Among 40 Coats' disease patients presenting foveal exudation without foveal detachment (Stages 2B-3A1, median follow-up: 4.7 years), 21 eyes (52.5%) developed a subfoveal nodule. These eyes were significantly associated with macular fibrosis progression (P < 0.0001, mean onset time: 11.0 ± 2.6 months) and worse visual outcome (P = 0.01). These observations suggested an updated classification.
Key words: Coats' disease, subfoveal nodule, macular fibrosis, outcome, prognosis, retina
Purpose:
To determine the prevalence, clinical characteristics and nature of subfoveal nodules in Coats' disease and the associated impact on the long-term visual outcome.
Methods:
Consecutive cases of Coats' disease with foveal exudation were retrospectively reviewed. The presence of a subfoveal nodule or macular fibrosis was recorded. Clinical characteristics, retinal imaging, and outcome were analyzed by comparative analysis. The histopathological description of an enucleated eye with subfoveal nodule was performed.
Results:
Among 40 patients presenting unilateral Stage 2B or 3A1 Coats' disease, a subfoveal nodule was detected in 21 patients (52.5%). The median follow-up was 4.7 years. Nineteen patients (47.5%) did not present a subfoveal nodule. Three patients (15.8%) without subfoveal nodule and 21 patients (100%) with subfoveal nodule progressed to a macular fibrotic scar (P < 0.0001), and the mean time of macular fibrosis onset was 11.0 ± 2.6 months. Final visual acuity was significantly worse in patients who presented a subfoveal nodule at diagnosis (P = 0.01). Of 18 cases with subfoveal nodule who underwent fluorescein angiography, retinal–retinal anastomosis and neovascularization were detected in 13 (72.2%) and 2 eyes (11.1%), respectively. Histopathological analysis of a subfoveal nodule revealed an aggregate of proteinaceous material including fibrin, spindle cells, macrophages, and pigmented cells.
Conclusion:
The presence of a subfoveal nodule at presentation is a predictive factor for macular fibrosis development and worse visual outcome in patients with Coats' disease. These observations suggest an updated classification introducing two subcategories within Stage 2B: without subfoveal nodule (Stage 2B1) and with subfoveal nodule (Stage 2B2).
Coats' disease is a congenital condition predominantly affecting young male subjects and characterized by unilateral retinal telangiectasia and exudation.1 Shields et al2 have proposed a classification system aimed at predicting the visual prognosis by stratifying cases according to the presence of foveal exudation and exudative retinal detachment at presentation. A more favorable visual prognosis is expected in the absence of foveal exudates at the initial evaluation, while a thick foveal exudation usually predicts a worse functional outcome. In addition, Shields et al have mentioned that poor vision is particularly expected when this exudation presents as a fibroglial foveal nodule, yet this observation was not included in their classification system.
The terms “subfoveal nodule,”3 “subretinal mounds,”4 “gray nodule,”2 and “macular fibrosis”5 have been employed to describe a nodular fibrotic lesion usually associated with a vascular component within the fovea or the posterior pole of patients with Coats' disease. Notably, Chang et al6 reported in a histological description of eyes with Coats' disease the presence of fibrous nodules in the macular area with variable calcified content.
The aim of this study is to determine the prevalence and clinical characteristics of subfoveal nodules in Coats' disease and the associated impact on the long-term visual outcome, as well as its histopathological nature.
Methods
This study was designed in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Swiss Federal Department of Health (Authorization CER-VD n° 95/15). Records of patients diagnosed with Coats' disease between August 1989 and June 2015 at Jules-Gonin Eye Hospital were retrospectively reviewed at presentation and for each subsequent visit. General data included patient age, sex, laterality of Coats' disease, and symptoms at presentation. Visual acuities, slit-lamp observations and tonometry measures were noted. Fundus examination data were obtained from drawings and color fundus photographs with RetCam II (Clarity Medical Systems, Inc, Pleasanton, CA), Optos 200 Tx (Optos, Dunfermline, United Kingdom), and Panoret-1000 (CMT Medical Technologies Inc, Valley Stream, NY). The amount of peripheral telangiectasia and retinal exudation at presentation was quantified in terms of clock hours, from 1 to 12. Coats' disease was classified according to Shields et al2 into Stage 1, telangiectasia only; Stage 2, telangiectasia and exudation (2A, extrafoveal exudation; 2B, foveal exudation); Stage 3, exudative retinal detachment (3A1, extrafoveal subtotal retinal detachment; 3A2, foveal subtotal retinal detachment; 3B, total retinal detachment); Stage 4, total detachment and secondary glaucoma and Stage 5, advanced end-stage disease.
