10.4) as the PAHs in the present study (log Koa range 5.0–13.7). For a compound in a given environment, log Koa and log foc are unchanged by the sampling equipment. In this present study, relative humidity was significantly different between soil air and air, while temperatures were not significantly different. Using Eq. 5, the temperature and RH differences observed in the soil air boxes are expected to correspond to Ksoil-air decreases of 1.5-fold (0.17 log units) for both Anniston and Wyckoff, and 1.8-fold (0.26 log units) for Mosier. These fold differences by location were constant across compounds for all molecular weights. As a conservative estimate, the expected differences in Ksoil-air would correspond to, at most, a 1.8-fold increase in both fsoil air and fratio. For any compounds close to equilibrium, the effect of the sampling equipment altering soil-air partitioning may change the direction of flux. Under the conditions measured in the present work, volatilization may be underestimated. Additionally, a 1.8-fold change is likely an overestimate because temperature and RH were not measured on the exterior of the soil air box at ground level, where conditions are expected to be more similar to the soil air box’s interior, but at 1.5m above the soil air box. We recommend monitoring temperature and RH in future applications.
Organic carbon content correlates with a soil’s capacity to sorb semi-volatile contaminants (Dalla Valle et al., 2005; Davie-Martin et al., 2015; Schwarzenbach et al., 2003). It might therefore be expected that soils rich in organic matter would favor partitioning to soil through deposition. Among our sampling locations, Mosier had the highest organic carbon content as well as the highest rates of deposition. Wykcoff had the lowest organic carbon content and no evidence of deposition. We predict that these associations are coincidences in this study, as there are other important variables that lead to deposition or volatilization, such as the time elapsed since contamination events.
4.5 Detection limits and relative PRC diffusion rates
Performance reference compounds (PRCs) are used to estimate how compounds are approaching equilibrium. Lighter, more volatile compounds generally reach equilibrium more quickly and are estimated using PRCs with similar physicochemical properties. Greater amounts of PRCs diffused from air samplers than from soil air samplers suggesting that, in comparison, compounds in air samplers are closer to equilibrium with the environment. On average, only 2% of fluorene-d10 remained in air samplers across all sites, while 36% remained in soil air samplers (Figure S1). Similar but less dramatic differences in dissipation occurred with the remaining PRCs. Accordingly, the air boxes sampled a larger volume of air than the soil air, and this discrepancy is expected because the design of the soil air box allows only limited exchange with ambient air. The different sampling rates do not affect air and soil air concentrations, because the calculations incorporate PRC loss on a per sample basis.
The volume of air sampled, however, does affect the environmental detection limit. Lower-molecular weight PAHs have similar limits in both air and soil air, but the quantitation limit for the heaviest PAHs is about 6 times greater in soil air (Table S1). As an example, consider a compound at equilibrium has the same concentration in both air and soil air. The passive samplers must have sequestered a concentration at least equal to the detection limit for it to be measured at the instrument. Because more air passes over the air sampler than the soil air sampler (inferred from PRC loss), it is possible that the soil air sampler has not accumulated enough of the compound for it to be above detection limit. For this reason, we only calculated fugacity ratios and flux if compounds were above quantitation limit in all air and soil air samplers, on a site-by-site basis. This ensures that we are not falsely assuming that air concentration is greater than soil air when sampling rates may be affecting detection limits.
4.6 Limitations and Advantages
Passive sampling is advantageous because the technique does not require electricity or maintenance while deployed. The novel soil air sampler and previous adaptations of passive sampling yield time-weighted average concentrations that are measured in situ. Compared to active samplers which can be deployed for hours to days at a time, passive samplers must often be deployed longer. The heaviest and least volatile compound detected in any sample was dibenzo[e,l]pyrene (log Kow = 7.28, log Koa = 12.77), which was found in approximately half of the samplers from Wyckoff. The detection of this compound indicates that the 2-week deployment period was sufficient for appreciable accumulation above detection limits at the most contaminated site. This PAH or other heavier compounds may also be present at the other sites, but require longer than two weeks to appreciably accumulate in the passive sampler material in lower environmental concentrations. The length of deployment and mass of passive sampling material should be tailored for the site of interest.
Previous soil-air partitioning research has revealed diurnal fluctuations (Degrendele et al., 2016). Measurements made with passive samplers are time-weighted averages over the scale of weeks and are not suitable for discerning variation within a day. Seasonal variations have also been reported, where volatilization of semi-volatile contaminants is greater in warmer temperatures (Wang et al., 2017; Wang et al., 2011; Wang et al., 2016). Wyckoff had the lowest average temperature yet had the highest PAH concentrations of all three sites. Direction and magnitude of soil-air partitioning is likely affected seasonally, and the results presented do not represent an annual average. Repeated measures at a site are necessary to determine seasonal variation. Results provided herein are an estimate of average flux over the duration of deployment only.
