Abstract
Histones are known for their ability to bind to and regulate expression of DNA. However, histones are also present in cytoplasm and extracellular fluids where they serve host defense functions and promote inflammatory responses. Histones are a major component of neutrophil extracellular traps that contribute to bacterial killing but also to inflammatory injury. Histones can act as antimicrobial peptides and directly kill bacteria, fungi, parasites and viruses, in vitro and in a variety of animal hosts. In addition, histones can trigger inflammatory responses in some cases acting through Toll-like receptors or inflammasome pathways. Extracellular histones mediate organ injury (lung, liver), sepsis physiology, thrombocytopenia and thrombin generation and some proteins can bind histones and reduce these potentially harmful effects.
KEYWORDS : antimicrobial peptides, histones, innate immunity, neutrophils, platelets
Histones: overview
Histones are evolutionary conserved basic proteins, present in all eukaryotic cells. As important parts of chromosomes, they play an essential role in organization and regulation of DNA. The positive charge of histones facilitates binding to the negatively charged DNA, forming the basic structural unit of chromatin known as the nucleosome. Each nucleosome core consists of superhelical DNA wound around an octamer of histones, composed of two copies of each of the core histones H2A, H2B, H3 and H4 [1]. The linker histone H1 binds to the complete nucleosome core particle and forms higher order structures [2].
Histones can be grouped into two classes: lysine-rich (H1, H2A, H2B) and arginine-rich (H3, H4) [3]. However, despite classification in different groups, all histones share a similar structure, comprised of a long central helix accompanied by a C-terminal histone fold, comprising three α-helices connected by loops, on each end [4].
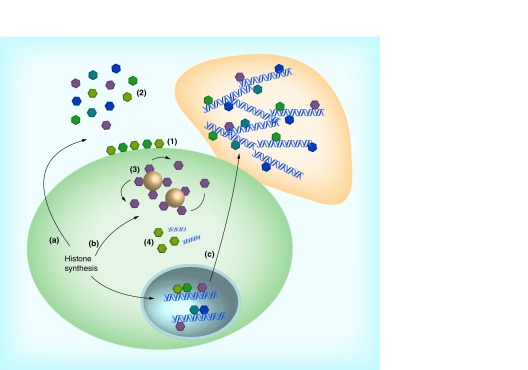
In addition to their role in DNA condensation, histones have long been known to be important regulators of gene transcription [5], through post-translational modifications, such as methylation, acetylation or phosphorylation, of histone N-terminal tails [6]. A recent study showed that through methylation, histone H3 can carry epigenetic information over multiple cell divisions in yeast [7]. Other novel roles for histones are emerging. The linker histone H1 has been proposed to play a role in regulating apoptosis induced by DNA damage [8] or T-cell cytokine deprivation [9]. After biosynthesis in the cytoplasm, histones are imported into the nucleus [10]. However, a significant amount of histones accumulates in the cytoplasm [11] and on the plasma membrane [12] where they have other functions, such as antimicrobial activity. The extranuclear functions of histones are summarized in Figure 1.
Figure 1. . Antimicrobial action of histones.
After synthesis in the cytoplasm, histones are transported to the nucleus where they regulate DNA condensation and gene transcription. Alternatively, histones can have extranuclear functions, either in the cytoplasm (b) or in the extracellular space (a). Histones transported from the cytoplasm either remain membrane-bound (1) or are released into the extracellular space (2), where they exert broad-spectrum antimicrobial activity against bacteria, viruses, parasites and fungi. Within the cytoplasm, histone H1 is bound to lipid droplets and is released upon stimulation with endotoxin or lipotechoic acid (3). In addition, histone H2B functions as a sensor for viral dsDNA (4). Nuclear histones end up in neutrophil extracellular traps (c), where they play an important part in neutrophil extracellular trap-mediated bacterial killing.
Antimicrobial activity of histones
Histones and histone-like proteins with broad-spectrum antimicrobial activity have been found in many different species, including insects [13], chickens [14], fish [15], frogs [16] and mammals, such as mice [17], cows [18], rats [18] and humans [19]. Functioning as antimicrobial peptides, histones and their derivatives form an important part of the skin defense [16], but are also found in other tissues, such as the stomach [15] or intestine [20], reproductive tissue [21] as well as in blood [22]. In addition, histones are an important component of neutrophil extracellular traps (NETs) as discussed below. Table 1 summarizes the reports on antimicrobial activity of histones.
Table 1. . Antimicrobial activity of full-length and fragmented histones.
| Histone | Pathogen | ||
|---|---|---|---|
| H1 | Full-length | Gram-positive bacteria | L. monocytogenes, S. epidermidis, S. aureus, M. fortuitum, B. subtilis [13,17,21] |
| Gram-negative bacteria | E. coli, S. typhimurium [13,17,20,21,23] | ||
| Virus | Norwalk virus [24] | ||
| Fungi | C. neoformans, C. tropicalis [17,25] | ||
| Parasites | Unknown | ||
| Fragmented | Gram-positive bacteria | M. luteus, P. citreus, B. subtilis, L. ivanovii [26,27] | |
| Gram-negative bacteria | E. coli, L. anguillarum, A. salmonicida, V. anguillarum, S. enterica [26–28] | ||
| Virus | Unknown | ||
| Fungi | Unknown | ||
| |
|
Parasites |
Unknown |
| H2A | Full-length | Gram-positive bacteria | B. subtilis, S. aureus, M. luteus [14,29–30] |
| Gram-negative bacteria | E. coli, S. flexniri [14,30] | ||
| Virus | Norwalk virus [24], Influenza virus [31] | ||
| Fungi | Unknown | ||
| Parasites | L. amazonensis L. major, L. braziliensis, L. mexicana [32,33] | ||
| Fragmented | Gram-positive bacteria | B. subtilis, S. aureus, S. mutans, M. luteus, L. garvieae, L. ivanovii, S. epidermis[29,34–36] | |
| Gram-negative bacteria | P. putida, E. coli, S. typhimurium, A. salmonicida, C. aquatilis, Y. ruckeri, E. ictaluri, V. anguillarum [34,35,37,38] | ||
| Virus | Unknown | ||
| Fungi | C. neoformans, S. cerevisae, C. albicans [35] | ||
| |
|
Parasites |
Unknown |
| H2B | Full-length | Gram-positive bacteria | B. subtilis, S. aureus, L. monocytogenes, M. fortuitum, M. luteus [14,17,21,29,30] |
| Gram-negative bacteria | E. coli, S. typhimurium, S. flexniri [14,17,21,30,39] | ||
| Virus | Norwalk virus [24], human papillomavirus [40], Influenza virus [31] | ||
| Fungi | C. neoformans [17] | ||
| |
|
Parasites |
L. amazonensis, L. major, L. braziliensis, L. Mexicana [32,33] |
| H3 | Full-length | Gram-positive bacteria | S. aureus [41] |
| Gram-negative bacteria | E. coli [39] | ||
| Virus | Norwalk virus [24], Influenza virus [31] | ||
| Fungi | Unknown | ||
| |
|
Parasites |
Unknown |
| H4 | Full-length | Gram-positive bacteria | S. aureus, P. acnes, M. luteus [19,29,41] |
| Gram-negative bacteria | E. coli [39] | ||
| Virus | Norwalk virus [24], Influenza virus [31] | ||
| Fungi | Unknown | ||
| Parasites | Unknown | ||
| Fragmented | Gram-positive bacteria | B. subtilis and S. aureus [42] | |
| Gram-negative bacteria | E. coli and P. aeruginosa [43] | ||
| Virus | Unknown | ||
| Fungi | Unknown | ||
| Parasites | Unknown |
• Direct antimicrobial effects of histones
The antimicrobial activity of histones has been long known, with first reports of antimicrobial activity of histones and histone-like proteins originating from 1942 [44]. In 1958, Hirsch showed the bactericidal activity of arginine-rich histones against both Gram-positive and Gram-negative bacteria [45].
