Abstract
The meiotic cell cycle is required for production of fertilization-competent gametes. Germ cell meiotic commitment requires expression of Stimulated by retinoic acid gene 8 (Stra8), which is transcriptionally activated by retinoic acid (RA). Meiotic suppression in embryonic male germ cells is believed to result from sex-specific differences in CYP26B1-catalyzed RA metabolism in the developing gonads. Here we show in mice that RA-induced Stra8 transcription is epigenetically controlled and requires a co-activator that binds proximal to the RA response elements (RAREs) in the Stra8 promoter. Embryonic male germ cells exposed in utero to the class I/II histone deacetylase (HDAC) inhibitor, trichostatin-A (TSA), show premature Stra8 activation and meiotic entry without altered Cyp26bl expression. We also show that Stra8 expression is detectable and physiologically regulated in adult mouse ovaries. Further, oogenesis induction in adult females using TSA is associated with Stra8 activation, and these events are absent in mice deficient in the RA precursor vitamin A. Finally, all of the actions of TSA in premeiotic germ cells in vitro and in mouse ovaries in vivo can be reproduced with the small molecule HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA). Thus, the ability of RA to transcriptionally induce expression of the meiosis-commitment gene, Stra8, is epigenetically controlled and this process involves a novel co-activator that functions upstream of the RAREs. These events not only coordinate the sex-specific timing of meiotic entry during embryogenesis, but also contribute to the regulation of oogenesis in adult female mammals.
Keywords: histone deacetylase, epigenetics, retinoic acid, Stra8, germ cell, oogenesis, spermatogenesis, meiosis, ovary, testis
Introduction
Germline development in mammals has traditionally been viewed under the assumption that females differ from males in their inability to produce new germ cells during postnatal life.1 However, a growing body of evidence has challenged the belief that a non-renewable stock of oocytes enclosed within follicles is endowed in the ovaries at birth.2 For example, recent studies with mice have demonstrated that the resting (primordial) follicle pool, once established, remains stable through the first 100 days of life despite the loss of hundreds, if not thousands, of follicles through atresia during this time period.3,4 This stability of the follicle reserve is likely explained by findings that the primordial follicle pool is replenished in adult mice during each 4–5 day reproductive cycle.5,6 Evidence for the production of new oocytes during adulthood has also been derived by monitoring primordial follicle regeneration following an insult that initially depletes the pool, using either doxorubicin-exposed mice or etoposide-treated rats as models.6,7 Further, injection of adult female mice with the class I/II histone deacetylase (HDAC) inhibitor, trichostatin-A (TSA), increases primordial follicle numbers, with the greatest effects observed in aging mice that possess a diminished follicle reserve.6
In addition to this body of work, genetic studies have identified cyclin-dependent kinase-5 and Abl enzyme substrate-1 (Cables1), which encodes a cell cycle-regulatory protein, as a critical gene involved in constraining the rate of oocyte renewal in adult mouse ovaries.8 Interestingly, the increased oocyte generation observed in adult Cables1-null females is offset by decreased oocyte quality, as reflected by elevated atresia of immature oocytes and increased fragmentation of mature oocytes retrieved after superovulation.8 Despite the clear implications of all of these studies, isolation of premeiotic germ cells capable of supporting oogenesis in adult ovaries remained elusive until just recently. Using criteria established in an earlier study to mark replicating germline cells in ovarian tissue of juvenile and young adult mice,3 Ji Wu and colleagues successfully purified germline stem cells (GSCs) from neonatal and adult mouse ovaries.9 In addition to showing that these cells can be propagated in vitro for months, transplantation of these cells into chemotherapy-conditioned adult female recipients leads to generation of developmentally-competent oocytes that produce viable offspring.9 Although representing an important step in further establishing that adult female mammals retain the capacity to produce new oocytes, the signaling pathways that control oocyte formation during adulthood remain obscure.
The events that coordinate the transition of premeiotic germ cells into oocytes during fetal ovarian development remain incompletely described as well. However, several studies have proposed that retinoic acid (RA) serves as the key molecular switch that underpins the sex-specific timing of meiotic entry in the mammalian embryonic gonads.10,11 The central premise of this theory is that the relatively synchronous meiotic entry of germ cells observed to occur in mouse fetal ovaries between embryonic days (e) 12.5 and e16.5 is triggered by the presence of RA.10–12 In contrast, meiosis is believed to be actively prevented in germ cells of the embryonic testes by a sex-specific elevation in expression of the retinoid-metabolizing enzyme, CYP26B1 (also known as P450RAI-2), which degrades RA and thus prevents meiotic entry in males until puberty.13 Other evidence suggests that this current explanation for the sex-specific timing of meiotic entry based on local availability of RA may be oversimplified.
For example, e13.5 female gonads contain approximately 4-fold higher levels of RA than e13.5 male gonads, when measured by an F9 premeiotic germline cell bioassay.10 While supportive of the aforementioned theory, bioactive RA is still detectable in embryonic male gonads at levels that can activate an RA-responsive promoter when Cyp26b1 expression is at its peak and meiosis is thought to be kept at bay by the resulting degradation of RA.10 It may be that a threshold level of RA, which is not met in the embryonic testes, is needed to activate meiosis; however, as little as 10 nM RA can trigger the events needed for meiotic commitment in germ cells.13 In addition, embryonic testes reportedly show an 80% decline in Cyp26b1 expression between e13.5 and e14.5,10 at which time increased expression of Nanos2 is believed to take over the active suppression of meiosis in male germ cells in the face of waning RA metabolic activity.14 Although such a model is reasonable to explain how male germ cells continue to escape meiotic entry during the latter stages of embryonic development when Cyp26b1 expression levels decline, it does not clarify why male germ cells in e12.5 gonads, which exhibit the same low level of Cyp26b1 expression as that observed at e14.5,10 fail to enter meiosis. Along these same lines, germ cells isolated from XY gonads at e11.5 versus e12.5 contrast sharply in their ability to enter meiosis when maintained in non-gonadal tissue aggregates, with e11.5, but not e12.5, male germ cells capable of meiotic entry.15 Such findings, when considered with the fact that Cyp26b1 is expressed at comparable levels in male gonads at e11.5 and e12.5,10 further suggest that a genetic or epigenetic switch, rather than or in addition to simply changes in RA metabolic capacity, somehow deactivates the molecular machinery required for RA-induced meiotic entry in XY, but not in XX, germ cells.