We present two metrics of soil-air partitioning: fugacity ratio and flux. Comparing these two metrics highlights an important difference, particularly with the Wyckoff data (Figure 3). Flux compares the difference between two concentrations (Eq. 3), while fratio is a ratio of two concentrations (Eq. 1). For example, at Wyckoff, indeno[1,2,3-cd]pyrene levels in air (0.000739 ng m−3) and soil air (0.00464 ng m−3) were both low, but significantly different. The soil air level is ~6 times greater than air, although the difference in these values is only 0.00390 ng m−3. These data yield high fratio, but low magnitude of flux. A weight-of-evidence approach should be considered when concluding direction and magnitude of soil-air partitioning.
The height of boundary layer was estimated as 0.001 m and used in flux calculations across all three sites. In windier locations, the boundary layer might be expected to be smaller, thereby increasing the magnitude of flux. Importantly, however, the samplers in the present study were each deployed in areas without large influence of wind. If another value were used in flux calculations, the profile and relative trends of flux among the three sites would be unchanged, and only the magnitude would be different. Computing the fugacity ratio does not require an estimated value for boundary layer.
A major advantage of the recent in situ methods for measure soil-air partitioning is that they use the same sampling technique for measuring both air and soil air. Traditional method of measuring soil-air flux requires the measurement of several soil parameters and the estimation of compound-specific physicochemical properties including foc and Ksoil-air. The overlying air samplers, whether active or passive must also be effectively calibrated. Numerous estimated values are also used in the present work, however the sampling equipment and calculations for both environmental matrices are nearly identical. Any input parameters for the environmental concentration calculations that are over- or under-estimated would affect both matrices in the same manner.
5. Conclusions
The passive soil air sampler described here is suitable for measuring concentrations of semi-volatile organic contaminants in air that are in equilibrium with soil. When co-deployed with ambient air passive samplers, direction and magnitude of soil-air partitioning can be measured. Along with the advantages of in situ sampling, the described passive sampling method requires no electricity and allows for longer, maintenance-free deployment periods. We have demonstrated its performance in three unique environments where compounds were found to be differentially partitioning between air and soil. Variability among soil air samplers is predictably greater than air samplers, and sensitivity can be adjusted with length of deployment and mass of passive sampling material. Environmental conditions under the novel soil air box do not substantially change soil-air partitioning behavior and should be monitored in future uses. The passive soil-air fugacity sampler is a candidate for use in numerous sites with new or historical contamination, or in locations where conventional soil sampling techniques are challenging.
Supplementary Material
Highlights.
We measured PAH and PCB soil-air flux with a novel passive sampling device
PAH deposition was greatest at the site of a recent oil train derailment and fire
Humidity and temperature under the soil air sampler box should be monitored
Variability was higher for soil air samplers, likely because of soil heterogeneity
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) award number P42 ES016465 and the associated Chemistry Facility Core, P30 ES000210. CE Donald was supported in part by NIEHS Training Grant Fellowship T32ES007060 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not represent the official views of the NIEHS or NIH. We greatly appreciate the help of Helen Bottcher, Stanley Warner, Keith Allers, Henry and Leslie Donald, the City of Mosier, Oregon and the permission of Forever Wild Land Trust Program of the Alabama Department of Conservation and Natural Resources. Alan Bergmann, Jamie Minick, Ricky Scott, Holly Dixon, Amber Barnard, Jessica Scotten, Gary Points, and Josh Willmarth of the Food Safety and Environmental Stewardship Program provided valuable help in field sampling and laboratory analysis.