Antibacterial activity
In villus epithelial cells, located in the small intestine, cytoplasmic H1 provides protection against infection by Salmonella typhimurium CS105, but not wild-type S. typhimurium [20]. While undergoing apoptosis, detached epithelial cells also release both full-length and fragmented H1, both of which display anti-staphylococcal activity similar to cellular H1, possibly providing extracellular protection [20]. More recently, it was shown that H1 bound to lipid droplets inside Drosophila cells is released upon cellular stimulation with endotoxin or lipotechoic acid, resulting in killing of both intracellular Gram-positive and Gram-negative bacteria [13].
While Hirsch suggested that lysine-rich histones did not possess antibacterial activity, many different observations have proven otherwise since his report in 1958. Histone H2A and H2B are present on the epithelial surface of the placenta, providing the placenta and the fetus protection against microbial infection [46]. Both H2A and H2B possess the capacity to neutralize endotoxin, and show antimicrobial activity against Escherichia coli [46]. In addition, antibacterial activity of H2A and H2B against both Gram-positive and Gram-negative bacteria has been shown in chicken [14], trout [47] and shrimp [29].
The antibacterial activity of the arginine-rich histones is less well studied. While histone H3 shows antibacterial activity against both E. coli and Staphylococcus aureus (although less potent than H2B) [39], not much is known about other antimicrobial effects. Human sebocytes (sebum-producing epithelial cells) release H4, which displays bactericidal activity against S. aureus and Propionibacterium acnes, a Gram-positive human skin commensal [19]. The antibacterial activity of H4 is enhanced by the presence of free fatty acids present on the skin [19]. These findings were strengthened by the observation that calf thymus H4 kills both E. coli and S. aureus [39]. By contrast, post-translationally modified H4 isolated from the skin of a Japanese tree frog did not show antibacterial activity against these bacteria [48]. In the case of other cationic antimicrobial peptides, very small differences in amino acid sequence can result in major differences in antimicrobial activity (e.g., the human neutrophil defensins differing by only one amino acid have major differences in activity vs Candida albicans) [49]. Further mechanistic studies are needed to understand the role of specific domains or amino acids in antimicrobial activity of histones.
Mechanism of action in E. coli
Lysine-rich and arginine-rich histones have different mechanisms by which they induce bacterial killing. The lysine-rich histone H2B can penetrate the bacterial cell membrane and bind to bacterial DNA. In contrast, the arginine-rich H3 and H4 mediate their antimicrobial activity through destruction of the cell membrane [39]. The lysine-rich histone H1 also disrupts the bacterial membrane, as shown in salmon [23]. The antimicrobial activity of some histones seems to depend in part on cleavage by the bacterial protease OmpT [39]. Interestingly, in the absence of OmpT, H2B is no longer able to penetrate the bacterial cell membrane and all histones are found on the cell membrane. However, although the MIC values of all histones increase significantly (with H2B most affected) in the absence of OmpT, the antibacterial activity of histones remains, indicating the presence of a separate mechanism [39]. One hypothesis suggested by Tagai et al. [39] to explain the antimicrobial action in the absence of OmpT is disruption and destabilization of cellular function by binding to endotoxin and possibly other cell membrane structures [39,50].
Mechanism of action in S. aureus
In contrast to E. coli, no bacterial protease of S. aureus is involved in the antimicrobial activity of histones [41]. H2B, H3 and H4 all remain on the bacterial cell membrane. Both H3 and H4 are capable of disrupting cell membrane structure (resembling the activity of pore-forming AMPs). Histone H2B does not cause any morphological abnormalities and binds to lipotechoic acid, thereby destabilizing cell integrity [41].
Modulation of antibacterial activity of histones by bacteria
As with most other host defense mechanisms, it seems that bacteria have developed methods to resist killing by histones. Finegoldia magna, a Gram-positive commensal of the skin and mucous membranes, is able to bind histones using a surface protein known as FAF. In addition, it secretes a protease, known as SufA, capable of degrading histones [51].