Despite these questions regarding the importance of RA metabolic activity in sex-specific meiotic entry during embryo-genesis, other work has established an unequivocal role for the protein encoded by Stimulated by retinoic acid gene 8 (Stra8) as an inducer of meiotic commitment.13,16 In addition to data showing exclusive expression of Stra8 in fetal ovarian germ cells just prior to their transition into meiosis,12 gene knockout studies have demonstrated that Stra8 is required for premeiotic DNA synthesis, meiotic initiation and meiotic progression in germ cells of both sexes.17–19 Consistent with the central role that RA is believed to play in vertebrate germline development and the sex-specific timing of meiotic entry, RA appears to function as the principal inducer of Stra8 expression in early germ cells.10,11,13,18 A recent study reported that oogenesis can be induced in adult female mice by the HDAC inhibitor TSA,6 indicating that his-tone acetylation status may dictate whether germ cells can enter meiosis. Therefore, the present study was designed to explore this possibility in more detail and to assess whether HDACs and RA interact to control the meiotic cell cycle.
Results
Epigenetic regulation of Stra8 expression in premeiotic germ cells
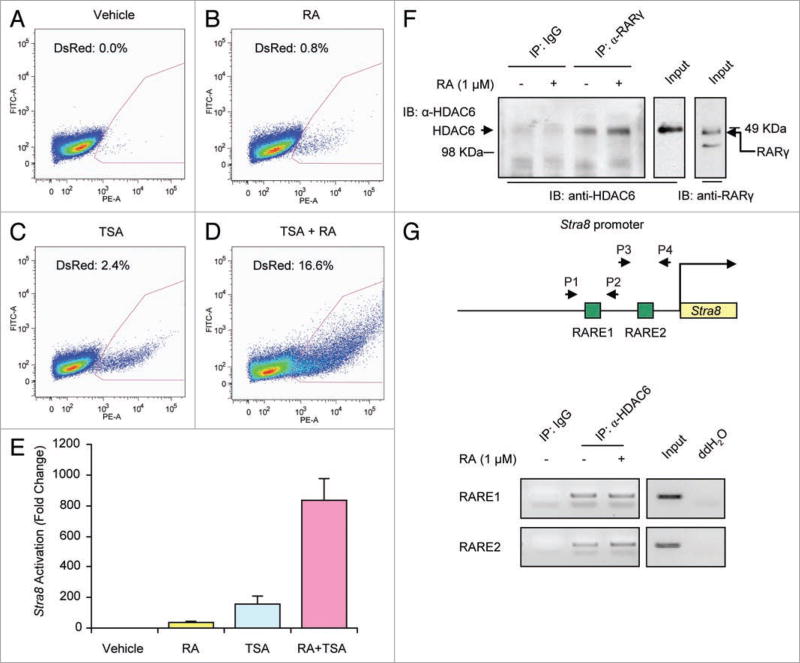
We first determined if expression of Stra8, which is required for gametogenesis,17–19 is under epigenetic control. We isolated 1.4 kb of the murine Stra8 promoter (pStra8) and cloned this fragment into a DsRed2 reporter vector that was stably introduced into F9 premeiotic germ cells. Treatment of F9pStra8-DsRed2 cells with either RA or TSA resulted in a small but detectable level of Stra8 activation (Fig. 1A – C). When added together, RA and TSA synergistically activated the Stra8 promoter (Fig. 1D and E). Mutagenesis of the RAREs abolished the induction of Stra8 by RA and TSA (Suppl. Fig. S1), indicating that HDAC activity modulates RA signaling at the level of the Stra8 promoter. No effects were noted in F9 cells expressing the DsRed2 reporter vector alone (Suppl. Fig. S1), confirming that the effects TSA and RA in F9 cells reflected actions on the Stra8 promoter. Biochemical assays showed that endogenous HDACs interact with endogenous RA receptors (RARs) (Fig. 1F) and co-precipitate with the endogenous Stra8 promoter (Fig. 1G) in premeiotic germ cells. These findings further support the notion that HDACs and RA interact to regulate Stra8 transcription, possibly through direct epigenetic silencing of the Stra8 locus by HDAC activity.
Figure 1. HDAC inhibition amplifies RA-induced Stra8 promoter activation.
(A–E) Flow cytometric analysis of DsRed2 expression (red quadrant, percent positive cells indicated) in F9pStra8-DsRed2 cells treated with vehicle, RA (1 µM), TSA (50 nM), or RA plus TSA for 24 h (E, complied data; n = 3). (F) Co-immunoprecipitation analysis of endogenous HDAC6 and RARγ interaction in F9 cells treated with vehicle or RA (I µM) for 4 h (Ip, immunoprecipitation antibody; IB, immunoblot antibody; Input, cell lysate). (G) ChIP analysis showing interaction of HDAC6 with the endogenous Stra8 promoter in soluble chromatin from lysates of F9 cells treated with vehicle or RA (1 µM) for 4 h (P1 and P2, primers for RARE1 region; P3 and P4, primers for RARE2 region).
Stra8 promoter deletion mutants uncover a broader mechanism for TSA action
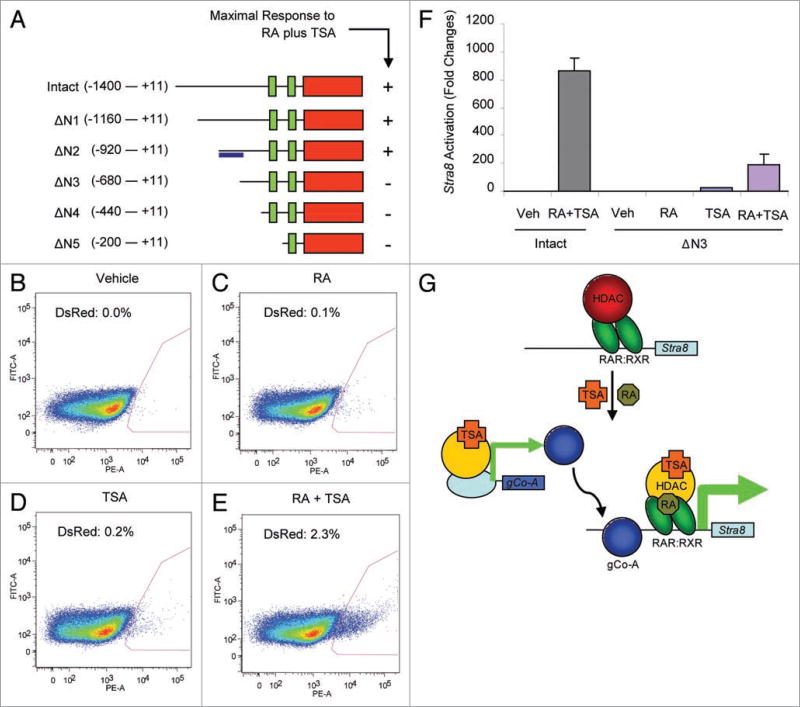
To gain further mechanistic insight into how HDAC activity might influence Stra8 gene expression, we performed deletion mutation analysis of the Stra8 promoter. In F9 cells stably expressing progressively-truncated Stra8 promoter constructs, we identified a 240-bp region just upstream of the RAREs that is required for TSA and RA to maximally activate Stra8 expression (Fig. 2A). However, elimination of this region did not lead to robust activation of Stra8 in the presence of RA alone (Fig. 2B – F). These findings indicate that this region of the Stra8 promoter likely houses a binding site for a TSA-sensitive co-activator of RA-mediated Stra8 transcription in germ cells that is under epigenetic constraint in the presence of HDAC activity. A more complicated model for meiotic commitment therefore emerged, likely involving not just HDAC-dependent modification of the Stra8 locus but also a novel HDAC-repressed co-activator that maximizes RA-induced Stra8 transcription when HDAC activity is alleviated (Fig. 2G).