Abbreviations
- PAH
polycyclic aromatic hydrocarbon
- PCB
polychlorinated biphenyl
- LDPE
low-density polyethylene
- RH
relative humidity
- PRC
performance reference compound
- PTFE
polytetrafluoroethylene
- GC
gas chromatography
- SI
- PELMO
pesticide leaching model
- RSD
relative standard deviation
- NAPL
non-aqueous phase liquid
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Toxic Substances and Disease Registry. Health Consultation: Anniston PCB Air Sampling. Division of Community Health Investigations; 2015. [Google Scholar]
- 2.Anderson KA, Sethajintanin D, Sower GJ, Quarles L. Field trial and modeling of uptake rates of in situ lipid-free polyethylene membrane passive sampler. Environmental Science & Technology. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. Journal of Chromatography A. 2015;1419:89–98. doi: 10.1016/j.chroma.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidleman TF, Leone AD. Soil–air exchange of organochlorine pesticides in the Southern United States. Environmental Pollution. 2004;128:49–57. doi: 10.1016/j.envpol.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Brenner RC, Magar VS, Ickes JA, Abbott JE, Stout SA, Crecelius EA, Bingler LS. Characterization and FATE of PAH-Contaminated Sediments at the Wyckoff/Eagle Harbor Superfund Site. Environmental Science & Technology. 2002;36:2605–2613. doi: 10.1021/es011406u. [DOI] [PubMed] [Google Scholar]
- 6.Cabrerizo A, Dachs J, Barcelo D. Development of a soil fugacity sampler for determination of air-soil partitioning of persistent organic pollutants under field controlled conditions. Environmental Science & Technology. 2009;43:8257–8263. doi: 10.1021/es9020525. [DOI] [PubMed] [Google Scholar]
- 7.Dalla Valle M, Jurado E, Dachs J, Sweetman AJ, Jones KC. The maximum reservoir capacity of soils for persistent organic pollutants: implications for global cycling. Environmental Pollution. 2005;134:153–164. doi: 10.1016/j.envpol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Davie-Martin CL, Hageman KJ, Chin YP. An improved screening tool for predicting volatilization of pesticides applied to soils. Environmental Science & Technology. 2013;47:868–876. doi: 10.1021/es3020277. [DOI] [PubMed] [Google Scholar]
- 9.Davie-Martin CL, Hageman KJ, Chin YP, Rouge V, Fujita Y. Influence of Temperature, Relative Humidity, and Soil Properties on the Soil-Air Partitioning of Semivolatile Pesticides: Laboratory Measurements and Predictive Models. Environmental Science & Technology. 2015;49:10431–10439. doi: 10.1021/acs.est.5b02525. [DOI] [PubMed] [Google Scholar]
- 10.Degrendele C, Audy O, Hofman J, Kucerik J, Kukucka P, Mulder MD, Pribylova P, Prokes R, Sanka M, Schaumann GE, Lammel G. Diurnal variations of air-soil exchange of semi-volatile organic compounds (PAHs, PCBs, OCPs and PBDEs) in a central European receptor area. Environmental Science & Technology. 2016;50:4278–4288. doi: 10.1021/acs.est.5b05671. [DOI] [PubMed] [Google Scholar]
- 11.Donald CE, Elie MR, Smith BW, Hoffman PD, Anderson KA. Transport stability of pesticides and PAHs sequestered in polyethylene passive sampling devices. Environmental Science and Pollution Research. 2016;23:12392–12399. doi: 10.1007/s11356-016-6453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eek E, Cornellissen G, Breedveld GD. Field measurement of diffusional mass transfer of HOCs at the sediment-water interface. Environmental Science & Technology. 2010;44:6752–6759. doi: 10.1021/es100818w. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez LA, Lao W, Maruya KA, Burgess RM. Calculating the diffusive flux of persistent organic pollutants between sediments and the water column on the Palos Verdes Shelf Superfund Site using polymeric passive samplers. Environmental Science & Technology. 2014;48:3925–3934. doi: 10.1021/es404475c. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari F, Trevisan M, Capri E. Predicting and measuring environmental concentration of pesticides in air after soil application. Journal of Environmental Quality. 2003;32:1623–1633. doi: 10.2134/jeq2003.1623. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson KE, Dickhut RM. Molecular Diffusivity of Polycyclic Aromatic Hydrocarbons in Air. Journal of Chemical and Engineering Data. 1994;39:286–289. [Google Scholar]
- 16.Harner T, Bidleman T, Jantunen L, Mackay D. Soil-air exchange model of persistent pesticides in the United States cotton belt. Environmental Toxicology and Chemistry. 2001;20:1612–1621. doi: 10.1897/1551-5028(2001)020<1612:saemop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Harner T, Mackay D, Jones KC. Model of the long-term exchange of PCBs between soil and the atmosphere in the southern U.K. Environmental Science & Technology. 1995;29:1200–1209. doi: 10.1021/es00005a010. [DOI] [PubMed] [Google Scholar]
- 18.Hermanson MH, Scholten CA, Compher K. Variable Air Temperature Response of Gas-Phase Atmospheric Polychlorinated Biphenyls near a Former Manufacturing Facility. Environmental Science & Technology. 2003;37:4038–4048. doi: 10.1021/es030332e. [DOI] [PubMed] [Google Scholar]