Antiviral activity
In contrast to the antibacterial activity of histones, the antiviral activity is not as well investigated. The first report originates from 1966, when Connolly et al. reported that calf and fowl histones reduce infectivity of Semliki virus in chick embryo fibroblasts [52]. A more recent report from 2003 shows both the linker histone H1 and the core histones can inhibit attachment of Norwalk virus by binding to both the viral particles and the cell membrane [24]. This interaction of histones on Norwalk virus seems to be specific, as infection of hepatitis E, poliovirus or the insect virus AcNPV were not inhibited. Another role for cytosolic histone H2B in the recognition of viral dsDNA showed where H2B acts as a cytosolic sensor for viral dsDNA in human cells [40]. Binding of viral dsDNA by H2B results in activation of innate antiviral pathways, as well as inhibition of viral multiplication. We have recently found that histones are able to neutralize influenza A viruses with H3 and H4 having greater antiviral activity than H1, H2A and H2B [31]. We reported on the activity of histone H4 most extensively since it was the most potent at inhibiting viral infectivity. In our study the antiviral effect of the H4 was mediated by direct interaction with the virus rather than the host cells. In addition, H4 did not cause cell injury in a wide range of concentrations that reduced viral infectivity. H4 was able to strongly aggregate viral particles and this was associated with reduced uptake of the virus by target cells. Further studies of antiviral mechanisms of histones are warranted.
Antiparasitic activity
Histones have also been shown to display antiparasitic activity. Leishmania promastigotes, the infectious form transmitted through the insect vector, are trapped in NETs, resulting in rapid killing [32]. Immunoneutralization of histones can abrogate killing of the parasites, supporting the antiparasitic activity of histones. In addition, purified H2A and H2B, but not H1, can kill Leishmania promastigotes [33].
Antifungal activity
Although Candida albicans can be trapped and killed by NETs, histones may not be involved in this process. Up to 200 µg/ml of H2A or a mixture of both core and linker histones is not enough to kill C. albicans [53] while other Candida species, such as Candida tropicalis, have been shown to be susceptible to histone killing [25]. In addition, histone H1 and H2B display fungicidal activity against Cryptococcus neoformans A in mice [17]. Similar results have been obtained using histone extracts in dogs, mice and calves. In these cases lysine-rich histones were more fungicidal while arginine-rich histones were fungistatic [54].
Cleavage products of histones
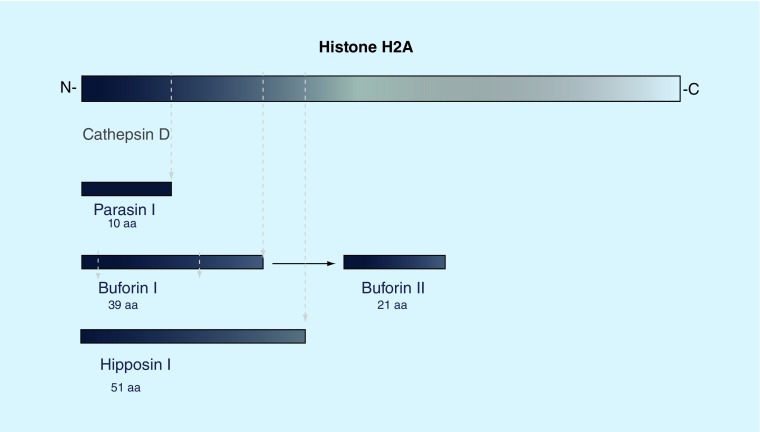
We refer readers to an excellent review of anti-bacterial properties of naturally occurring fragments of histone H1, H2A and H2B was provided by Kawasaki and Iwamuro for a detailed discussion of this topic [55]. Figure 2 illustrates fragments of H2A that have been described. Fragments of H1 have been found in fish skin and fragments of H2A have been found prominently in frog stomach and fish skin and these fragments in natural or synthetic form have strong antibacterial activity. Recent studies have evaluated the mechanisms of anti-bacterial activity of histone fragments. Like H2A, the H2A fragment buforin II found in frog stomach can penetrate the cell membrane, after which it is proposed to bind to bacterial DNA or RNA without causing cell lysis, resulting in rapid cellular death [56]. This suggestion is supported by the general correlation between DNA affinity and antimicrobial activity of buforin II [57]. Using mutational studies, the H2A fragment parasin (from fish skin) was shown that a basic residue at the N-terminal end is required for binding to the cell membrane, while the α-helical structure of the peptide is responsible for maintaining antimicrobial activity [58]. A slightly larger N-terminal cleavage product (51 aa) of histone H2A, known as hipposin I, was discovered in Atlantic halibut [34]. A recent study found that the N-terminal domain of hipposin is responsible for bacterial membrane permeabilization and killing, while a C-terminal fragment (termed HipC) can enter cells without killing the bacteria [59]. Such studies raise interest in the potential of selective use of fragments of histones, or novel combinations of histone fragments, for antimicrobial therapy as proposed by Kawasaki et al. [55]. The mechanisms through which histone fragments are generated in vivo are unclear and this also deserves more study.
Figure 2. . Cleavage products of histone H2A.
Cleavage products of histone H2A have been found in a number of species. In Parasilus asotus, an N-terminal 19aa fragment known as Parasin I is cleaved from full-length H2A by cathepsin D upon epidermal injury. Buforin I has been identified as a 39aa cleavage product and can be isolated from the stomach of Bufo bufo gargarizans. A smaller, 21aa peptide can be derived from Buforin I. A larger N-terminal fragment, hipposin I, was found in Hippoglossus hippoglossus L.
To our knowledge, no natural fragments of histone H3 have been identified yet. However, H4 fragments with antimicrobial activity have been isolated. Histogrannin, a slightly modified fragment corresponding to the C-terminal aa86–100 was first isolated from bovine adrenal medulla [18]. Unlike all other fragments, histogrannin is not derived from full-length H4, but is actually synthesized from a separate mRNA variant known as H4-v.1 [42]. More recently, histogranin was shown to inhibit growth of Gram-positive and Gram-negative bacteria by inhibiting the activity of ATP-dependent DNA gyrase, strongly resembling the activity of quinolone antibiotics [43].
Histones & neutrophil extracellular trap
Using phagocytosis, neutrophils take up pathogens, which are rapidly killed after fusing of the phagosome with cytoplasmic granules containing a variety of antimicrobial components [60]. Beside their phagocytic function, neutrophils can also induce extracellular killing of microorganisms using NETs [30]. The formation of NETs is generally a cell-destructive process, in which nuclear material disassembles and intracellular organelle membranes are disintegrated. The rupture of the plasma membrane results in the release of a mixture of DNA and several granular and cytoplasmic proteins (such as myeloperoxidase and elastase), which is then used to trap and kill a broad variety of pathogens. Since NETs and histones in NETs can mediate injurious effects as well (see below), it is felt that a balance is needed between host defense benefits and inflammatory injury caused by NETs [61].