Figure 2. Mechanism for HDAC repression of Stra8 expression in germ cells.
(A) Deletion mutation analysis of the Stra8 promoter identifies a small region (blue bar) necessary for activation by RA plus TSA in F9 cells stably expressing the indicated constructs and treated for 24 h (response measured, DsRed2 expression). (B–F) Flow cytometric analysis of DsRed2 expression (red quadrant, percent positive cells indicated) in F9 cells stably expressing the truncated ΔN3-Stra8 promoter-DsRed2 vector after treatment with vehicle, RA (1 µM), TSA (50 nM), or RA plus tSA for 24 h (F, complied data, n = 3). (G) Working model for epigenetic control of meiotic commitment in germ cells (gCo-A, putative germ cell-specific co-activator).
TSA disrupts sex-specific timing of embryonic germ cell meiotic commitment
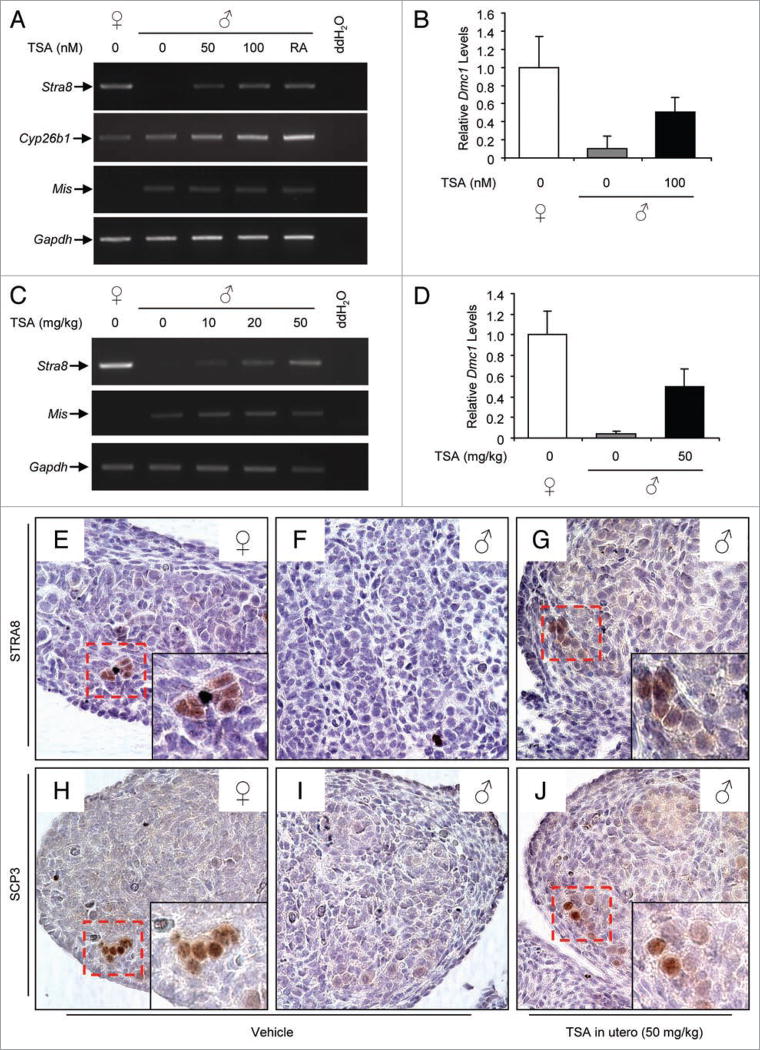
To assess the consequences of HDAC inhibition on embryonic germ cell differentiation, male gonads were isolated at e13.5, which marks the developmental window that a testis-specific elevation in Cyp26b1 expression is believed to protect male germ cells from the meiosis-promoting actions of RA.13 Following in-vitro culture for 24 h with vehicle, Stra8 expression was undetectable. However, exposure of e13.5 male gonads to TSA resulted in a dose-related increase in Stra8 expression without an alteration in Cyp26b1 expression (Fig. 3A); the latter finding indicates that RA-metabolic capacity of the embryonic testes was unaffected by HDAC inhibition. Moreover, e13.5 ovaries cultured in parallel and run initially as a negative control were found to express relatively high levels of Cyp26b1 (Fig. 3A), contrasting an earlier report that Cyp26b1 expression is not detectable in this tissue.11 Timed-pregnant female mice were then injected with vehicle or TSA at e13.5 and fetuses were collected 24 h later for analysis. Stra8 expression was detected in ovaries, but not testes, of e14.5 fetuses exposed in utero to vehicle (Fig. 3C, E and F). However, testes of e14.5 fetuses exposed to TSA showed a dose-dependent increase in Stra8 mRNA levels (Fig. 3C), and possessed STRA8-positive germ cells (Fig. 3G; Suppl. Fig. S2). The effects of TSA appeared specific for Stra8, since no change in expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) or Müllerian-inhibiting substance (Mis), which is expressed in embryonic male but not female gonads, was observed in TSA-exposed testes (Fig. 3C). The induction of Stra8 in embryonic male germ cells following HDAC inhibition was also associated with increased expression of Dmc1 (Fig. 3B and D), and the appearance of synaptonemal complex protein 3 (SCP3)-positive germ cells (Fig. 3J), both of which mark meiotic progression.20 We then performed gene profiling analysis of e14.5 gonads from both males and females to assess the possibility of sex-specific Hdac family member gene expression. These studies showed that Hdac5 is more abundantly expressed in male versus female embryonic gonads (Suppl. Fig. S3), uncovering a potential role for HDAC5 in coordinating the sex-specific timing of meiotic entry during embryogenesis.
Figure 3. HDAC inhibition induces premature Stra8 activation and meiotic entry in embryonic male germ cells.