The presence of a subfoveal nodule or macular fibrosis was recorded. A subfoveal nodule was defined on fundus examination as a yellow, exudative, protruding, spheroidal lesion. Nodule size was estimated in papillary diameter. The lesion was confirmed by B-scan ultrasonography (OTI SCAN 1000; Ophthalmic Technologies Inc, ON, Canada) and the height of the nodule was measured. On fundoscopy, the presence of an associated vascular component and the presence of hyperpigmentation were also noted. Macular fibrosis was defined as a subretinal white-gray scar at the fovea. Findings from fluorescein angiography (FA) on RetCam II were recorded. The presence of retinal–retinal anastomosis and/or neovascularization (retinal or choroidal) was determined by two different observers (A.D. and F.M.). Data from spectral-domain optical coherence tomography (SD-OCT) on Spectralis (Heidelberg Engineering, Heidelberg, Germany) were also recorded when available. The nodule height was measured as the axial distance between retinal pigment epithelium (RPE) and the apex of the nodule by a single examiner (A.M.a).
Patients presenting without foveal exudation (stage < 2A) or with foveal exudative retinal detachment (>3A2) at presentation and those with follow-up <12 months were excluded from the study.
Amblyopia Therapy and Visual Acuity Evaluation
Amblyopia therapy was initiated as needed after full correction of any refractive error. The patching regimen was adapted to age and was prescribed part-time to full-time (up to 10 hours daily) depending on collaboration and results. Maintenance therapy was continued after stabilization. Parents were encouraged to stimulate the child during patching hours, with eye–hand coordination activities. Visual acuity was assessed at the last control, with a minimum indication of the presence/absence of light perception, then recordable measurements using Teller cards for the youngest children, distance best corrected visual acuity (BCVA) with LH (Lea) symbols from 30 months to 4 years, Snellen “E” chart between 4 years and 5 years, and numbers or letters from 5 years to 6 years of age.
Statistical Analysis
Comparative analyses were performed between eyes with or without subfoveal nodule. Baseline clinical characteristics were compared using Mann–Whitney test for quantitative variables and Chi-square or Fisher's exact test for categorical variables where appropriate, on GraphPad Prism (version 5.0f; GraphPad Software, La Jolla, CA). Visual acuity levels were reported in decimal unit and the logarithm of the minimal angle of resolution (LogMAR) was used to calculate means. In all statistical comparisons, differences with a P value inferior to 0.05 were considered significant.
Pathology
Sections of formalin-fixed enucleated eyes with the diagnosis of Coats' disease, archived between August 1989 and June 2015, were retrieved from the histopathological files of Jules-Gonin Eye Pathology Laboratory. Nine cases were identified and reviewed for the presence of subfoveal nodules as well as macular fibrosis. In all cases, hematoxylin eosin stain was performed. Martius Scarlett Blue stain was realized additionally on one eye presenting a subfoveal nodule. Immunohistochemical analyses were performed in this case with the following antibodies from Dako (Agilent Technologies Inc, Santa Clara, CA): cytokeratins (clone AE1-AE3, dilution 1:200), CD68 (clone PGM-1, dilution 1:100), CD31 (clone JC70A, dilution 1:100).
