Fifteen patients with exudative age-related macular degeneration were treated with intravitreal injections of ziv-aflibercept (1.25 mg). Ziv-aflibercept improved the best-corrected visual acuity, multifocal electroretinography amplitudes, and central retinal thickness from baseline to 26 weeks. No retinal toxicity on full-field electroretinography or adverse events occurred during the follow-up period.
Key words: ziv-aflibercept, age-related macular degeneration, full-field electroretinography, multifocal electroretinography
Purpose:
To evaluate the 6-month safety and efficacy of ziv-aflibercept intravitreal injections for treating exudative age-related macular degeneration.
Methods:
Fifteen patients with unilateral exudative age-related macular degeneration were enrolled. The best-corrected visual acuity was measured and spectral domain optical coherence tomography was performed at baseline and monthly. Full-field electroretinography and multifocal electroretinography were obtained at baseline and 4, 13, and 26 weeks after the first injection. All patients received three monthly intravitreal injections of ziv-aflibercept (1.25 mg) followed by as-needed treatment.
Results:
Between baseline and 26 weeks, the mean logMAR best-corrected visual acuity improved (P = 0.00408) from 0.93 ± 0.4 (20/200) to 0.82 ± 0.5 (20/160) logarithm of the minimum angle of resolution, respectively; the central retinal thickness decreased significantly (P = 0.0007) from 490.3 ± 155.1 microns to 327.9 ± 101.5 microns; the mean total macular volume decreased significantly (P < 0.0001) from 9.51 ± 1.36 mm3 to 8.08 ± 1.34 mm3, and the a-wave implicit time increased, with no differences in the other full-field electroretinography parameters. The average multifocal electroretinography macular responses within the first central 15° showed significantly (P < 0.05) increased P1 amplitudes at 26 weeks. No systemic or ocular complications developed.
Conclusion:
Intravitreal ziv-aflibercept significantly improved the best-corrected visual acuity, multifocal electroretinography amplitudes, central retinal thickness, and total macular volume from baseline to 26 weeks. No retinal toxicity on full-field electroretinography or adverse events occurred during the follow-up period.
Age-related macular degeneration (AMD) is the leading cause of legal blindness and central visual loss in industrialized nations, with patients above the age of 65 years primarily affected.1 Aflibercept, a recombinant fusion protein engineered to bind all isoforms of VEGF-A, VEGF-B, and placental growth factor, received U.S. Food and Drug Administration approval in November 2011 for treating exudative AMD.2,3 Ziv-aflibercept (Zaltrap; codeveloped by Sanofi-Aventis and Regeneron Pharmaceuticals, Inc), a fully humanized soluble recombinant fusion protein created by fusing extracellular Ig domain 2 of VEGFR-1 and extracellular Ig domain 3 of VEGFR-2 to the Fc (constant) region of human IgG1, was approved by the Food and Drug Administration in 2012 for treating metastatic colorectal carcinoma under intravenous administration.4,5 However, ziv-aflibercept has higher osmolarity compared with aflibercept, which was hypothesized to cause retinal toxicity.6,7
In an experimental rabbit model used to evaluate the retinal safety of intravitreal ziv-aflibercept, our research group showed that all eyes had normal funduscopic, tomographic, and electrophysiologic findings 24 hours and 7 days after one intravitreal injection of 0.05 mL (25 mg/mL).8 Intravitreal ziv-aflibercept also has been studied to treat various exudative macular disorders, including exudative AMD, diabetic macular edema (DME), and vein occlusion–related macular edema.9–17 Although the efficacy and lack of toxicity have been reported, a longer follow-up study of the treatment of exudative AMD has not been conducted.
Until now, intravitreal injections of various anti-VEGF drugs, including aflibercept, have been the best strategy for treating exudative AMD, but the price of the currently approved drugs must be considered. Ziv-aflibercept recently drew attention as an alternative treatment strategy for exudative AMD because it is cheaper than aflibercept. When a 4-mL bottle of ziv-aflibercept is fractioned in 30 doses (0.05 mL), it costs almost 30 times less than aflibercept. The aim of this study was to evaluate the safety and efficacy of intravitreal injections of ziv-aflibercept for treating exudative AMD during a 6-month follow-up period.
Methods
Study Design
This study was a prospective, interventional, nonrandomized clinical trial that adhered to the tenets of the Declaration of Helsinki. The Ethics Committee of the Federal University of São Paulo (Institutional Review Board number 707.034) and the National Research Ethics Commission (CONEP) approved the study protocol. This study was registered at clinicaltrials.gov (NCT02556723). All patients provided informed consent for the research before screening and after explanation of the nature and possible consequences of participation. The primary outcome was assessed at 26 weeks (approximately 6 months), and a longer follow-up is planned through 52 weeks (approximately 12 months). This study reports only the 26-week data.