• Composition of NETs
All core histones are found in NETs and 70% of NET-associated proteins comprise histones [62]. Some studies report the presence of H1 in NETs [30], while other studies indicate that H1 is degraded during NET formation [63]. In line with these findings, the presence of all core histones, but not linker histones, was demonstrated using immunofluorescence [62]. In unstimulated neutrophils, all core histones are present in equal amounts. However, in NETs, H3 and H4 are found in lower concentrations than H2A and H2B [62]. Antibodies against H2A and H2B prevent NET-mediated bacterial killing, indicating the importance of histones [30]. The linker histone H1 has also recently been found in NETs where it can form an epitope for auto-antibody formation [64]. Of note, a recent study reached somewhat different conclusions through showing that DNA itself plays a key role in the antibacterial activity of NETs through calcium chelation [65].
Histones in NETs are generally citrullinated by the enzyme peptidylarginine deiminase 4 (PAD4). PAD4 appears to be essential for chromatin decondensation and NET formation in most situations [66,67]. Of note, in one study citrullination of histones reduced their antibacterial activity [67]. There is also evidence that elastase and neutrophil oxidant production are needed for NET formation. Mice lacking PAD4 cannot form NETs and had worse outcome in a model of necrotizing fasciitis infection [67]. In contrast, these mice had similar response to sepsis induced by cecal ligation and puncture and were partially protected from endotoxin-induced shock [68]. An interesting recent report raised some question about the importance of NETs in human defense against infection [69,70]. Sorensen et al. described a patient with Papillon–Lefevre syndrome resulting from lack of neutrophil serine proteases. The patient presented with severe periodontal disease but otherwise did not have pronounced history of recurrent infections. Neutrophils in the patient lacked elastase and could not produce NETs, but were able to kill bacteria normally in vitro. These results suggest that NETs (and by extension NET-associated histones) do not play a major role in defense against bacteria in humans. This conflict may be in part resolved by recent findings that NETs can be formed by more than one process [71–74]. The ‘classic’ mechanism of NET formation involves death of the neutrophil (recently termed ‘suicidal NETosis’). However, another mechanism was recently described called ‘vital NETosis’ in which the cells survive and continue to carry out other typical neutrophil functions. The triggers and in vivo importance of the ‘vital NETosis’ are less well understood. The role of histones in ‘vital NETosis’ also need to be clarified. It should also be noted that NETs contain both nuclear and mitochondrial DNA and the relative contribution of each to their functional activities is not clear [75]. Clearly there is much yet to be learned about the host defense or injurious effects (see below) of NETs.
The role of NETs in viral infection is receiving increasing attention [76]. Influenza viruses induce NET formation in vivo and in vitro [77,78]. The role of NETs in host defense against influenza virus is open to question since PAD4-/- mice clear the virus as well as wild-type mice, although the PAD4-/- mice had less weight loss in this setting [79]. NETs and histones deposited in the liver vasculature have been found to contribute to clearance of intravenous pox viruses [80]. There is some evidence that virus-induced NET formation is mediated by distinct mechanisms from NETs formed in response to bacteria or classic neutrophil stimuli like phorbol ester [76,77]. As in the case of NET production during bacterial infection, there is evidence of NETs both in viral clearance and virus-related tissue injury. Furthermore, the specific role of histones within NETs in their antiviral or pro-inflammatory effects has not been dissected out as yet.
Histones as inducers of inflammation & thrombosis
As with many mediators of innate immunity, histones also have been shown to trigger inflammatory responses and even host cell injury under certain circumstances. For a summary, see Figure 3. As noted, histones are major components of neutrophil NETs. NETs are found to contribute to adverse inflammatory responses in auto-immune diseases such as systemic lupus erythematosus [81–83] or respiratory impairment in cystic fibrosis [61,84,85] or chronic obstructive pulmonary disease [86]. Some studies have histones of 50 µg/ml or more have been found to induce death of endothelial and lung epithelial cells [87]. In this study, NETs were also shown to have this effect and histones were found to be the major component of NETs responsible for cell death. Free histones in blood have been implicated as major mediators of sepsis physiology [88]. Extracellular histones can also induce thrombin generation and promote thrombosis [89–91]. In addition, histones can induce thrombocytopenia [92]. Extracellular histones have also been found to mediate liver injury [93,94]. Lung injury occurring during major trauma, after transfusions or after C5a generation, has also found to be mediated by histones [22,95–97]. In the case of transfusion-related acute lung injury, platelet activation resulted in NET formation in blood and lungs and anti-histone antibody or DNase were protective [97]. As noted above, PAD4 gene-deleted mice which cannot citrullinate histones or form NETs were protected from LPS-induced sepsis physiology [68].
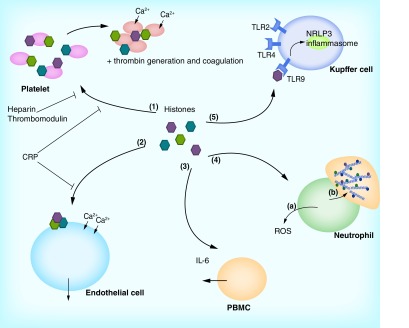
Figure 3. . Mechanisms of histone-induced cellular damage.
When released from cells, histones can damage host cells and modulate inflammatory responses. Histones can bind to platelets (1), which results in calcium influx, platelet activation and aggregation. In addition, histones induce coagulation and the generation of thrombin. Histone-mediated injury can be reduced by administration of heparin and thrombomodulin, as well as CRP, which binds directly to histones. Direct cellular toxic effects can be observed in endothelial cells (2). Histones bind to the endothelial cell membrane, resulting in cell permeabilization, calcium influx and cell death. This can be inhibited by CRP. In addition, histones can activate phagocytic cells (3–5). PBMCs can be triggered to secrete IL-6 (3). When neutrophils are stimulated with histones, neutrophils release ROS as part of the neutrophil respiratory burst response (4a). Moreover, histones trigger NET formation in neutrophils (4b). Last, histones mediate activation of NRLP3 inflammasomes in Kupffer cells (5), which results in sterile liver injury. This inflammasome activation is thought to be activated through binding of histones to TLR9. In addition, histones can also activate TLR2 and TLR4.