Results
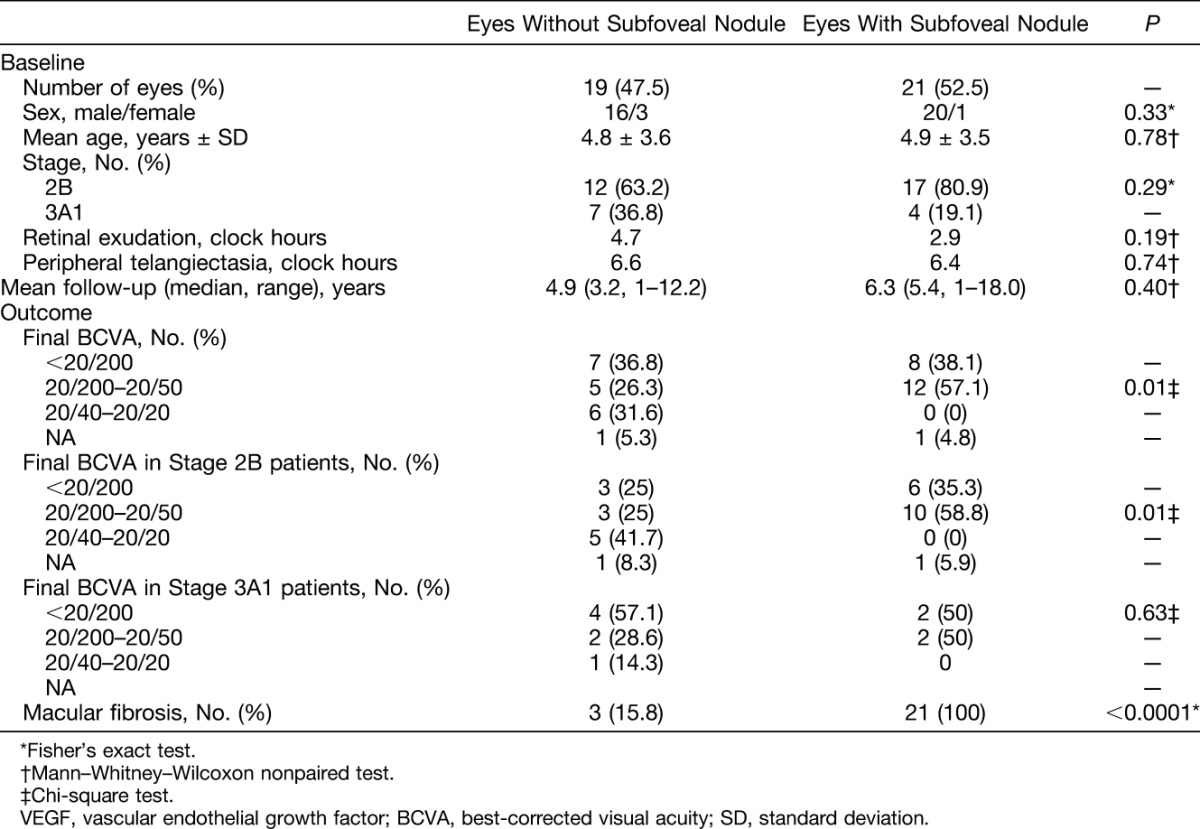
Records of 101 patients with Coats' disease were reviewed. Sixty-one patients were excluded of which 51 cases presented stage ≤2A or ≥3A2 and 10 cases had a follow-up shorter than 12 months. Among the remaining 40 patients presenting Stage 2B or 3A1, 19 patients (47.5%) did not present with subfoveal nodule nor developed it during follow-up (Figure 1). A subfoveal nodule was detected in 21 patients (52.5%): the diagnosis was made at presentation in 19 cases, and 2 months later in 2 cases, after regression of initially extended foveal exudates. The median follow-up was 4.7 years (range, 1–18 years). Figure 1 displays 2 cases, with and without subfoveal nodule. Comparative analyses between patients with and without subfoveal nodule are shown in Table 1. There were no significant differences in age, sex, extension of telangiectasia or exudation at presentation, or follow-up duration between groups. The development of macular fibrosis was significantly more frequent in patients with subfoveal nodule (P < 0.0001). All 21 patients (100%) with subfoveal nodule and 3 patients (15.8%) without subfoveal nodule progressed to a macular fibrotic scar. In cases with and without subfoveal nodule, the mean time from diagnosis to the detection of macular fibrosis was 11.0 ± 2.6 months and 13.6 ± 9.2 months, respectively. Visual acuity levels at the last recorded visit were significantly worse in patients with subfoveal nodule (P = 0.01). No patient with subfoveal nodule and 6 patients without subfoveal nodule (31.6%) showed final visual acuities higher than 20/40. When analyzing separately final visual acuity levels in Stage 2B and Stage 3A1 patients, a significant difference between eyes with and without subfoveal nodules was found only in Stage 2B patients (P = 0.01). The main causes of severe visual loss (<20/200) in patients without subfoveal nodule were: macular atrophy in n = 3/3 eyes with Stage 2B and tractional retinal detachment (n = 2/4), macular fibrosis (n = 1/4) and macular atrophy (n = 1/4) in eyes with Stage 3A1.