The major eligibility criteria included patients above 50 years and the presence in the study eye (1 eye per patient) of active subfoveal or juxtafoveal choroidal neovascularization secondary to AMD. The main outcome measure was the safety of intravitreal ziv-aflibercept. The best-corrected visual acuity (BCVA) was not a major eligibility criterion, and patients with a low visual acuity were included. The first four patients included in this study were consecutive patients from the Retina Sector of the Department of Ophthalmology of the Federal University of São Paulo who have already been treated with bevacizumab at least 6 months before the beginning of the study. The other subjects were consecutive patients presenting exudative AMD coming from the Ophthalmology Emergency Room of the Federal University of São Paulo.
Patients were excluded if any of the following were present: other causes of choroidal neovascularization in either eye; bilateral active choroidal neovascularization; previous laser therapy or ocular surgery in the study eye such as macular translocation surgery, pars plana vitrectomy, glaucoma filtering surgery, verteporfin photodynamic therapy (Visudyne; QLT Inc, Vancouver, BC, Canada), and subfoveal focal laser photocoagulation; history of active uveitis; uncontrolled diabetes mellitus and hypertension; history of cerebrovascular accident or myocardial infarction within 6 months of study entry; renal failure requiring dialysis or renal transplant; pregnancy or lactation; or history of allergy to fluorescein or povidone-iodine.
Assessments
All patients were evaluated every 4 weeks. A retina specialist (V.F.K.) performed a comprehensive ophthalmic examination of both eyes that included measurement of the BCVA measured using the Early Treatment Diabetic Retinopathy Study chart, intraocular pressure measurements by Goldmann applanation tonometry, dilated fundus examination, slit-lamp examinations, and imaging with fundus photography, spectral domain optical coherence tomography (OCT), and fluorescein angiography (FA). Indocyanine green angiography was performed in doubtful cases to rule out other causes of choroidal neovascularization. Optical coherence tomography was performed at every visit; FA and fundus photography were performed at baseline and 26 weeks after treatment initiation.
Optical coherence tomography was performed with the Spectralis spectral domain OCT instrument (Heidelberg Engineering, Heidelberg, Germany). The central retinal thickness (CRT) was defined as the mean thickness of the neurosensory retina in a central 1-mm-diameter area, and total macular volume (TMV) was defined as the total volume of the scanned neurosensory retina covering a 20-degree × 20-degree (5.7 × 5.7 mm) macular volume cube. The eye-tracking feature of the Spectralis spectral domain OCT was used to evaluate the same area during the follow-up visits. Because the instrument error was inexact identification of the retinal layer segmentation, the incorrectly placed points were manually repositioned.
The Heidelberg Retina Angiograph 2 (Heidelberg Engineering) was used to perform FA and, in some cases, indocyanine green angiography. Fundus photography was performed using the Topcon TRC-50IX or Topcon TRC-50DX (Topcon, Tokyo, Japan). Multifocal (mfERG) and full-field electroretinography (ffERG) were performed at baseline and 4, 13, and 26 weeks after the treatment initiation (Veris Pro 6.3.2; EDI, Redwood City, CA). Adverse events were recorded at each visit.
The cataract status was recorded at baseline and 12 weeks and 26 weeks as a safety measure and as a possible confounding factor for visual acuity assessment. The intraocular pressure was measured at baseline and at 4, 8, 13, 17, 21, and 26 weeks.
The ffERGs were performed after minimal pupillary diameter of 6 mm obtained after instillation of 1 drop each of tropicamide 1% and phenylephrine 10%, and the subjects were dark-adapted for 30 minutes. The corneal surface was anesthetized with drops of tetracaine (1.0%) and, under dim red illumination, a bipolar contact lens electrode (Burian-Allen; Hansen Ophthalmic Laboratories, Iowa City, IA) was placed on the corneal surface. A drop of 2% methylcellulose was placed on the inside surface of the contact lens for protection and to ensure good electrical contact. A gold cup to ground the electrode was applied to the earlobe. The visual response imaging system software was used with the FMSIII stimulator running at 75 Hz (EDI). Dark-adapted 0.01 ERGs (rod response), dark-adapted 3.0 ERGs (combined responses), dark-adapted 3.0 oscillatory potentials, light-adapted 3.0 ERGs (cone response), and light-adapted 3.0 flicker responses (cone-pathway response) were recorded. The ERGs were recorded based on the International Society for Clinical Electrophysiology of Vision guidelines. The peak-to-peak amplitudes (microvolts) and implicit times (milliseconds) from each step of the standard protocol were determined.17
The mfERGs were recorded monocularly after full pupillary dilation and at least 10 minutes of light adaption in ambient room illumination (illuminance of testing room, 255 lux). A fundus camera was used to monitor fixation, and the optics corrected the refractive error for the test distance. Recordings were acquired with the same type of bipolar contact lens electrode used to record the ffERGs. The amplifier gain was 50,000, and the bandpass was 10 Hz to 300 Hz. The visual stimuli for the mfERGs consisted of 103 black and white hexagons (0.45 and 280 cd/m2, respectively), alternating in a semirandom sequence, modulated according to a binary m-sequence. The visual stimuli were scaled in size with eccentricity (Veris Hexagon 103) and displayed on a high-resolution black-and-white camera-refractor monitor, driven at a frame rate of 75 Hz. The surround luminance was 100 cd/m2. Each m-sequence lasted approximately 7 minutes and was divided into 16 segments lasting 28 seconds each. The mfERGs were extracted from the ERG recording and digitized by an analog-to-digital interface at a sampling rate of 1,200 Hz. The Veris algorithm was used to extract the local retinal responses.