(A) expression of Stra8, Cyp26b1, Mis or Gapdh in gonads of e14.5 male fetuses cultured for 24 h with increasing concentrations of TSA or a high concentration of RA (0.8 µM). Analysis of e14.5 ovaries cultured in parallel are provided as a control (ddH2O, negative control water blank for PCR amplification). (B) Quantitative PCR analysis of Dmc1 expression in male gonads exposed to TSA (β-actin used as sample loading control). (C) expression of Stra8, Mis or Gapdh mRNA levels in gonads of e14.5 female and male fetuses exposed in utero to increasing doses of TSA 24 h earlier. (D) Quantitative PCR analysis of Dmc1 expression in gonads of male fetuses exposed to TSA in utero (β-actin used as sample loading control). (E–J) Immunohistochemical detection of STRA8 (E–G) or SCp3 (H–J) expressing cells (brown) in gonads of e14.5 female (E and H) or male (F, G, I and J) fetuses exposed in utero to vehicle (E, F, H and I) or 50 mg/kg TSA (G and J) 24 h earlier, highlighting the premature induction of STRA8 and meiotic entry in embryonic male germ cells by TSA (insets).
Expression of endogenous Stra8 in adult mouse ovaries
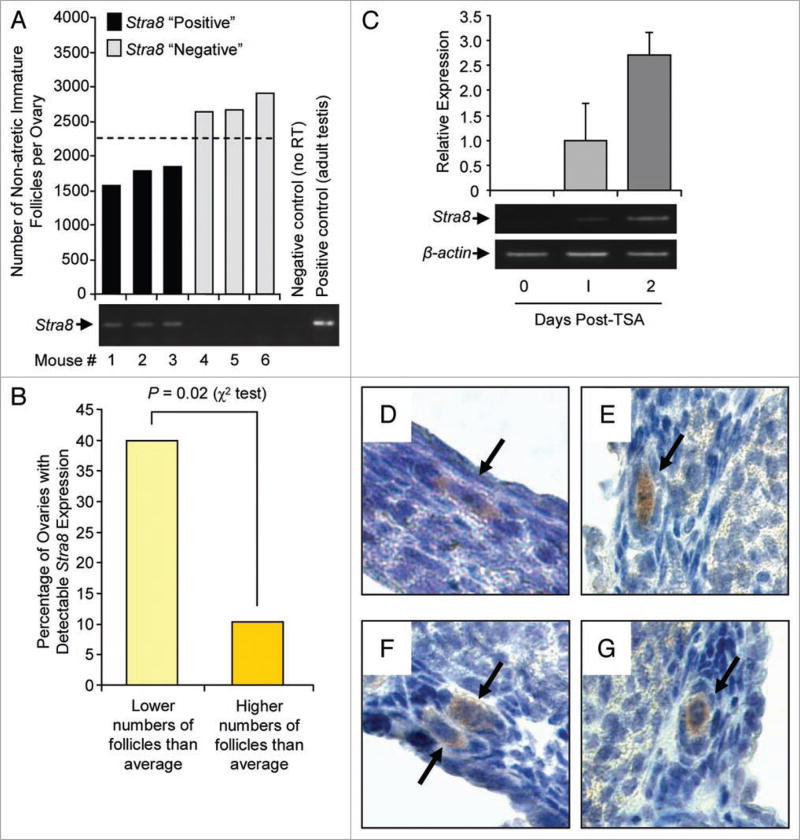
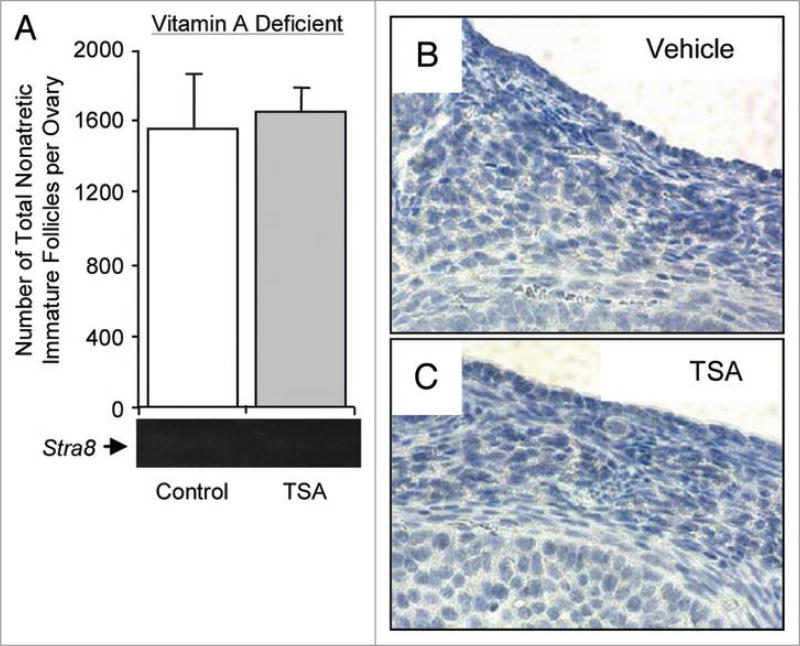
To next assess if fundamental signaling pathways involved in the control of embryonic germ cell differentiation are also operative in the regulation of postnatal oogenesis, we collected ovaries from sixty female mice at 60 days of age. One ovary from each mouse (right and left randomized) was used to quantify oocyte-containing immature (primordial, primary, preantral) follicle numbers, from which we determined a mean (±SEM) number of 2,360 (±815) per ovary. Each ovary was then designated as having a higher or lower than average number of follicles. The contralateral ovary from each mouse was used for gene expression analysis. We observed that Stra8 mRNA was detectable in about 25% of the 60 ovaries examined. When follicle numbers in contralateral ovaries were compared, 40% of ovaries with lower than average follicle counts expressed detectable levels of Stra8 mRNA, whereas only 10% of ovaries with higher than average counts showed detectable Stra8 expression (Fig. 4A and B). Thus, Stra8 is expressed more frequently in ovaries with lower than average follicle numbers, presumably on the verge of estrous cycle-related replenishment.5,6 Induction of oogenesis in adult female mice with TSA was associated with increased Stra8 expression (Fig. 4C; Suppl. Fig. S4) and the appearance of STRA8-immunopositive cells that were either not contained within discernible follicles or resembled early oocytes in primordial follicles (Fig. 4D–G; Suppl. Fig. S5; see also Suppl. Fig. S6 for antibody specificity controls). Quantitative estimates identified between 20–35 STRA8-positive cells per ovary, indicating that TSA did not cause a generalized inductive response in preexisting oocytes since less than 2% of the most immature (primordial) follicle pool contained STRA8-positive oocytes. Further, many STRA8-positive cells detected after TSA exposure exhibited an elongated morphology (Fig. 4D and E) that was distinct from the ovoid appearance of follicle-enclosed STRA8-positive oocytes (Fig. 4F and G). These endpoints were absent in female mice maintained on a diet deficient in the RA precursor, vitamin A (Fig. 5).
Figure 4. Stra8 is expressed and regulated in adult mouse ovaries.