Fig. 1.
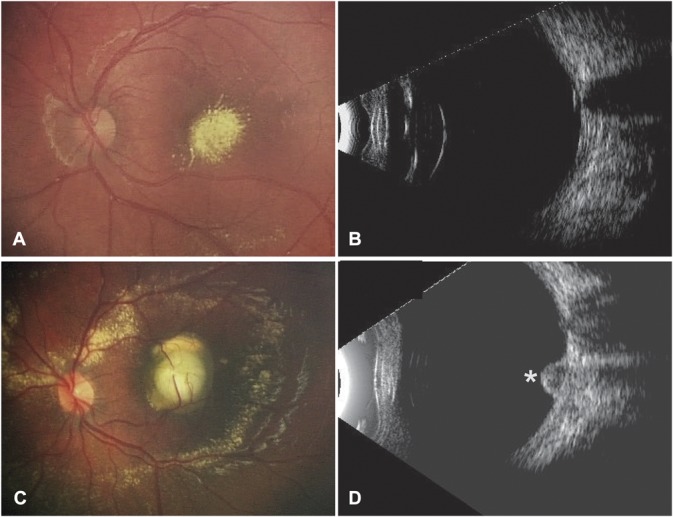
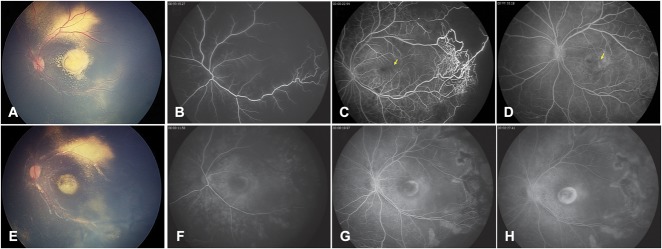
A and B. Two-year-old boy presenting with Stage-2B Coats' disease. Color fundus photograph (A) showed a yellow lesion suggestive of retinal exudates, and B-scan ultrasonography (B) confirmed the absence of subretinal nodule. C and D. Four-year-old boy presenting with Stage-2B Coats' disease. On color fundus photograph (A), a yellow, exudative, protruding, subfoveal spheroidal lesion was visible and was associated with two second-order arteries and a third-order vein entering the lesion. B-scan ultrasonography (B) confirmed the presence of a subretinal nodule (star).
Table 1.
Comparative Analysis Between Eyes With and Without Subfoveal Nodule Among Patients With Stage 2B or 3A1 Coats' Disease
Clinical and imaging characteristics of patients with subfoveal nodule are summarized in Table 2. On fundus examination, a vascular element formed by a second-to-third-order artery and a second-to-third-order vein was detected entering the subfoveal nodule in all cases (Figure 1C). Fluorescein angiography was available for 18 patients with subfoveal nodule, 13 of whom underwent FA at presentation and 15 at the last visit, after the development of macular fibrosis. At presentation, FA showed a hypofluorescent area corresponding to the nodule during the early phase (n = 13/13), which remained hypofluorescent (n = 6/13) or became hyperfluorescent with minimal leakage (n = 7/13) during the late phase (Figure 2). After the development of macular fibrosis, FA showed in most cases an increasing hyperfluorescence with well-delimited borders, corresponding to staining (n = 13/15), or less frequently an increasing and extending hyperfluorescence, corresponding to leakage (n = 2/15) (Figure 2). Of 18 patients with subfoveal nodule who underwent fluorescein angiography, retinal–retinal anastomosis was detected in n = 13 eyes (72.2%) and neovascularization in n = 2 eyes (11.1%) (Figure 3). Spectral-domain optical coherence tomography was performed in 14 patients with subfoveal nodule at the last visit. Spectral-domain optical coherence tomography at the level of the fibrotic nodule showed a hyperreflective, sharply demarcated subfoveal lesion associated with a posterior shadowing effect. There was a moderate retinal layer disorganization and retinal thickening, with abrupt elevation of retinal layers by the underlying hyperreflective nodule (Figure 4). The mean height of fibrotic nodules measured on spectral-domain optical coherence tomography was 0.7 ± 0.3 mm.