The mfERG recordings were analyzed by measuring the N1 and P1 (N1 indicated the first negative wave and P1, the first positive wave) amplitudes (in nanovolts/degrees2), and the implicit times (in milliseconds) for local ERG responses, in six equally spaced, concentric rings around a central disk at 0, 5, 10, 15, 20, and 25°. The Veris software by default calculates an array of first-order focal ERG kernel traces, one from each stimulated area, by cross-correlation. The trace array contains the information about cone function within the tested areas.18,19 Response densities (nanovolts/degree2) at each eccentricity from fixation were analyzed and compared with the normative data from our laboratory.
Intervention
All patients received three monthly 0.05-mL intravitreal injections of ziv-aflibercept (25 mg/mL) (total dose, 1.25 mg). Commercially acquired ziv-aflibercept was repackaged in glass vials in an aseptic filling facility with no dilution maintaining the same original chemical properties, such as the osmolarity of 1,000 mOsm. All injections were administered according to a standardized method.20 No topical or systemic antibiotics were prescribed before or after the injections.
Retreatment Criteria
After a 3-month loading phase, patients were reassigned to an as-needed treatment with the same dose of ziv-aflibercept administered during the loading phase. The as-needed treatment regimen was administered if the patient had any of the following criteria in the study eye: a greater than 100-mm increase in CRT compared with the lowest previous treatment; loss of 5 or more letters of BCVA; new, recurrent, or persistent subretinal or intraretinal fluid based on OCT; or new macular hemorrhage or a hemorrhagic area exceeding more than 50% of the disk area. Decisions about retreatment were also made based on the investigator's evaluation of the BCVA, ophthalmic examination results, and OCT and FA images, according to a Phase 2 study that evaluated a novel intravitreal anti-VEGF drug.21
All patients were instructed to return 1 month after the injection for an ophthalmologic evaluation or to return promptly if signs and symptoms of endophthalmitis, retinal detachment, or vitreous hemorrhage developed.
Outcomes
The primary safety outcome was the evaluation of ffERG changes over time when the baseline findings were compared with those at 4, 13, and 26 weeks. The secondary outcomes at 26 weeks were the changes in the N1-P1 amplitudes and implicit times on the mfERGs, mean changes in the BCVA values from baseline over time, incidence rates of adverse events, mean changes in the CRT and TMV, and the mean number of injections. The electrophysiologic assessors (J.M.P. and S.E.S.W.) were masked to the patient treatments. An unmasked physician (J.R.d.O.D.) evaluated all OCT and FA images.
Statistical Methods
For categorical variables, the absolute and relative frequencies are reported, and for numerical variables, the means and SDs are reported.
The mean BCVA, CRT, TMV, mfERG parameters (N1-P1 amplitudes, N1 and P1 implicit times), and ffERG parameters (amplitudes and implicit times of the rod responses, b- and a-waves of the dark-adapted combined responses, oscillatory potential responses, cone responses, and 30-Hz flicker responses) were compared between baseline and 26 weeks.
For some ffERG parameters (scotopic b-wave amplitudes, combined maximal a-wave amplitudes, combined maximal b-wave amplitudes, and cone-response b-wave amplitudes), we calculated the differences between baseline and 26 weeks and then compared those differences between the examined and control eyes. All the comparisons were performed using the Student's t-test for paired samples. P < 0.05 was considered significant. Statistical analyses were performed using Stata/SE 12.0 statistical software (StataCorp, College Station, TX).
Results
Patient Characteristics
Between July 2014 and July 2015, 16 patients with exudative AMD in one eye were enrolled. One patient was excluded because of concomitant branch retinal vein occlusion in the study eye. Fifteen patients (10 men, 5 women; mean age ± SD, 70.2 ± 8.2 years) met the study criteria and all completed the 26-week follow-up. Thirteen (86.7%) eyes were phakic and 2 (13.3%) were pseudophakic.