(A and B) Relationship between immature follicle numbers and Stra8 expression in ovaries of individual mice at 60 days of age (6 randomly selected mice are shown; mean immature follicle number per ovary from analysis of 60 mice is indicated by the dashed line). Stra8 mRNA is more frequently detected in ovaries with lower than average immature follicle numbers. (C) elevated ovarian Stra8 expression, as assessed by quantitative pCR (β-actin, sample loading control), following oogenesis induction in adult female mice with TSA (10 mg/kg body weight). (D–G) Immunohistochemical detection of STRA8-positive cells (brown) in adult mouse ovaries 24 h after injection of TSA (10 mg/kg body weight). Confirmation of antibody specificity was performed using Stra8-null testis tissue analyzed in parallel (Suppl. Fig. S6).
Figure 5. In vivo induction of Stra8 expression and oogenesis in adult ovaries by HDAC inhibition requires RA.
(A) TSA fails to induce oogenesis or ovarian Stra8 expression in 6-month-old female mice maintained on a vitamin A-deficient diet (mean ± SEM, n = 4–6 mice per group). (B and C) Immunohistochemical analysis of STRA8 expression (brown) in ovaries of vehicle. (B) or TSA-treated (C) adult female mice maintained on a vitamin A-deficient diet, showing an absence of immunopositive cells in the cortical region (compare with Fig. 4D–G).
Confirmation that HDAC inhibition promotes Stra8 expression and oogenesis
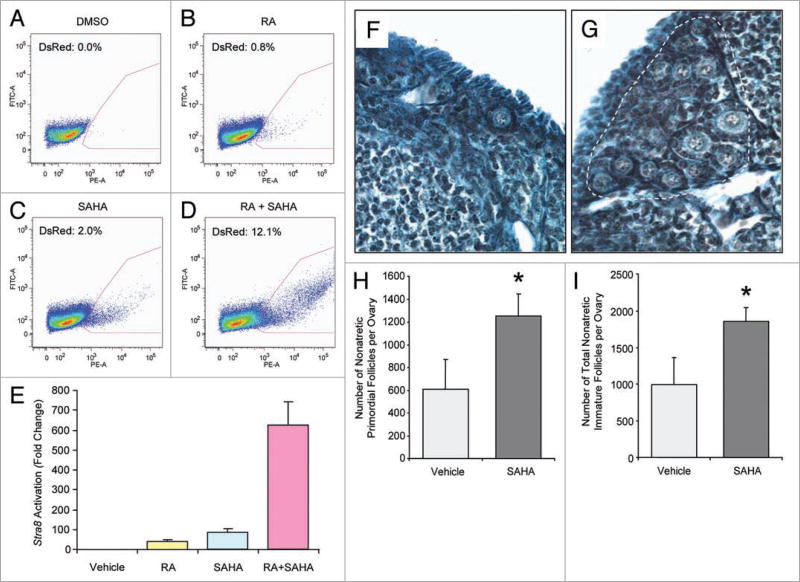
In support of the specificity of the TSA effects, a second HDAC inhibitor termed suberoylanilide hydroxamic acid (SAHA)21 was found to reproduce the actions of TSA on Stra8 activation in premeiotic germ cells (Fig. 6A – E) and on the oogenic activity of TSA in adult female mice (Fig. 6F – I). Moreover, in serially-sectioned ovaries of adult females exposed to vehicle or TSA, we observed that the early oocyte marker SOHLH1 (ref. 22), the diplotene stage-specific oocyte marker MSY2 (ref. 23), and the oocyte-specific maternal effect gene product MATER (ref. 24) were uniformly detected in all oocytes in both vehicle- and TSA-treated animals (Suppl. Fig. S7). Although the fate of newly generated oocytes in adult females injected with HDAC inhibitors remains unknown, these cells possess the same morphological and gene expression characteristics of follicle-enclosed immature oocytes present prior to TSA treatment.
Figure 6. SAHA mimics the effects of TSA on Stra8 expression and oogenesis.
(A–E) Flow cytometric analysis of DsRed2 expression (red quadrant, percent positive cells indicated) in F9pStra8-DsRed2 cells treated with vehicle, RA (1 µM), SAHA (1.6 µM), or RA plus SAHA for 24 h (E, complied data; n = 3). (F and G) Histological appearance of ovaries from 6-month-old female mice 24 h after injection with vehicle (F) or SAHA (G; 150 mg/kg body weight), with large clusters of primordial oocytes in SAHA-treated ovaries highlighted (white dashed line). (H and I) Number of oocyte-containing primordial (H) or total immature (I) follicles in 6-month-old female mice 24 h after injection with vehicle or SAHA (mean ± SEM, n = 5 mice per group; *p < 0.05 versus vehicle).
Discussion
While still incompletely described, progress has been made over the past several years in elucidating mechanisms involved in germ cell differentiation and meiotic competency. Recent studies of mammalian (principally mouse) and avian systems has produced a basic working model where RA metabolic activity is believed by many to be the key rate-limiting factor that dictates whether early germ cells enter meiosis.10,11,13,25 One of the main target genes of RA in this context is Stra8, which encodes a germline-specific protein required for meiotic competency in both male and female germ cells.17–19 Our results add a new level of complexity and fine-tuned control to this model by showing that activation of the Stra8 promoter in premeiotic germ cells is repressed through an epigenetic mechanism involving histone deacetylation. When this tonic repression is alleviated by suppressing HDAC function, RA is able to fully induce transcription of the Stra8 gene. Hence, the decision of germ cells to enter meiosis is determined not only by the local availability of RA but also by epigenetic changes in chromatin conformation within germ cells that reflect histone acetylation status.
This conclusion is fully supported by the premature activation of Stra8 observed in germ cells of embryonic testes following TSA exposure, at a developmental time point when sex-specific Stra8 expression and meiotic commitment are thought to be governed by local RA metabolism.10,11,13 In fact, our assessment of Cyp26b1 in embryonic gonads failed to show the large sex-specific difference in expression reported in previous studies.10,11 Although the basis of this discrepancy is unknown, these data along with our new observations that HDAC activity profoundly influences Stra8 expression raise questions over past claims that RA metabolic capacity serves as the principal determinant underlying the sex-specific entry of germ cells into meiosis during embryogenesis. It should also be noted that recent studies using adult male mice reported that TSA exposure disrupts spermatogenesis, although this outcome was attributed to global changes in gene expression associated with germ cell development and survival.26,27
Our results also offer a reasonable explanation of how RA-mediated signaling, which is pleiotropic and operative in so many tissues during embryogenesis, can selectively induce expression of the Stra8 gene only in the germline. This has been one of the most puzzling questions regarding mammalian germ cell development since the identification of the germline-specificity of RA-driven Stra8 gene expression more than a decade ago.13,16 In fact, our findings provide the first direct experimental data supporting recent suggestions that a unique epigenetic configuration in germ cells may explain why RA cannot induce Stra8 in somatic cells.13 Determination of how HDAC activity represses Stra8 activation was derived from several observations. The initial clue was provided by co-immunoprecipitation experiments showing that HDACs directly interact with RARs in premeiotic germ cells. Past studies have shown that unbound RARs can repress gene transcription by recruiting HDACs through two co-repressors, termed Silencing mediator of retinoid and thyroid receptor (SMRT) and nuclear receptor co-repressor (N-CoR). This interaction can be reversed by the presence of high levels of RA, leading to a relaxed chromatin state permissive to transcription.28–30 Our finding that embryonic male gonads exposed in vitro to high concentrations of RA induce Stra8 expression to levels comparable to those observed when e13.5 testes are exposed to an HDAC inhibitor is consistent with this model.