Table 2.
Clinical and Imaging Characteristics of Subfoveal Nodules in 21 Patients With Stage 2B or 3A1 Coats' Disease Presenting a Subfoveal Nodule
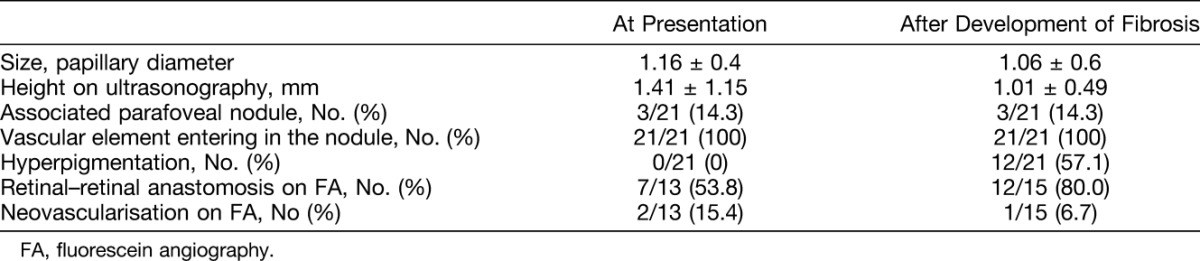
Fig. 2.
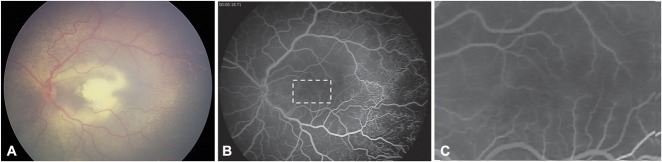
A. Color fundus photograph of a two-year-old boy presenting with Stage-2B Coat's disease and a subfoveal nodule. B and D. Fluorescein angiography at presentation showed a hypofluorescent subfoveal lesion during early phase and midphase (B and C), which became hyperfluorescent with minimal leakage during the late phase (D) because of the presence of a retinal–retinal anastomosis (C and D, yellow arrows). Note the peripheral retinal telangiectasias and nonperfusion areas (C) characteristic of Coats' disease. E. Color fundus photograph from the same patient acquired 6 months later after he developed macular fibrosis. F and H. Fluorescein angiography showed an increasing hyperfluorescence with well-delimited borders corresponding to staining. Note the scars resulting from peripheral laser photocoagulation of nonperfused areas (G and H).
Fig. 3.
A. Color fundus photograph of a one-year-old boy presenting with Stage-3A1 Coat's disease and a subfoveal nodule. B. Fluorescein angiography showed a retinal–retinal anastomosis at the level of the subfoveal nodule (dotted-line rectangle). C. Magnification of the rectangular area (B) showing the retinal–retinal anastomosis.
Fig. 4.