The mean intraocular pressure was 14.7 ± 2.8 mmHg before treatment and 13.4 ± 2.1 mmHg after treatment (P = 0.3506). Up to and including Week 26, 8 patients received 3 injections (loading dose), 5 patients received four injections, and 2 patients received 6 intravitreal injections of ziv-aflibercept in the study eye. Two patients received the injection on Week 26 (Patients 5 and 10) because of subretinal fluid, and one patient due to new intraretinal fluid (Patient 13) on this week. Table 1 shows the baseline characteristics and the number of injections each patient received.
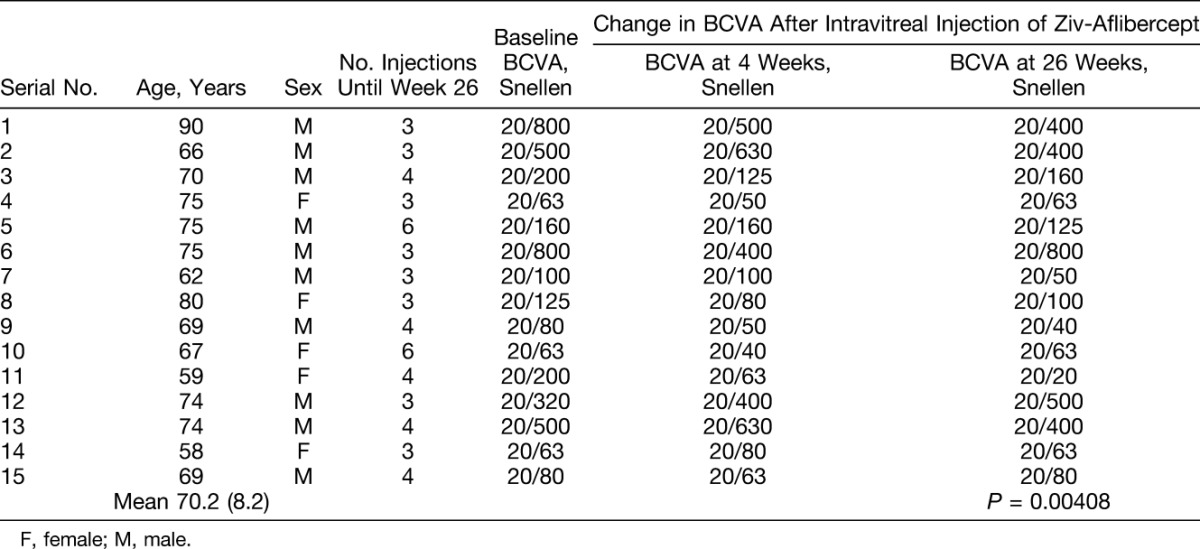
Table 1.
Patient Demographics, Number of Intravitreal Ziv-Aflibercept Injections, and Change in BCVA After Intravitreal Ziv-Aflibercept
Efficacy
Before treatment, the mean Snellen BCVA of the eyes in the study group was 20/200 (logarithm of the minimum angle of resolution [logMAR], mean ± SD, 0.93 ± 0.4; median, 20/160 [logMAR 0.88]; range, 20/800 ± 1.6 to 20/63 ± 0.5). During the 26-week follow-up, the mean BCVA improved to 20/160 (logMAR, mean ± SD, 0.82 ± 0.47; median, 20/125 [logMAR 0.74]; P = 0.00408; Student's t-test; range, 20/800 ± 1.54 to 20/20 ± 0.06) (Table 1).
Morphologic improvement was monitored based on the CRT and TMV measurements. The CRT (mean ± SD) measured by OCT decreased significantly (P = 0.0007) from the baseline value of 490.3 ± 155.1 microns to 327.9 ± 101.5 microns 26 weeks after treatment, representing an overall decrease of 33.13%. The mean TMV decreased significantly (P < 0.0001) from 9.51 ± 1.36 mm3 at baseline to 8.08 ± 1.34 mm3 at 26 weeks (Table 2, Figure 1).
Table 2.