ROS: Reactive oxygen species.
• Mechanisms of injury caused by histones
Direct toxic effects on cells
Histones bind to endothelial cells and cause cell permeabilization leading to calcium influx and cell death at concentrations in the range of 50 µg/ml [22,95,96]. Levels of free histones in this range or higher were found in serum of patients with severe trauma, pancreatitis or sepsis [95]. In these cases it is unclear if the histones originated in NETs or just from death of other cells. H4 has been demonstrated in bronchoalveolar lavage fluid as well in patients with acute lung injury due to bacterial pneumonia or sepsis [96], and in this case neutrophils appear to be the major source. As noted histones have been implicated as a major mediator of epithelial and endothelial cell death induced by NETs [84,87].
Direct activation of phagocytic cells by histones
Histones have been shown to directly stimulate production of IL-6 by peripheral blood mononuclear cells and to trigger NET formation by neutrophils [22]. We have recently found that histones H3 and H4 are strong, direct stimulators of neutrophil respiratory burst responses [Hoeksema et al., Unpublished Data]. Sterile liver injury has been shown to be mediated by the activation of NLRP3 inflammasomes in Kupffer cells by extracellular histones [93]. In this case binding of histones to TLR9 was implicated as the cause of inflammasome activation. In another study of fatal liver injury in mice, activation of TLR2 and TLR4 by histones was found to be critical [94].
Platelet activation & thrombin generation by histones
As noted above, histones can bind to platelets, cause platelet calcium influx, activation and aggregation and also can induce thrombin generation and trigger coagulation [88–90]. These events have been directly linked to lung injury after trauma or transfusions [22,97–99]. PAD4 and neutrophils have been shown to be critical for deep-vein thrombosis in mice [100].
• Mechanisms through which histone-induced coagulation & inflammation are (or can be) modulated
A variety of endogenous or pharmacological mediators have been shown to protect against injury caused by histones. Inhibiting injury induced by histones also involve blocking of platelet activation, including depletion of platelets or administration of thrombomodulin, aspirin or antibodies to glycoprotein IIb/IIIa [92,97,99]. Heparin has been shown to prevent binding of histones to platelets and protect against thrombocytopenia, tissue injury and death caused by histone infusion [92]. Activated protein C cleaves histones and has been shown to be protective in models of sepsis and after severe trauma [88,101]. Of interest, histones present in NETs were shown to be protected against degradation by activated protein C [87].
Another mechanism of protection against histone-induced injury relates to proteins that directly bind to histones and inhibit their ability to bind to, activate or injure cells. Several studies have shown that anti-histone antibodies can be protective in mouse models of sepsis or injury [22,97,101]. CRP is an acute phase reactant that is elevated in infection and injury and contributes to innate defense in various ways. Recently, it was shown that CRP binds directly to histones and that it is present in complexes with histones in serum of patients with severe trauma [95]. Serum from trauma patients containing high levels of histones causes injury to endothelial cells and this effect is attenuated when high levels of CRP are also present. Histones that have bound to CRP cause less platelet aggregation and less thrombocytopenia and thrombin generation in mice. This effect also results in less lung injury and improved survival. Hence, CRP appears to be an endogenous modulator of the damaging effects of histones. The interalpha inhibitor protein in plasma and its associated glycosaminoglycans was also recently shown to bind to free histones and protect against injurious effects of histones in vitro and in vivo [102]. We have recently found that the host defense lectin, surfactant protein D, also binds to histones and reduces their ability to trigger neutrophil respiratory burst responses [Hoeksema et al. Unpublished Data].
Finally, since histones originate in NETs in various inflammatory states, and histones can in turn trigger neutrophil activation, depletion of neutrophils has been shown to be protective in some studies as noted above [96]. This is obviously not a practical solution in clinical situations; however, methods to inhibit neutrophil activation or oxidant release could be tested for their ability to reduce histone release. In addition, inhibition of neutrophil elastase or PAD4 enzyme activity are other means to reduce NET formation.
• Can histone-induced inflammation be protective?
Most studies thus far have focused on adverse effects of histone-induced inflammation. It is less clear if extracellular histones are a protective part of the innate immune response through immune-modulatory effects. Various cationic antimicrobial peptides contribute to innate defense not only through their direct antimicrobial effects but also through their ability to up or downregulate phagocyte activation [103,104] or modulate activation of lymphocytes or dendritic cells [105,106]. Activation of platelet aggregation and coagulation by histones could be protective against infection or sterile wounds. Similarly, activation of immune cells in a controlled manner by histones could contribute to host defense as well. Unfortunately there are currently little data to support or refute this hypothesis.
Conclusion
Histones are highly conserved among different species and they appear to represent a very ancient and ubiquitous element of the innate immune system in the living world. There is now extensive evidence of in vitro antibacterial activity and emerging evidence of antiviral, antifungal and antiparasitic activity for core histones and the mechanisms of action are beginning to be defined. Overall, extracellular histones exert their antimicrobial effects in a manner similar to other cationic antimicrobial peptides. One of the clearest in vivo contexts in which histones exert antimicrobial effects in vivo is as part of NETs. However, cytoplasmic or extracellular histones are present in a variety of contexts [55], such as lipid droplets [13], the skin [19,35] and the placenta [46], where they contribute to host defense.
Like many other innate defense mediators [107], histones have key functions unrelated to host defense. As examples, pulmonary surfactant protein D has roles in surfactant lipid homeostasis and some antimicrobial peptides (e.g., LL-37) have roles in wound healing and epithelial barrier function [108,109]. Also, as with other innate defense mediators, there is evidence that extracellular histones can contribute to cell injury, harmful inflammation and activation of thrombosis.
Future perspective
It is clear that a great deal more needs to be learned about the role of histones in host defense and inflammation. A key question for future studies is the mechanism of release of extracellular histones. The sources and regulation of extracellular histones other than those derived from NETs or cell necrosis are relatively unknown. Much of the current literature on histones derive from in vitro studies or studies of species other than humans. In addition, histones can be released in free form or complexed with DNA (with associated changes in structure), and can be post-translationally modified in various ways. These features have not been evaluated extensively in reports of anti-microbial activity of histones. It is hoped that future studies will focus on human models, specific mechanisms of bacterial or viral killing, specific receptors for histones on immune cells and mechanisms of clearing of histones. The roles of NETs in bacterial and viral infection, and of histones in NETs, need to be clarified. Important clinical outcomes that could result from these studies include adaptation of histones or active antimicrobial subdomains of histones [55] for therapeutic purposes and means to inhibit histone (or NET) release or abet clearance of histones to reduce inflammatory injury.