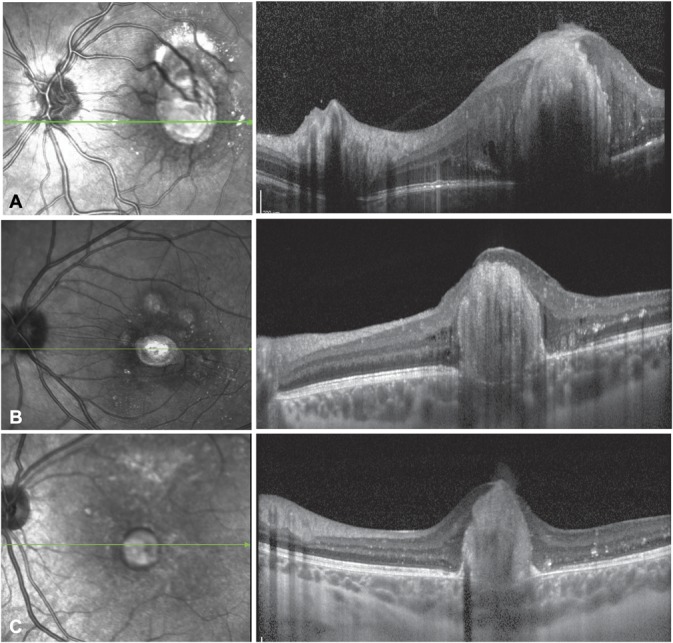
Spectral-domain optical coherence tomography horizontal sections in 3 patients with Coats' disease and a fibrotic subfoveal nodule observed 9 (A), 12 (B), and 26 (C) months after presentation, respectively.
Pathology
Among 101 patients with Coats' disease followed at Jules-Gonin Eye Hospital, 11 eyes underwent enucleation with subsequent pathologic evaluation and diagnosis confirmation. Nine procedures were performed at our institution and the corresponding eyes were available for analysis. In seven cases, there was a complete exudative retinal detachment with telengiectatic retinal vessels, retinal gliosis, and partial retinal atrophy. Several cases exhibited intraretinal and/or preretinal hemorrhages and retinal hard exudate. Inside exudates, numerous macrophages and cholesterol crystals were found. In two cases, a dense subretinal fibrotic lesion was identified with fibrous RPE metaplasia. The subretinal fibrosis also contained isolated cords and strands of hyperplastic RPE.
One case with Stage 4 Coats' disease exhibited a nodule underneath the fovea composed by an aggregate of proteinaceous material, including fibrin (best highlighted with Martius Scarlett Blue Stain) and spindle cells, as well as macrophages and some pigmented cells. The nodule dimensions were 288 µm × 358 µm. Immunohistochemistry showed that the spindle cells located at the periphery of the lesion expressed both low and high molecular weight cytokeratins consistent with a RPE origin. The nodule also contained CD163+ macrophages. There was no vascular component within the nodule and CD31 expression was not observed (Figure 5).
Fig. 5.
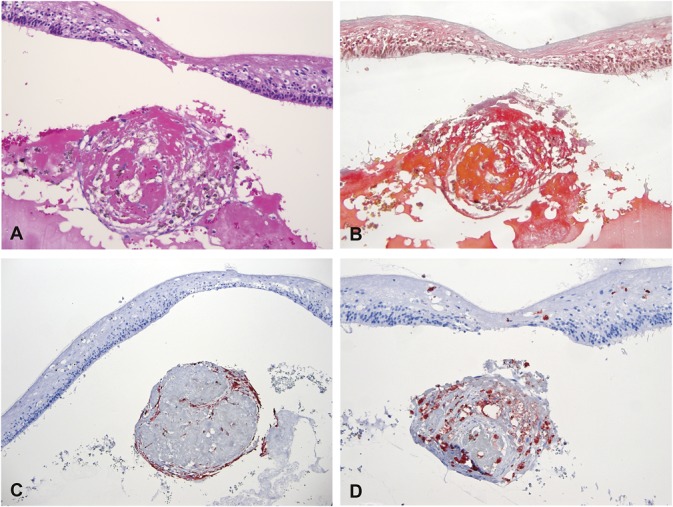
Histopathological analysis of a subfoveal nodule from an eye with Coats' disease. Specific stainings were performed for hematoxylin eosin (A, ×126), Martius Scarlett Blue (B, ×126), cytokeratins (AE1–AE3) (C, ×63), CD68 (D, ×126). The subfoveal lesion was formed by a round aggregate of proteinaceous material including fibrin (B) admixed with spindle cells, macrophages, and pigmented cells. Some of the cells within the lesion expressed pancytokeratins (C) consistent with a retinal pigment epithelium origin, while other expressed CD68 (D). Specific staining for endothelial cells was negative.