Change in CRT, and Change in TMV After Intravitreal Ziv-Aflibercept
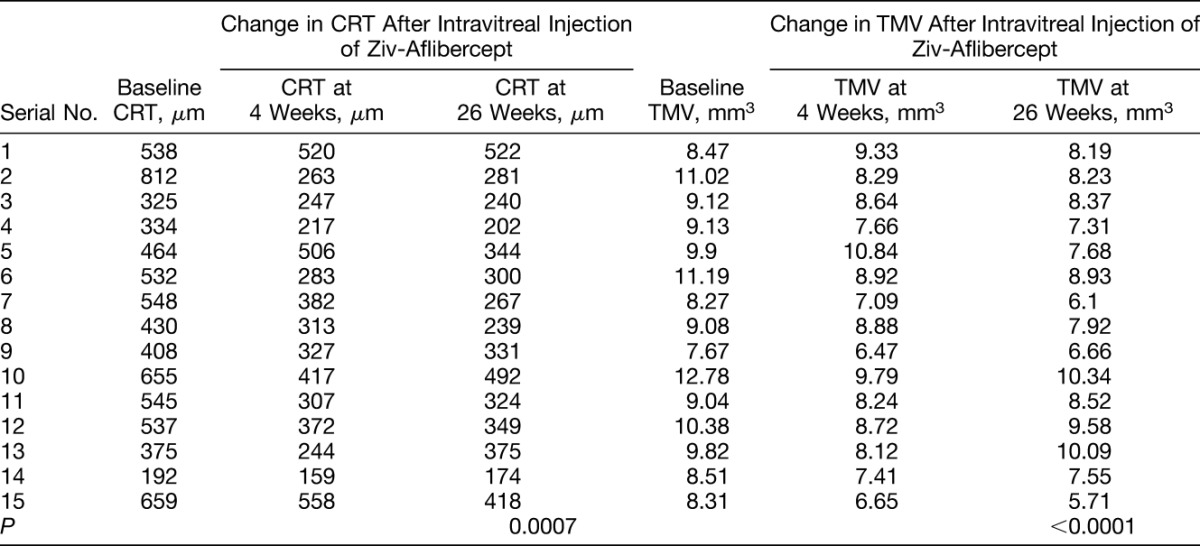
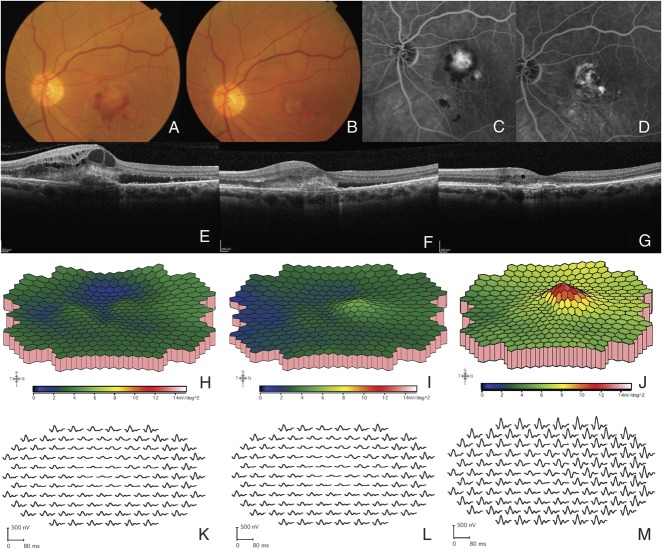
Fig. 1.
Representative examinations of the left eye of a patient submitted to intravitreal ziv-aflibercept (Patient 7). A. Fundus picture of the left eye at baseline reveals choroidal neovascularization secondary to AMD; (B) Fundus picture of the same eye 26 weeks after intravitreal ziv-aflibercept injections; (C) FA before treatment; (D) FA 26 weeks after treatment with intravitreal ziv-aflibercept; (E–G) OCT image (scan length: 6 mm, horizontal section) at baseline, 4, and 26 weeks after treatment initiation; (H–J) 3-Dimensional response density plots at baseline, 4, and 26 weeks, showing increase in response amplitude; (K–M) Multifocal electroretinography trace array at baseline, 4, and 26 weeks.
Multifocal Electroretinography
Comparison of the N1-P1 amplitudes and N1 and P1 implicit times of the mfERGs at baseline and 4, 13, and 26 weeks after the start of treatment showed significant differences in the N1-P1 amplitudes (Table 3, Figure 1) but not in the N1 and P1 latencies.