EXECUTIVE SUMMARY.
In addition to binding DNA, histones are released in cytoplasm and the extracellular space where they appear to serve functions unrelated to DNA homeostasis.
Histones directly kill a wide range of pathogens in a manner similar to other antimicrobial peptides.
Some bacteria have acquired methods to counteract antimicrobial activity of histones.
Histones are a major component of neutrophil extracellular traps (NETs) and mediate some of the beneficial and harmful effects of NETs. An important topic for future study will be to determine the extent to which histones are released and act in free form versus as part of NETs in vivo.
Histones promote inflammation and coagulation during infection sometimes leading to injury.
Several proteins and heparin bind to histones and modulate their procoagulant and proinflammatory effects, suggesting active regulation of histone-induced inflammation.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Allan J, Hartman PG, Crane-Robinson C, Aviles FX. The structure of histone H1 and its location in chromatin. Nature. 1980;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- 3.Delange RJ, Smith EL. Histones: structure and function. Annu. Rev. Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- 4.Arents G, Burlingame RW, Wang BC, Love WE, Moudrianakis EN. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc. Natl Acad. Sci. USA. 1991;88(22):10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005;83(3):344–353. doi: 10.1139/o05-041. [DOI] [PubMed] [Google Scholar]
- 6.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragunathan K, Jih G, Moazed D. Epigenetics. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science. 2015;348(6230):1258699. doi: 10.1126/science.1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi A, Shimizu S, Hirota J, et al. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell. 2003;114(6):673–688. doi: 10.1016/s0092-8674(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 9.Garg M, Perumalsamy LR, Shivashankar GV, Sarin A. The linker histone h1.2 is an intermediate in the apoptotic response to cytokine deprivation in T-effectors. Int. J. Cell. Biol. 2014;2014:674753. doi: 10.1155/2014/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhlhausser P, Muller EC, Otto A, Kutay U. Multiple pathways contribute to nuclear import of core histones. EMBO J. 2001;2(8):690–696. doi: 10.1093/embo-reports/kve168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlatanova JS, Srebreva LN, Banchev TB, Tasheva BT, Tsanev RG. Cytoplasmic pool of histone H1 in mammalian cells. J. Cell Sci. 1990;96(Pt 3):461–468. doi: 10.1242/jcs.96.3.461. [DOI] [PubMed] [Google Scholar]
- 12.Watson K, Edwards RJ, Shaunak S, et al. Extra-nuclear location of histones in activated human peripheral blood lymphocytes and cultured T-cells. Biochem. Pharmacol. 1995;50(3):299–309. doi: 10.1016/0006-2952(95)00142-m. [DOI] [PubMed] [Google Scholar]
- 13.Anand P, Cermelli S, Li Z, et al. A novel role for lipid droplets in the organismal antibacterial response. eLife. 2012;1:e00003. doi: 10.7554/eLife.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This reference shows a novel mechanism through which histones mediate host defense.
- 14.Li GH, Mine Y, Hincke MT, Nys Y. Isolation and characterization of antimicrobial proteins and peptide from chicken liver. J. Pept. Sci. 2007;13(6):368–378. doi: 10.1002/psc.851. [DOI] [PubMed] [Google Scholar]
- 15.Park CB, Kim MS, Kim SC. A novel antimicrobial peptide from Bufo bufo gargarizans . Biochem. Biophys. Res. Commun. 1996;218(1):408–413. doi: 10.1006/bbrc.1996.0071. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H, Isaacson T, Iwamuro S, Conlon JM. A protein with antimicrobial activity in the skin of Schlegel's green tree frog Rhacophorus schlegelii (Rhacophoridae) identified as histone H2B. Biochem. Biophys. Res. Commun. 2003;312(4):1082–1086. doi: 10.1016/j.bbrc.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 17.Hiemstra PS, Eisenhauer PB, Harwig SS, Van Den Barselaar MT, Van Furth R, Lehrer RI. Antimicrobial proteins of murine macrophages. Infect. Immun. 1993;61(7):3038–3046. doi: 10.1128/iai.61.7.3038-3046.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaire S, Shukla VK, Rogers C, et al. Isolation and characterization of histogranin, a natural peptide with NMDA receptor antagonist activity. Eur. J. Pharmacol. 1993;245(3):247–256. doi: 10.1016/0922-4106(93)90104-h. [DOI] [PubMed] [Google Scholar]
- 19.Lee DY, Huang CM, Nakatsuji T, et al. Histone H4 is a major component of the antimicrobial action of human sebocytes. J. Invest. Dermatol. 2009;129(10):2489–2496. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose FR, Bailey K, Keyte JW, Chan WC, Greenwood D, Mahida YR. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect. Immun. 1998;66(7):3255–3263. doi: 10.1128/iai.66.7.3255-3263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silphaduang U, Hincke MT, Nys Y, Mine Y. Antimicrobial proteins in chicken reproductive system. Biochem. Biophys. Res. Commun. 2006;340(2):648–655. doi: 10.1016/j.bbrc.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 22.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013;187(2):160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards RC, O'Neil DB, Thibault P, Ewart KV. Histone H1: an antimicrobial protein of Atlantic salmon (Salmo salar) Biochem. Biophys. Res. Commun. 2001;284(3):549–555. doi: 10.1006/bbrc.2001.5020. [DOI] [PubMed] [Google Scholar]
- 24.Tamura M, Natori K, Kobayashi M, Miyamura T, Takeda N. Inhibition of attachment of virions of Norwalk virus to mammalian cells by soluble histone molecules. Arch. Virol. 2003;148(9):1659–1670. doi: 10.1007/s00705-003-0143-4. [DOI] [PubMed] [Google Scholar]
- 25.Kashima M. H1 histones contribute to candidacidal activities of human epidermal extract. J. Dermatol. 1991;18(12):695–706. doi: 10.1111/j.1346-8138.1991.tb03160.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes JM, Molle G, Kemp GD, Smith VJ. Isolation and characterisation of oncorhyncin II, a histone H1-derived antimicrobial peptide from skin secretions of rainbow trout, Oncorhynchus mykiss . Dev. Comp. Immunol. 2004;28(2):127–138. doi: 10.1016/s0145-305x(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 27.Luders T, Birkemo GA, Nissen-Meyer J, Andersen O, Nes IF. Proline conformation-dependent antimicrobial activity of a proline-rich histone h1 N-terminal Peptide fragment isolated from the skin mucus of Atlantic salmon. Antimicrob. Agents Chemother. 2005;49(6):2399–2406. doi: 10.1128/AAC.49.6.2399-2406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrzykat A, Zhang L, Mendoza V, Iwama GK, Hancock RE. Synergy of histone-derived peptides of coho salmon with lysozyme and flounder pleurocidin. Antimicrob. Agents Chemother. 2001;45(5):1337–1342. doi: 10.1128/AAC.45.5.1337-1342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patat SA, Carnegie RB, Kingsbury C, Gross PS, Chapman R, Schey KL. Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. Eur. J. Biochem. 2004;271(23–24):4825–4833. doi: 10.1111/j.1432-1033.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]; •• This is a seminal paper providing the first detailed description of neutrophil extracellular traps (NETs).