Discussion
In this retrospective series of 40 patients with Stage 2B or 3A1 Coats' disease, 52.5% presented a subfoveal nodule that progressed in all affected cases to macular fibrosis and was associated with a worse visual outcome. Among patients presenting subfoveal exudation without subfoveal nodule, only 15.8% developed macular fibrosis, leading to higher final visual acuities in this group.
The classification system of Coats' disease by Shields et al2 takes into account the presence and location of lipid exudates at presentation to stratify the visual prognosis of the disease. Thus, patients without foveal exudation (Stage 2A) have better functional outcome than patients presenting with foveal exudation at diagnosis (Stage 2B). In this study, we showed that the presence of a subfoveal nodule is a predictor of worse visual outcome because of the subsequent development of macular fibrosis. However, the prognosis value of subfoveal nodules regarding final visual function was only confirmed in Stage 2B patients. The lack of prognosis value in Stage 3A1 is because of the possible occurrence of other causes of severe visual loss during the course of the disease, such as tractional retinal detachment. On the basis of these observations, the presence of a subfoveal nodule should be included in the current classification to better detect those patients at higher risk of macular fibrosis and poor visual outcome. We suggest to update the classification system of Coats' disease by introducing two subcategories within Stage 2B (Foveal exudation): without subfoveal nodule (Stage 2B1) and with subfoveal nodule (Stage 2B2) (Table 3).
Table 3.
A Proposed Update for Shields' Classification System of Coats' Disease

The analysis of FA showed that the vascular elements associated with subfoveal nodules were composed in a majority of cases of a retinal–retinal anastomosis and of a neovascular complex in some cases. Interestingly, although Coats' disease is mainly characterized by alterations of the peripheral retinal vasculature, the observation of retinal–retinal anastomosis in the macular area of these patients confirms that the disease is related to a wider spectrum of congenital retinal telangiectasis including Type 1 idiopathic macular telangiectasia, as previously suggested.7–9 Moreover, it was recently shown that Type 1 macular telangiectasia patients harbored abnormal retinal capillary anastomoses in the perifoveal area.10 However, because there has been no report of subfoveal nodule in the absence of peripheral telangiectasia, it is not possible to confirm that all subfoveal nodules arise independently of the peripheral exudation. Some could be caused by severe accumulation or exudation in the macula from the peripheral vascular anomalies. Jumper et al5 have described the development of macular fibrosis in 11 of 47 patients (23%) with Coats' disease and its association with intraretinal anastomosis and neovascularization. These authors proposed that macular fibrosis resulted from a neovascular process in response to the accumulation of lipid exudates. Recently, Sigler et al4 reported the presence of retinal angiomatous proliferation with chorioretinal anastomosis,11,12 using spectral-domain optical coherence tomography only, in 5 of 21 (24%) patients with Coats' disease treated by intravitreal bevacizumab. The authors hypothesized that Coats' disease involves an abortive defect during the angiogenesis of the retinal midcapillary plexus, and that this aberrant vascular development in the macula could lead to Type 3 neovascularization with chorioretinal anastomosis although indocyanine green angiography was not performed. In the present study, we report the presence of intraretinal or subretinal neovascularization visible on FA in 27.8% of patients with a subfoveal nodule. However, the presence of a choroidal component was not possible to identify using only spectral-domain optical coherence tomography because of the lack of indocyanine green angiography, not routinely performed in children and adolescent patients with Coats' disease. Because macular fibrosis is the possible result of lipid accumulation from aberrant retinal vessels or neovascularization, treatment by intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents seems to be a relevant strategy. However, the possible contribution of anti-VEGF therapy to the development of fibrosis in Coats' disease is still controversial.13,14 Whether anti-VEGFs should be systematically indicated when a subfoveal nodule is present remains to be determined.