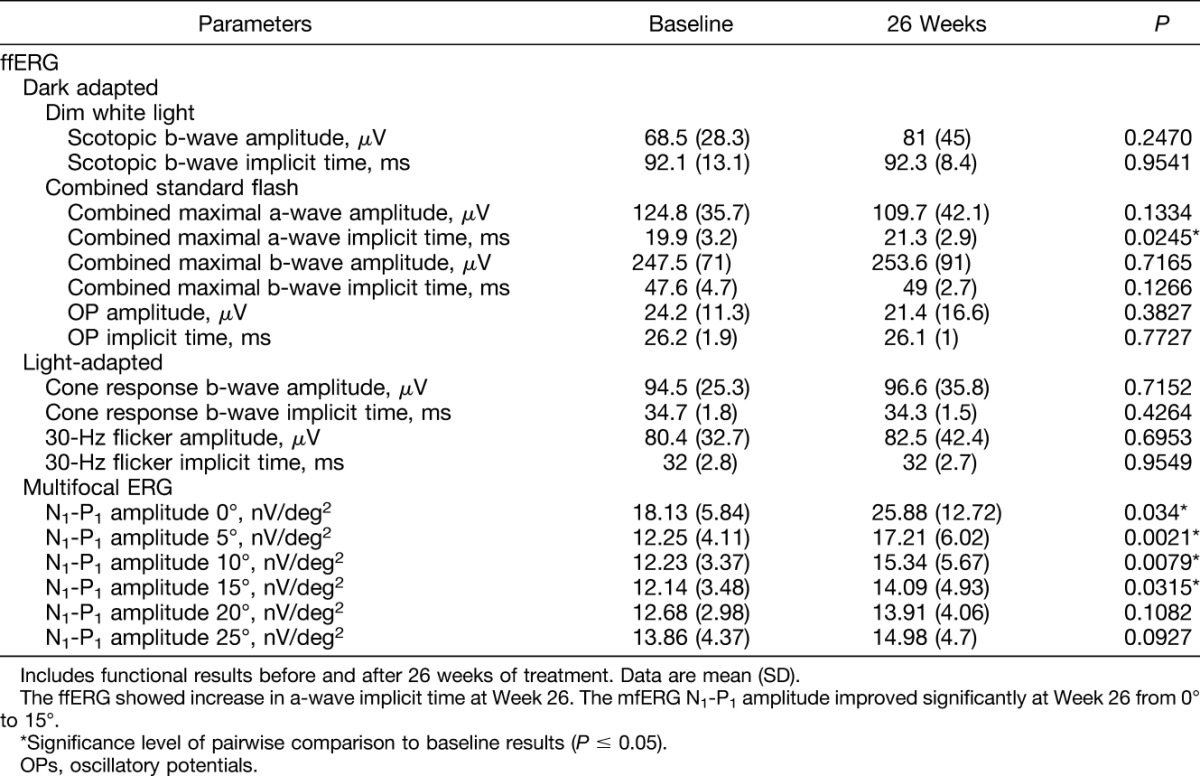
Table 3.
Mean ffERG and Mean mfERG Data at Baseline and 26 Weeks After Intravitreal Ziv-Aflibercept
Safety
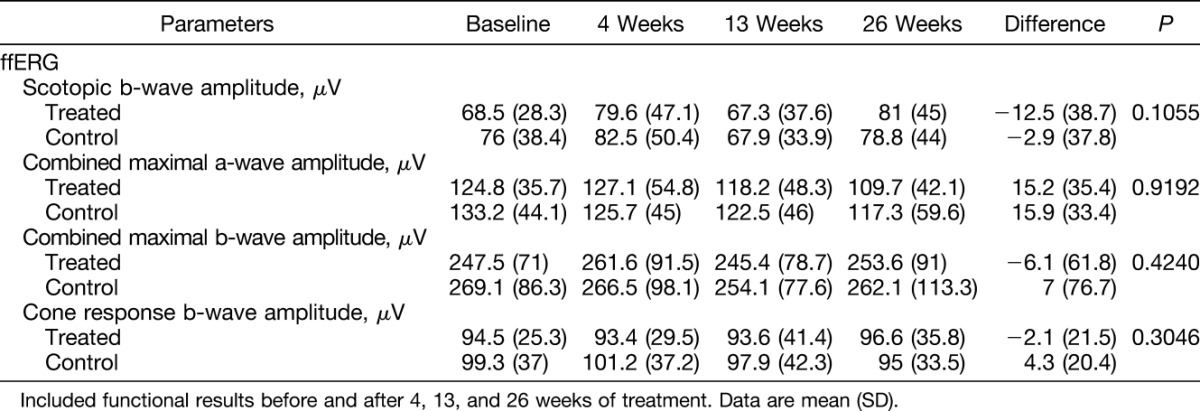
Thirteen patients underwent 4 ffERGs each at baseline and 4, 13, and 26 weeks after the start of treatment. One patient underwent only two ffERGs (baseline and 4 weeks) and refused the other two ERG examinations. A total of 58 ffERGs were performed, and a significant increase in the a-wave implicit times was observed 26 weeks after treatment. No significant changes between baseline and the 26-week results were observed for the other ffERG parameters (Table 3). No significant (P < 0.05) differences were seen when the ffERG components of the treated and fellow eyes were compared during the 26-week follow-up period (Table 4).
Table 4.
Comparison Between the Treated and Control Eye Mean ffERG Parameters at Baseline and 26 Weeks After Intravitreal Ziv-Aflibercept
Adverse Events
Intravitreal ziv-aflibercept was well tolerated. The most common ocular adverse events (0.08%) were associated with the intravitreal injection procedure, that is, vitreous floaters (one patient), punctate keratitis, and conjunctival abrasion related to povidone-iodine (two patients). None of the patients presented anterior chamber or vitreous inflammation.