- 31.Hoeksema M, Tripathi S, White M, et al. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immun. 2015;21(7):736–745. doi: 10.1177/1753425915593794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimaraes-Costa AB, Nascimento MT, Froment GS, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl Acad. Sci. USA. 2009;106(16):6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Chen Y, Xin L, et al. Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 2011;79(3):1124–1133. doi: 10.1128/IAI.00658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birkemo GA, Luders T, Andersen O, Nes IF, Nissen-Meyer J. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.) Biochim. Biophys. Acta. 2003;1646(1–2):207–215. doi: 10.1016/s1570-9639(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 35.Park IY, Park CB, Kim MS, Kim SC. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus . FEBS Lett. 1998;437(3):258–262. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- 36.Cho JH, Sung BH, Kim SC. Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta. 2009;1788(8):1564–1569. doi: 10.1016/j.bbamem.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Cho JH, Park IY, Kim HS, Lee WT, Kim MS, Kim SC. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002;16(3):429–431. doi: 10.1096/fj.01-0736fje. [DOI] [PubMed] [Google Scholar]
- 38.Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC. Structure–activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl Acad. Sci. USA. 2000;97(15):8245–8250. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an important paper that describes a mechanism of antibacterial activity of a histone fragment.
- 39.Tagai C, Morita S, Shiraishi T, Miyaji K, Iwamuro S. Antimicrobial properties of arginine- and lysine-rich histones and involvement of bacterial outer membrane protease T in their differential mode of actions. Peptides. 2011;32(10):2003–2009. doi: 10.1016/j.peptides.2011.09.005. [DOI] [PubMed] [Google Scholar]; • This paper is important for beginning to define antibacterial mechanisms of histones.
- 40.Kobiyama K, Takeshita F, Jounai N, et al. Extrachromosomal histone H2B mediates innate antiviral immune responses induced by intracellular double-stranded DNA. J. Virol. 2010;84(2):822–832. doi: 10.1128/JVI.01339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This reference is one of the few that defines a specific antiviral mechanism for histones.
- 41.Morita S, Tagai C, Shiraishi T, Miyaji K, Iwamuro S. Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against Gram-positive Staphylococcus aureus . Peptides. 2013;48:75–82. doi: 10.1016/j.peptides.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Lemaire S, Rogers C, Dumont M, et al. Histogranin, a modified histone H4 fragment endowed with N-methyl-D-aspartate antagonist and immunostimulatory activities. Life Sci. 1995;56(15):1233–1241. doi: 10.1016/0024-3205(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 43.Lemaire S, Trinh TT, Le HT, et al. Antimicrobial effects of H4-(86–100), histogranin and related compounds-possible involvement of DNA gyrase. FEBS J. 2008;275(21):5286–5297. doi: 10.1111/j.1742-4658.2008.06659.x. [DOI] [PubMed] [Google Scholar]; • This paper is one of the few describing an antibacterial mechanism for a histone fragment.
- 44.Miller BF, Abrams R, Dorfman A, Klein M. Antibacterial properties of protamine and histone. Science. 1942;96(2497):428–430. doi: 10.1126/science.96.2497.428. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch JG. Bactericidal action of histone. J. Exp. Med. 1958;108(6):925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HS, Cho JH, Park HW, Yoon H, Kim MS, Kim SC. Endotoxin-neutralizing antimicrobial proteins of the human placenta. J. Immunol. 2002;168(5):2356–2364. doi: 10.4049/jimmunol.168.5.2356. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes JM, Kemp GD, Molle MG, Smith VJ. Anti-microbial properties of histone H2A from skin secretions of rainbow trout, Oncorhynchus mykiss . Biochem. J. 2002;368(Pt 2):611–620. doi: 10.1042/BJ20020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawasaki H, Iwamuro S, Goto Y, Nielsen PF, Conlon JM. Characterization of a hemolytic protein, identified as histone H4, from the skin of the Japanese tree frog Hyla japonica (Hylidae) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;149(1):120–125. doi: 10.1016/j.cbpb.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Raj PA, Antonyraj KJ, Karunakaran T. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem. J. 2000;347 Pt 3:633–641. [PMC free article] [PubMed] [Google Scholar]
- 50.Augusto LA, Decottignies P, Synguelakis M, Nicaise M, Le Marechal P, Chaby R. Histones: a novel class of lipopolysaccharide-binding molecules. Biochemistry. 2003;42(13):3929–3938. doi: 10.1021/bi0268394. [DOI] [PubMed] [Google Scholar]
- 51.Murphy EC, Mohanty T, Frick IM. FAF and SufA: proteins of Finegoldia magna that modulate the antibacterial activity of histones. J. Innate Immun. 2014;6(3):394–404. doi: 10.1159/000356432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connolly JH. Effect of histones and protamine on the infectivity of Semliki Forest virus and its ribonucleic acid. Nature. 1966;212(5064):858. doi: 10.1038/212858a0. [DOI] [PubMed] [Google Scholar]
- 53.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 54.Gadebusch HH, Johnson AG. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J. Infect. Dis. 1966;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- 55.Kawasaki H, Iwamuro S. Potential roles of histones in host defense as antimicrobial agents. Infect. Disord. Drug Targets. 2008;8(3):195–205. doi: 10.2174/1871526510808030195. [DOI] [PubMed] [Google Scholar]; •• This paper provides an excellent review of antimicrobial activities of vertebrate histones.