In assessing how HDACs might repress Stra8 activation, we also unintentionally uncovered evidence of a critical regulatory region in the Stra8 promoter, just upstream of the RAREs, that likely binds a co-activator needed for RA to maximally induce Stra8 gene transcription in germ cells. Although the identity of this putative co-activator remains to be established, our findings along with past work from others lend strong support for its existence and perhaps its germline specificity. For example, transgenic mice expressing a truncated region of the Stra8 promoter spanning nucleotides −371 to +29 and driving luciferase show extragonadal expression of the transgene,31 contrasting the gonadal-specific expression observed when the larger (1.4-kb) promoter fragment is used for transgenesis.32 Interestingly, the 0.4-kb truncated promoter that drives transgene expression outside of the gonads31 possesses both RAREs but lacks the same upstream region deleted in our study that is needed for RA to maximally induce Stra8 activation in premeiotic germ cells. It therefore seems reasonable to postulate that this putative co-activator is a germ cell-specific protein that interacts with the Stra8 promoter not only to facilitate RA-induced transcription but also to convey germline specificity of Stra8 expression following induction by a ubiquitous signaling molecule like RA. Our current efforts are directed at isolation and characterization of this putative co-activator to address these questions.
Another important implication of this work relates the increasing body of evidence countering the longstanding belief that mammalian females are incapable of generating new oocytes during adulthood.2 In addition to follicle-counting studies demonstrating the mathematical improbability of a non-renewable oocyte pool being established at birth in rodents and primates,3,4,33 evidence supporting postnatal oocyte renewal in mammals has been derived by a variety of other approaches,3,5–8 including most recently the purification of what are believed to be GSCs from neonatal and adult mouse ovaries.9 The present investigation has further bolstered this conclusion by providing insights into the molecular cues that control the activation of Stra8 and oogenesis in ovaries of adult female mice. Perhaps not surprisingly, RA emerged as a regulator of both processes in the adult ovary, similar to its role in promoting Stra8 expression and oocyte formation during fetal ovarian development.13 However, our findings also indicate that HDAC activity, in addition to RA bioavailability, is a key determinant of oogenesis. Further, we have observed significant increases in HDAC activity in the ovaries as females age (Suppl. Fig. S8). These findings predict an even greater tonic repression of Stra8 expression and, consequently, oogenic potential might occur in aging females, and this may contribute to the rapid decline in follicle numbers observed later in life that ultimately drives age-related ovarian failure.
Finally, it is worth mentioning that the induction of Stra8 promoter activity, as measured by DsRed2 expression in F9pStra8-DsRed2 cells, not only produced novel mechanistic insight into the epigenetic control of RA-regulated Stra8 expression but accurately predicted the oogenic potential of pharmacologic agents in adult female mice injected in vivo. The future use of F9pStra8-DsRed2 cells in high-throughout screening assays may therefore provide a novel means to identify additional small molecules that can be tested for their ability to promote de-novo oocyte formation during adulthood. Such experiments, coupled with our current efforts to isolate and characterize the putative co-activator of RA-induced Stra8 expression in germ cells, will help continue to help assemble a more complete molecular blueprint for how such key events as Stra8 expression, the sex-specific timing of embryonic germ cell meiotic commitment, and postnatal gametogenesis are regulated.
Materials and Methods
Animals and treatments
Wild-type C57BL/6 female mice (non-pregnant or timed-pregnant) and male mice were obtained from Charles River Laboratories (Wilmington, MA) or the National Institute on Aging (NIH, Bethesda, MD). Vitamin A deficiency was induced by maintaining female mice on a vitamin A-deficient diet (TD88407; Harlan Laboratories, Indianapolis, IN) for 5 months and 1 week, starting at 3 weeks of age. All-trans RA and TSA were obtained from Sigma (St. Louis, MO), whereas SAHA was obtained from Biomol (Plymouth Meeting, PA). All three agents were dissolved in dimethylsulfoxide (DMSO) and given as a single intraperitoneal injection at the following concentrations: RA, 100 mg/kg body weight; TSA, 10–50 mg/kg body weight, depending on the model studied (see figure legends); SAHA, 150 mg/kg body weight. The institutional animal care and use committee of Massachusetts General Hospital reviewed and approved all animal procedures.
Histology and oocyte (follicle) counts
Ovaries were fixed in a solution composed of 0.34 N glacial acetic acid, 10% formalin and 28% ethanol, and embedded in paraffin. Serial sections were cut (8 µm), aligned in order on glass slides, and stained with hematoxylin-picric acid methyl blue for morphometry-based determination of the number of non-atretic primordial and total immature (primordial, primary, preantral) follicles per ovary.34,35
Organ cultures
Embryonic gonad cultures were performed essentially as described.11,36,37 Briefly, timed-pregnant mice female were euthanized on e13.5, and embryos were collected into ice-cold 1X-concentrated phosphate-buffered saline (PBS). Gonads were dissected from each embryo and cultured atop agar blocks, which had been pre-equilibrated with culture medium consisting of DMEM, 10% bovine calf serum (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin for at least 1 h. Embryonic gonads were cultured for 24 h in the presence of the indicated treatments, and then collected in RNA Later extraction buffer (Ambion, Austin, TX). Total RNA was purified using RNeasy Micro plus kit (Qiagen, Valencia, CA).