During the 26-week follow-up, no retinal detachment, anterior or posterior uveitis, vitreous hemorrhage, or endophthalmitis developed. No systemic (nonocular) adverse events were related to the study drug. There were no cardiovascular or cerebrovascular events such as heart failure, stroke, or arterial thrombosis.
Discussion
This study evaluated the safety and efficacy profiles of intravitreal ziv-aflibercept, a systemic chemotherapeutic agent approved to treat metastatic colorectal cancer.5,22 Ziv-aflibercept and aflibercept have the same structure but aflibercept undergoes a different purification process and contains different buffer solutions that are less irritating when injected intravitreally.23 Intravitreal aflibercept also comes in an iso-osmotic aqueous solution, and it is approved to treat exudative AMD, DME, and retinal vein occlusion–related macular edema. However, ziv-aflibercept is hyperosmolar (1,000 mOsm/L) compared with the vitreous, and it is administered intravenously.23,24
No current patients had increased intraocular pressure or cataract progression 26 weeks after intravitreal injections of ziv-aflibercept, and no severe ocular adverse events developed, which agrees with other reports.9–16 Mansour et al10 studied the safety profile of 1.25 mg of intravitreal ziv-aflibercept in four patients with exudative AMD and two patients with DME and reported a significant decrease in the height of the foveolar detachment of the retinal pigment epithelium and improved vision. In this study, the follow-up period was longer, the mean CRT decreased, and the BCVA improved. However, the BCVA decreased in one patient (Case 12) from 20/320 to 20/500. It could be explained by the subretinal fibrosis this patient already presented at baseline, and which persisted at 26 Weeks.
Our patients underwent mfERG recordings, an electrophysiologic method that evaluates localized retinal or macular areas and can be used as a parameter of efficacy after intravitreal injections.25,26 The current result showed that eyes treated with ziv-aflibercept had increased mfERG amplitudes derived from the central retina (0–15°), whereas no changes were seen in the bioelectrical responses of the other retinal areas (20 and 25°) or in the N1 and P1 implicit times. We believe that the improved mfERG central amplitudes, associated with decreases in central macular thickness and TMV showed the electrophysiological and structural effectiveness of ziv-aflibercept. Moschos et al reported a linear relationship between the visual acuity and P1 response amplitudes in eyes with AMD treated with bevacizumab, suggesting that this anti-VEGF drug improved the retinal function.27 A correlation between mfERG retinal response density in the central 15° and retinal thickness also was seen in 4 eyes after bevacizumab treatment, resulting in improved mfERG macular function responses with decreased CRT measured by OCT.28
Other studies have reported that ffERG can be a functional tool for evaluating retinal toxicity after intravitreal injections.14,28,29 In this study, an increase in the a-wave combined response implicit time was the only ffERG parameter that changed significantly during follow-up. We believe that this might have occurred because of our small sample, because the main ffERG markers of retinal toxicity (rod response, oscillatory potentials, and 3.0 flicker) remained unchanged over time. In a study in which patients with DME underwent monthly intravitreal ziv-aflibercept injections, no significant differences were found in the amplitudes or implicit times of any ffERG components, and the visual acuity improved with decreases in CRT observed over 24 weeks.14
We believe the low number of injections per patient could be related to the late AMD stage that most of the patients already presented at baseline, especially because of the presence of subretinal fibrosis. It somewhat reflects the reality of developing countries in which patients with exudative AMD wait several months for evaluation and treatment in a tertiary care system. Patients with a recently diagnosed exudative AMD would be expected to need more injections. As some of the patients only received three intravitreal injections of ziv-aflibercept, a study with monthly injections in patients with exudative AMD is needed to confirm the absence of toxicity of ziv-aflibercept. A recently published article in which patients presenting DME were submitted to monthly intravitreal ziv-aflibercept injections during 6 months did not show any ffERG signs of retinal toxicity. According to this study, escalating doses would not lead to retinal toxicity.14
The limitations of this study were the small number of patients, with 11 treatment-naive patients; the nonrandomized design; and the short follow-up period. Except for one patient, all patients underwent 4 ffERGs and 4 mfERGs from baseline to 26 weeks, which may be a good strategy for monitoring the treatment safety and efficacy, respectively. To the best of our knowledge, this study is the only one that has evaluated the treatment efficacy of intravitreal ziv-aflibercept with mfERGs.
Ziv-aflibercept may be a safe and effective alternative treatment for exudative AMD. A longer follow-up is required to confirm if repeated injections or dose escalations increase the risk of ocular adverse events or retinal toxicity.
Footnotes
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasília, Brazil), and the Pan–American Association of Ophthalmology/Pan–American Ophthalmological Foundation, Paul Kayser Global Award, Arlington, TX.
None of the authors has any conflicting interests to disclose.