- 19.Huckins JN, Tubergen MW, Manuweera GK. Semipermeable membreane devices containing model lipid: a new approach to monitoring the bioavailability of liphphilic contaminants and estimating their bioconcentration potential. Chemosphere. 1990;20:533–552. [Google Scholar]
- 20.Liu HH, Bao LJ, Feng WH, Xu SP, Wu FC, Zeng EY. A multisection passive sampler for measuring sediment porewater profile of dichlorodiphenyltrichloroethane and its metabolites. Analytical Chemistry. 2013a;85:7117–7124. doi: 10.1021/ac400589a. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Ming LL, Nizzetto L, Borga K, Larssen T, Zheng Q, Li J, Zhang G. Critical evaluation of a new passive exchange-meter for assessing multimedia fate of persistent organic pollutants at the air-soil interface. Environmental Pollution. 2013b;181:144–150. doi: 10.1016/j.envpol.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wang S, McDonough CA, Khairy M, Muir D, Lohmann R. Estimation of Uncertainty in Air-Water Exchange Flux and Gross Volatilization Loss of PCBs: A Case Study Based on Passive Sampling in the Lower Great Lakes. Environmental Science & Technology. 2016;50:10894–10902. doi: 10.1021/acs.est.6b02891. [DOI] [PubMed] [Google Scholar]
- 23.McDonough CA, Puggioni G, Helm PA, Muir D, Lohmann R. Spatial Distribution and Air- Water Exchange of Organic Flame Retardants in the Lower Great Lakes. Environmental Science & Technology. 2016 doi: 10.1021/acs.est.6b02496. [DOI] [PubMed] [Google Scholar]
- 24.Meijer S, Shoeib M, Jantunen L, Jones K, Harner T. Air-soil exchange of organochlorine pesticides in agricultural soils. 1. Field measurements using a novel in situ sampling device. Environmental Science & Technology. 2003;37:1292–1299. [Google Scholar]
- 25.Minick D, Anderson KA. Diffusive flux of PAHs across sediment-water and water-air interfaces at urban superfund sites. Environmental Toxicology and Chemistry. 2017 doi: 10.1002/etc.3785. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulik LB, Donald CE, Smith BW, Tidwell LG, Hobbie KA, Kincl L, Haynes EN, Anderson KA. Emissions of Polycyclic Aromatic Hydrocarbons from Natural Gas Extraction into Air. Environmental Science & Technology. 2016;50:7921–7929. doi: 10.1021/acs.est.6b02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. 2. John Wiley & Sons; Hoboken, New Jersey: 2003. [Google Scholar]
- 28.Simonich SL, Hites RA. Importance of vegetation in removing polycyclic aromatic hydrocarbons from the atmosphere. Nature. 1994;370:49–51. [Google Scholar]
- 29.Stogiannidis E, Laane R. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. Reviews of Environmental Contamination and Toxicology. 2015;234:49–133. doi: 10.1007/978-3-319-10638-0_2. [DOI] [PubMed] [Google Scholar]
- 30.Tidwell LG, Allan SE, O’Connell SG, Hobbie KA, Smith BW, Anderson KA. PAH and OPAH Flux during the Deepwater Horizon Incident. Environmental Science & Technology. 2016;50:7489–7497. doi: 10.1021/acs.est.6b02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Wang X, Gong P, Yao T. Polycyclic aromatic hydrocarbons in surface soil across the Tibetan Plateau: spatial distribution, source and air-soil exchange. Environmental Pollution. 2014;184:138–144. doi: 10.1016/j.envpol.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Wang X, Ren J, Gong P, Yao T. Using a passive air sampler to monitor air-soil exchange of organochlorine pesticides in the pasture of the central Tibetan Plateau. Science of the Total Environment. 2017;580:958–965. doi: 10.1016/j.scitotenv.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Simonich S, Giri B, Chang Y, Zhang Y, Jia Y, Tao S, Wang R, Wang B, Li W, Cao J, Lu X. Atmospheric concentrations and air-soil gas exchange of polycyclic aromatic hydrocarbons (PAHs) in remote, rural village and urban areas of Beijing-Tianjin region, North China. Science of the Total Environment. 2011;409:2942–2950. doi: 10.1016/j.scitotenv.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Luo C, Wang S, Cheng Z, Li J, Zhang G. The Abandoned E-Waste Recycling Site Continued to Act As a Significant Source of Polychlorinated Biphenyls: An in Situ Assessment Using Fugacity Samplers. Environmental Science & Technology. 2016;50:8623–8630. doi: 10.1021/acs.est.6b01620. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Luo C, Wang S, Liu J, Pan S, Li J, Ming L, Zhang G, Li X. Assessment of the air-soil partitioning of polycyclic aromatic hydrocarbons in a paddy field using a modified fugacity sampler. Environmental Science & Technology. 2015;49:284–291. doi: 10.1021/es5040766. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Deng S, Liu Y, Shen G, Li X, Cao J, Wang X, Reid B, Tao S. A passive air sampler for characterizing the vertical concentration profile of gaseous phase polycyclic aromatic hydrocarbons in near soil surface air. Environmental Pollution. 2011;159:694–699. doi: 10.1016/j.envpol.2010.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.