In a previous histopathological description of 62 eyes enucleated for severe Coats' disease, Chang et al6 observed in 6 eyes a subfoveal fibrotic nodule with RPE proliferation, which occasionally contained calcium or bone. Our histopathological analysis did not reveal similar features within subfoveal fibrotic lesions, but it identified in one case a nodular aggregate of partially fibrinous material admixed with RPE-derived cells and macrophages. It is possible that this aggregate represents an early and incipient phase of a subsequent subfoveolar fibrosis. We speculate that the nodule might then adhere to the RPE and to the sensory retina as local tissue remodeling and fibrosis progress. We also hypothesize that the composition of these nodules varies with disease progression, which is supported by the differential FA behaviors at diagnosis and after the development of fibrosis, as described in the present study. At diagnosis, the nodule has most likely a high lipid content, which is consistent with the early and late hypofluorescence, in the absence of leakage from abnormal vessels. With disease progression, the nodule becomes fibrotic, with frequent hyperpigmentation and possible calcification, modifying its histological characteristics and increasing its fluorescein affinity that manifest as a late staining during FA.
Limitations of this study include its retrospective nature, the limited number of patients because of the low prevalence of Coats' disease, and the absence of certain imaging modalities in a minority of patients because of the long inclusion period covering different eras of modern ocular imaging. Moreover, certain modalities were not evaluated because they were technically challenging in young subjects with difficulties to maintain fixation, such as fundus autofluorescence that could contribute to investigate the possible calcified component of subfoveal nodules. However, the strong statistical associations reported in the study support our observations.
In conclusion, the presence of a subfoveal nodule at presentation is a predictive factor for the development of macular fibrosis and worse visual outcome in patients with Coats' disease and foveal exudation.
Acknowledgments
The authors thank Marie-Claire Gaillard, MD and Aude Ambresin, MD for clinical assistance.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
References
- 1.Coats G. Forms of retinal diseases with massive exudation. Roy Lond Ophthalmol Hosp Rep 1908;17:440–525. [Google Scholar]
- 2.Shields JA, Shields CL, Honavar SG, et al. Classification and management of Coats disease: the 2000 proctor lecture. Am J Ophthalmol 2001;131:572–583. [DOI] [PubMed] [Google Scholar]
- 3.Khurana RN, Samuel MA, Murphree AL, et al. Subfoveal nodule in Coats' disease. Clin Exp Ophthalmol 2005;33:301–302. [DOI] [PubMed] [Google Scholar]
- 4.Sigler EJ, Calzada JI. Retinal angiomatous proliferation with chorioretinal anastomosis in childhood Coats disease: a reappraisal of macular fibrosis using multimodal imaging. Retina 2015;35:537–546. [DOI] [PubMed] [Google Scholar]
- 5.Jumper JM, Pomerleau D, McDonald HR, et al. Macular fibrosis in Coats disease. Retina 2010;30:S9–S14. [DOI] [PubMed] [Google Scholar]
- 6.Chang MM, McLean IW, Merritt JC. Coats' disease: a study of 62 histologically confirmed cases. J Pediatr Ophthalmol Strabismus 1984;21:163–168. [DOI] [PubMed] [Google Scholar]
- 7.Smithen LM, Brown GC, Brucker AJ, et al. Coats' disease diagnosed in adulthood. Ophthalmology 2005;112:1072–1078. [DOI] [PubMed] [Google Scholar]
- 8.Cahill M, O'Keefe M, Acheson R, et al. Classification of the spectrum of Coats' disease as subtypes of idiopathic retinal telangiectasis with exudation. Acta Ophthalmol Scand 2001;79:596–602. [DOI] [PubMed] [Google Scholar]
- 9.Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology 1993;100:1536–1546. [PubMed] [Google Scholar]
- 10.Matet A, Daruich A, Dirani A, et al. Macular telangiectasia type 1: capillary density and microvascular abnormalities assessed by optical coherence tomography angiography. Am J Ophthalmol 2016;167:18–30. [DOI] [PubMed] [Google Scholar]
- 11.Freund KB, Ho I-V, Barbazetto IA, et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 2008;28:201–211. [DOI] [PubMed] [Google Scholar]
- 12.Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21:416–434. [DOI] [PubMed] [Google Scholar]
- 13.Daruich A, Matet A, Tran HV, Gaillard M-C, Munier FL. Extramacular fibrosis in Coats' disease. Retina 2016;36:2022–2028. [DOI] [PubMed] [Google Scholar]
- 14.Ramasubramanian A, Shields CL. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96:356–359. [DOI] [PubMed] [Google Scholar]