Gene expression
Total RNA was extracted using Tri-Reagent (Sigma) or RNA Later, and 1 µg of total RNA was reverse transcribed (Superscript II; Invitrogen) using oligo-dT primers. PCR amplification was performed using Taq polymerase and Buffer-D (Epicentre, Madison, WI) with primers for mouse Stra8 (NM_009292: forward, 5'-GCC AGA ATG TAT TCC GAG AA-3'; reverse, 5'-CTC ACT CTT GTC CAG GAA AC-3'; 651-bp product), mouse Mis (NM_007445: forward, 5'-TTG CTG AAG TTC CAA GAG CC-3'; reverse, 5'-TTC TCT GCT TGG TTG AAG GG-3'; 231-bp product) or mouse Cyp26b1 (NM_175475: forward, 5'-TTC TCT CTG CCA GTG GAC CT-3'; reverse, 5'-CGT GAG TGT CTC GGA TGC TA-3'; 538-bp product). As loading controls, mouse β-actin (NM_007393: forward, 5'-GAT GAC GAT ATC GCT GCG CTG-3'; reverse, 5'-GTA CGA CCA GAG GCA TAC AGG-3'; 440-bp product) and Gapdh (NM_008084: forward, 5'-GTG TTC CTA CCC CCA ATG TG-3'; reverse, 5'-GTC ATT GAG AGC AAT GCC AG-3'; 215-bp product) were amplified in parallel. The primer sequences for Hdac1 through Hdac9 are provided in Supplementary Table S1. All products were sequenced for confirmation. For quantitative analysis of Stra8 mRNA levels, PCR was performed using a Cepheid Smart Cycler II and primers specific for Stra8 (FAM-labeled D-LUX™ Pre-designed Gene Expression Assays, MLUX3312362) and β-actin (FAM-labeled certified LUX™ Primer Set for Mouse/Rat β-actin, 101M-01) obtained from Invitrogen. Expression ratios were calculated as described.6
Immunohistochemistry
Ovaries, testes and eviscerated e14.5 fetuses were fixed in 4% neutral-buffered paraformaldehyde and embedded in paraffin. Tissues were sectioned for immunohistochemical analysis using antibodies specific for STRA8 (rabbit polyclonal ab49602; Abcam, Cambridge, MA),19 SOHLH1 (rabbit polyclonal ab49272; Abcam),22 MSY2 (provided by N.B. Hecht)23 or MATER (provided by J. Dean)24 after high-temperature antigen unmasking,38 with slight modifications recommended by each supplier. Specificity of the STRA8 antibody was confirmed by western blot analysis, as well as by parallel immunostaining analysis of testis tissue from adult wild-type and Stra8-null mice (provided by D.C. Page).17
Plasmid construction
A 1.4-kb fragment of the murine Stra8 promoter (spanning nucleotides −1400 to +11; +1, transcription start site) was amplified from C57BL/6 mouse genomic DNA. This fragment was inserted in the XhoI/ EcoRI site of the pDsRed2-1 expression vector (Clontech, Mountain View, CA) containing a neomycin-resistance gene. Site-directed mutagenesis of the RAREs was performed using a PCR-based site-directed mutagenesis kit from Stratagene (La Jolla, CA). Deletion mutants were generated by PCR. All constructs were sequenced to confirm fidelity prior to use.
Cell culture and reagents
F9 germline cells (ATCC CRL-1720; American Type Culture Collection, Manassas, VA) were grown at 37°C in a humidified atmosphere of 5% CO2-95% air on 0.1% gelatin-coated tissue culture plates in DMEM containing 4.5 g/l glucose and supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mM L-glutamine, 50 µg/ml penicillin and 50 µg/ml streptomycin. Cells were transfected using lipofectamine (Invitrogen) and then selected by G418 (Geneticin) at a concentration of 350 µg/ml over a period of 2 weeks. Cells were then maintained in G418 for all remaining experiments.
Fluorescence-activated cell sorting (FACS) analysis of DsRed2 expression
Following culture under the conditions specified, F9 cells were collected by trypsinization, centrifuged (150 × g for 10 min at 4°C), washed with 1X-concentrated PBS, and re-centrifuged. Resultant cell pellets were resuspended in 1X-concentrated PBS containing 0.2% FBS, and analyzed with a FACSAria cytometer (Harvard Stem Cell Institute, Boston, MA) to determine the percentage of DsRed2-positive cells, essentially as described.39
Co-immunoprecipitation and ChIP assays
Cells were harvested in lysis buffer [137 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl (pH 7.6), 1% Triton X-100, 10% glycerol] supplemented with 1 mM PMSF and an EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN). Co-immunoprecipitation was performed by incubating lysates with RARγ antibody (mouse monoclonal sc-7387; Santa Cruz Biotechnology) overnight at 4°C, followed by incubation with protein G-agarose beads (Roche) for 1 h at 4°C. After centrifugation (50 ×g for 1 min at room temperature), resultant pellets were then analyzed by western blotting using a rabbit polyclonal anti-HDAC6 antibody. Lysates were also analyzed by standard western blotting with each antibody independently (anti-RARy and anti-HDAC6) to assure the presence of the endogenous proteins. Normal mouse IgG was used as a negative control for immunoprecipitations. For ChIP assays, lysates were processed using the EZ-ChIP kit (Millipore, Temecula, CA) along with a rabbit polyclonal anti-HDAC6 antibody (07–732; Millipore) for immunoprecipitation. Precipitated soluble chromatin was analyzed by PCR using one of the following primer sets designed to amplify two separate regions (approximately 100-bp each) of the mouse Stra8 promoter containing each RARE sequence: RARE1 forward (5'-TGG CAT TGC CCT GGT TGA GGG G-3') and RARE1 reverse (5'-CAA CTT GCC ACA GGT TGC AAG AGG-3'), spanning nucleotides −265 to −160; or, RARE2 forward (5'-GTG ACA GGG CTG TGA TTG GTT CGC-3') and RARE2 reverse (5'-CTC ACG ACT GCC CGT CGC AG-3'), spanning nucleotides −87 to +11.
Western blotting
Total protein was isolated using a dounce homogenizer in extraction buffer [20 mM Tris-HCl (pH 7.6), 0.5% NaDOC, 150 mM NaCl, 1% Triton X-100, 0.1% SDS] supplemented with 1 mM PMSF and an EDTA-free protease inhibitor cocktail (Roche). Lysates were centrifuged at 14,000 ×g for 10 min at 4°C, and protein concentrations in supernatants were determined (DC protein assay; BioRad, Hercules, CA). Ten µg of protein from each sample, or the entire protein yield from 40 oocytes, was mixed with 4X-concentrated LDS sample buffer (Invitrogen) plus 10X-concentrated sample reducing agent (Invitrogen), and denatured for 10 min at 70°C. Proteins were resolved in 4–12% Bis-Tris gels (Invitrogen), and transferred to PDVF membranes. Blots were probed with antibodies against STRA8, MATER, β-actin (MS-1295-P; NeoMarkers, Fremont, CA), RARy or HDAC6, washed and reacted with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG. Detection was performed with the Amersham ECL™ Plus kit (GE Healthcare, Buckinghamshire, UK).
HDAC activity assay
Nuclear extracts were obtained from mouse ovaries using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL), and assessed for HDAC activity using a fluorometric assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s specifications. Fluorometric detection was performed with a Perkin Elmer Wallac1420 (VICTOR3) multi-label counter.
Data presentation and analysis
All experiments were independently replicated at least three times (see figure legends for details), using different mice, tissues or cells in each experimental replicate. Assignment of mice to each experimental group was made randomly. Quantitative data from the experimental replicates were pooled and are presented as the mean ± SEM. Compiled data were analyzed by two-tailed Student’s t-test or the χ2 test. Representative results from the FACS, histological, RT-PCR, immunohistochemical and immunoblotting analyses are provided for qualitative assessment.