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99:933–943. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos N, Martin J, Ruan Q , et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis 2012;15:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schimidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six week results of the VIEW Studies. Ophthalmology 2014;121:193–201. [DOI] [PubMed] [Google Scholar]
- 4.Chu QS. Aflibercept (AVE 0005): an alternative strategy for inhibiting tumor angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther 2009;9:263–271. [DOI] [PubMed] [Google Scholar]
- 5.Sharma T, Dhingra R, Singh S, et al. Aflibercept: a novel VEGF targeted agent to explore the future perspectives of anti-angiogenic therapy for the treatment of multiple tumors. Mini Rev Med Chem 2013;13:530–540. [DOI] [PubMed] [Google Scholar]
- 6.Available at: http://products.sanofi.us/zaltrap/zaltrap.html. Accessed March 14, 2016.
- 7.Available at: http://www.regeneron.com/Eylea/eylea-fpi.pdf. Accessed February 24, 2014.
- 8.de Oliveira Dias JR, Badaró E, Novais EA, et al. Preclinical investigations of intravitreal ziv-aflibercept. Ophthalmic Surg Lasers Imaging Retina 2014;45:577–584. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira Dias JR, Xavier CO, Maia A, et al. Intravitreal injection of ziv-aflibercept in patient with refractory age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina 2015;46:91–94. [DOI] [PubMed] [Google Scholar]
- 10.Mansour AM, Al-Ghadban SI, Yunis MH, El-Sabban ME. Ziv-aflibercept in macular disease. Br J Ophthalmol 2015;99:1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Videkar C, Kapoor A, Chhablani J, Narayanan R. Ziv-aflibercept: a novel option for the treatment of polypoidal choroidal vasculopathy. BMJ Case Rep 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhablani J, Narayanan R, Mathai A, et al. Short-term safety profile of intravitreal ziv-aflibercept. Retina 2016;36:1126–1131. [DOI] [PubMed] [Google Scholar]
- 13.Chhablani J. Intravitreal ziv-aflibercept for recurrent macular edema secondary to central retinal venous occlusion. Indian J Ophthalmol 2015;63:469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade GC, Dias JR, Maia A, et al. Intravitreal injections of ziv-aflibercept for diabetic macular edema: a pilot study. Retina 2016;36:1640–1645. [DOI] [PubMed] [Google Scholar]
- 15.Mansour AM, Dedhia C, Chhablani J. Three-month outcome of intravitreal ziv-aflibercept in eyes with diabetic macular oedema. Br J Ophthalmol 2016. May 17. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Mansour AM, Chhablani J, Antonios RS, et al. Three-month outcome of ziv-aflibercept for exudative age-related macular degeneration. Br J Ophthalmol 2016 Mar 30. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Berezovsky A, Moraes NS, Nusinowitz S, Salomão SR. Standard full-field electroretinography in healthy preterm infants. Doc Ophthalmol 2003;107:243–249. [DOI] [PubMed] [Google Scholar]
- 18.Sutter EE. Imaging visual function with the multifocal m-sequence technique. Vis Res 2001;41:1241–1255. [DOI] [PubMed] [Google Scholar]
- 19.Salomão SR, Watanabe SE, Berezovsky A, Motono M. Multifocal electroretinography, color discrimination and ocular toxicity in tamoxifen use. Curr Eye Res 2007;32:345–352. [DOI] [PubMed] [Google Scholar]
- 20.Frenkel RE, Haji SA, La M, et al. A protocol for the retina surgeon's safe initial intravitreal injections. Clin Ophthalmol 2010;4:1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Xu G, Wang Y, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 2014;121:1740–1747. [DOI] [PubMed] [Google Scholar]
- 22.Cheng YD, Yang H, Chen GQ, Zhang ZC. Molecularly targeted drugs for metastatic colorectal cancer. Drug Des Devel Ther 2013;7:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trichonas G, Kaiser PK. Aflibercept for the treatment of age-related macular degeneration. Ophthalmol Ther 2013;2:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira Dias JR, Andrade GC, Novais EA, et al. Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept. IJRV 2016;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood DC. Evaluating retinal function with the multifocal technique. Prog Retin Eye Res 2000;19:607–646. [DOI] [PubMed] [Google Scholar]
- 26.Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. The electroretinogram: a useful tool for evaluating age-related macular disease? Doc Ophthalmol 2010;121:51–62. [DOI] [PubMed] [Google Scholar]
- 27.Moschos MM, Brouzas D, Apostolopoulos M, et al. Intravitreal use of bevacizumab (avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT study. Doc Ophthalmol 2007;114:37–44. [DOI] [PubMed] [Google Scholar]
- 28.Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (avastin) treatment. Retina 2006;26:270–274. [DOI] [PubMed] [Google Scholar]
- 29.Skaat A, Solomon A, Moroz I, et al. Increased electroretinogram a-wave amplitude after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Acta Ophthalmol 2011;89:e269–e273. [DOI] [PubMed] [Google Scholar]