- 56.Park CB, Kim HS, Kim SC. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998;244(1):253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 57.Uyterhoeven ET, Butler CH, Ko D, Elmore DE. Investigating the nucleic acid interactions and antimicrobial mechanism of buforin II. FEBS Lett. 2008;582(12):1715–1718. doi: 10.1016/j.febslet.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 58.Koo YS, Kim JM, Park IY, et al. Structure–activity relations of parasin I, a histone H2A-derived antimicrobial peptide. Peptides. 2008;29(7):1102–1108. doi: 10.1016/j.peptides.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 59.Bustillo ME, Fischer AL, Labouyer MA, Klaips JA, Webb AC, Elmore DE. Modular analysis of hipposin, a histone-derived antimicrobial peptide consisting of membrane translocating and membrane permeabilizing fragments. Biochim. Biophys. Acta. 2014;1838(9):2228–2233. doi: 10.1016/j.bbamem.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segal AW. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans . PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell. Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dwivedi N, Neeli I, Schall N, et al. Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity. FASEB J. 2014;28(7):2840–2851. doi: 10.1096/fj.13-247254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halverson TW, Wilton M, Poon KK, Petri B, Lewenza S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015;11(1):e1004593. doi: 10.1371/journal.ppat.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015;11(3):189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This and the preceding paper are important for clarifying the role of PAD4 and citrullination of histones in formation and activity of NETs.
- 68.Martinod K, Fuchs TA, Zitomersky NL, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125(12):1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nauseef WM. Proteases, neutrophils, and periodontitis: the NET effect. J. Clin. Invest. 2014;124(10):4237–4239. doi: 10.1172/JCI77985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorensen OE, Clemmensen SN, Dahl SL, et al. Papillon–Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J. Clin. Invest. 2014;124(10):4539–4548. doi: 10.1172/JCI76009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pilsczek FH, Salina D, Poon KK, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]; • This paper is important for describing an alternative mechanism of NET formation.
- 72.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 73.Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo . Nat. Med. 2012;18(9):1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl Acad. Sci. USA. 2015;112(9):2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper delineates another alternative mechanism of NET formation.
- 75.Keshari RS, Jyoti A, Dubey M, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE. 2012;7(10):e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenne CN, Kubes P. Virus-induced NETs-critical component of host defense or pathogenic mediator? PLoS Pathog. 2015;11(1):e1004546. doi: 10.1371/journal.ppat.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tripathi S, Verma A, Kim EJ, White MR, Hartshorn KL. LL-37 modulates human neutrophil responses to influenza A virus. J. Leukoc. Biol. 2014;96(5):931–938. doi: 10.1189/jlb.4A1113-604RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS ONE. 2011;6(7):e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenne CN, Wong CH, Zemp FJ, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13(2):169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013;190(3):1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl Med. 2011;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA. 2010;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saffarzadeh M, Preissner KT. Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr. Opin Hematol. 2013;20(1):3–9. doi: 10.1097/MOH.0b013e32835a0025. [DOI] [PubMed] [Google Scholar]
- 85.Marcos V, Zhou Z, Yildirim AO, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat. Med. 2010;16(9):1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 86.Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J. Trauma Acute Care Surg. 2012;73(6):1389–1394. doi: 10.1097/TA.0b013e318270d595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011;9(9):1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 90.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper is important for describing a mechanism of thrombogenesis by histones.
- 91.Johansson PI, Windelov NA, Rasmussen LS, Sorensen AM, Ostrowski SR. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J. Emerg. Trauma Shock. 2013;6(3):171–175. doi: 10.4103/0974-2700.115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang H, Chen HW, Evankovich J, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 2013;191(5):2665–2679. doi: 10.4049/jimmunol.1202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011;187(5):2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abrams ST, Zhang N, Dart C, et al. Human CRP defends against the toxicity of circulating histones. J. Immunol. 2013;191(5):2495–2502. doi: 10.4049/jimmunol.1203181. [DOI] [PubMed] [Google Scholar]; •• This reference describes the in vitro and in vivo role of CRP in neutralizing harmful effects of free histones.
- 96.Bosmann M, Grailer JJ, Ruemmler R, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27(12):5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caudrillier A, Looney MR. Platelet–neutrophil interactions as a target for prevention and treatment of transfusion-related acute lung injury. Curr. Pharm. Des. 2012;18(22):3260–3266. doi: 10.2174/1381612811209023260. [DOI] [PubMed] [Google Scholar]
- 99.Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE. 2013;8(9):e75961. doi: 10.1371/journal.pone.0075961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl Acad. Sci. USA. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is a key paper in which the role of histones in mediating sepsis physiology is demonstrated.
- 102.Chaaban H, Keshari RS, Silasi-Mansat R, et al. Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015;125(14):2286–2296. doi: 10.1182/blood-2014-06-582759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tripathi S, White MR, Wang G, Hartshorn K. LL-37 modulates human phagocyte responses to influenza A virus. J. Leukoc. Biol. 2014;96(5):931–938. doi: 10.1189/jlb.4A1113-604RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tecle T, Tripathi S, Hartshorn KL. Review: defensins and cathelicidins in lung immunity. Innate Immun. 2010;16(3):151–159. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- 105.Yang D, Liu ZH, Tewary P, Chen Q, De La Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr. Pharm. Des. 2007;13(30):3131–3139. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 106.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr. Opin Immunol. 2005;17(4):359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Tripathi S, White MR, Hartshorn KL. The amazing innate immune response to influenza A virus infection. Innate Immun. 2015;21(1):73–98. doi: 10.1177/1753425913508992. [DOI] [PubMed] [Google Scholar]
- 108.Otte JM, Zdebik AE, Brand S, et al. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul. Pept. 2009;156(1–3):104–117. doi: 10.1016/j.regpep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 109.Carretero M, Escamez MJ, Garcia M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Invest. Dermatol. 2008;128(1):223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]