Supplementary Material
Acknowledgments
We thank N.B. Hecht for MSY2 antibody; J. Dean for MATER antibody; D.C. Page for testes from adult wild-type and Stra8-null mice; J. Teixeira, K. Selesniemi and L. Prickett for technical assistance; and Y. Niikura for helpful discussions. This work was supported by National Institute on Aging MERIT Award R37-AG012279, the Rubin Shulsky Philanthropic Fund, the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial Research Funds. N. Wang is a recipient of a Massachusetts General Hospital Fund for Medical Discovery award.
Glossary
Abbreviations
- Cables1
cyclin-dependent kinase-5 and Abl enzyme substrate-1 gene
- ChIP
chromatin immunoprecipitation
- Dmc1
meiosis-specific homolog of E. coli RecA gene
- FBS
fetal bovine serum
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase gene
- GSC
germline stem cell
- HDAC
histone deacetylase
- MATER
maternal antigen that embryo requires
- Mis
müllerian-inhibiting substance gene
- MSY2
mammalian homologue of the Xenopus germ cell-specific nucleic acid-binding protein FRGY2
- N-CoR
nuclear receptor co-repressor
- PBS
phosphate-buffered saline
- pStra8
promoter region of stimulated by retinoic acid gene 8
- RA
retinoic acid
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- SAHA
suberoylanilide hydroxamic acid
- SCP3
synaptonemal complex protein 3
- SOHLH1
spermatogenesis and oogenesis specific basic helix loop helix transcription factor 1
- SMRT
silencing mediator of retinoid and thyroid receptor
- Stra8
stimulated by retinoic acid gene 8
- TSA
trichostatin-A
Footnotes
References
- 1.Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 2.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 4.Kerr JB, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 5.Allen E. Ovogenesis during sexual maturity. Am J Anat. 1923;31:439–82. [Google Scholar]
- 6.Johnson J, Bagley J, Skaznik-Wikiel M, Lee H-J, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells derived from bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borovskaya TG, Gol’dberg VE, Pakhomova AV, Perova AV, Timina EA. Morphological and functional state of rat ovaries in the early and late periods after injection of vepesid. Bull Exp Biol Med. 2006;141:645–57. doi: 10.1007/s10517-006-0242-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee H-J, Sakamoto H, Luo H, Skaznik-Wikiel ME, Friel A, Niikura T, et al. Loss of CABLES1, a cyclin-dependent kinase-interacting protein that inhibits cell cycle progression, results in germline expansion at the expense of oocyte quality in adult female mice. Cell Cycle. 2007;6:2678–84. doi: 10.4161/cc.6.21.4820. [DOI] [PubMed] [Google Scholar]
- 9.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 10.Bowles J, Knight D, Smith C, Wilhelm D, Richman JM, Mamiya S, et al. Sex-specific regulation of retinoic acid levels in developing mouse gonads determines germ cell fate. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 11.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–9. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–12. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 13.Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–11. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–5. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–13. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 16.Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, et al. Characterization of a pre-meiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1986;135:469–77. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baltus AE, Menke DB, Hu Y-C, Goodheart ML, Carpenter AE, de Rooij DG, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 18.Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AMM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–80. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Féret B, Vernet N, et al. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233–42. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- 20.Cohen PE, Pollack SE, Pollard JW. Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev. 2006;27:398–426. doi: 10.1210/er.2005-0017. [DOI] [PubMed] [Google Scholar]
- 21.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 22.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA. 2006;103:8090–5. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu W, Tekur S, Reinbold R, Eppig JJ, Choi YC, Zheng JZ, et al. Mammalian male and female germ cells express a germ cell-specific Y-box protein, MSY2. Biol Reprod. 1998;59:1266–74. doi: 10.1095/biolreprod59.5.1266. [DOI] [PubMed] [Google Scholar]
- 24.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26:267–8. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 25.Smith CA, Roeszler KN, Bowles J, Koopman P, Sinclair AH. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev Biol. 2008;8:85. doi: 10.1186/1471-213X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenic I, Hossain HM, Sonnack V, Tchatalbachev S, Thierer F, Trapp J, et al. In vivo application of histone deacetylase inhibitor trichostatin-A impairs murine male meiosis. J Androl. 2008;29:172–85. doi: 10.2164/jandrol.107.003848. [DOI] [PubMed] [Google Scholar]
- 27.Fenic I, Sonnack V, Failing K, Bergmann M, Steger K. In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis. J Androl. 2004;25:811–8. doi: 10.1002/j.1939-4640.2004.tb02859.x. [DOI] [PubMed] [Google Scholar]
- 28.Manggelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 29.Chambon P. A decade of molecular biology of retinoid receptors. FASEB J. 1996;10:940–54. [PubMed] [Google Scholar]
- 30.Minucci S, Ozato K. Retinoid receptors in transcriptional regulation. Curr Opin Genet Dev. 1996;6:567–74. doi: 10.1016/s0959-437x(96)80085-2. [DOI] [PubMed] [Google Scholar]
- 31.Giulli G, Tomljenovic A, Labrecque N, Oulad-Abdelghani M, Rassoulzadegan M, Cuzin F. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Reports. 2002;3:753–9. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayernia K, Li M, Jaroszynski L, Khusainov R, Wulf G, Schwandt I, et al. Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum Mol Genet. 2004;1:1451–60. doi: 10.1093/hmg/ddh166. [DOI] [PubMed] [Google Scholar]
- 33.Vermande Van-Eck G. Neo-ovogenesis in the adult monkey. Anat Rec. 1956;125:207–24. doi: 10.1002/ar.1091250205. [DOI] [PubMed] [Google Scholar]
- 34.Jones PB, Krohn PL. The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–95. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- 35.Skaznik-Wikiel M, Tilly JC, Lee H-J, Niikura Y, Kaneko-Tarui T, Johnson J, et al. Serious doubts over “Eggs Forever?”. Differentiation. 2007;75:93–9. doi: 10.1111/j.1432-0436.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 36.Morita Y, Manganaro TF, Tao X-J, Martimbeau S, Donahoe PK, Tilly JL. Requirement for phosphatidylinositol-3'-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology. 1999;140:941–9. doi: 10.1210/endo.140.2.6539. [DOI] [PubMed] [Google Scholar]
- 37.Morita Y, Tilly JL. Segregation of the actions of retinoic acid on fetal ovarian germ cell mitosis versus apoptosis by requirement for new macromolecular synthesis. Endocrinology. 1999;140:2696–703. doi: 10.1210/endo.140.6.6826. [DOI] [PubMed] [Google Scholar]
- 38.Matikainen T, Perez GI, Jurisicova A, Schlezinger JJ, Ryu H-Y, Pru JK, et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–60. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 39.Lee H-J, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.