Abstract
Viral vectors based on adeno-associated virus (AAV) are leading candidates for therapeutic gene delivery. Understanding rate-limiting steps in the entry of AAV vectors may be used in a rational approach to improve efficiency and specificity of transduction. This review describes our current understanding of AAV entry, a key step during infection. We discuss the identity and functions of AAV receptors and attachment factors, including the recently discovered multi-serotype receptor AAVR. We further provide an overview of other host factors that act during the trafficking stage of AAV vector transduction. In particular, we focus on cellular protein complexes associated with retrograde transport from endosomes to the trans-Golgi network. The novel insights in AAV-host interactions facilitated by technological advances in genetic screening approaches provide a greater depth in our understanding how AAV vectors exploit host factors to deliver its genetic cargo to the nucleus.
Introduction
The success of gene therapy relies on the efficacious means to transfer functional genes into cells to correct for dysfunctional, endogenous genes. In the last three decades, recombinant virus vectors such as those derived from adenoviruses, retroviruses and lentiviruses, have emerged as highly efficient gene delivery vehicles, although safety issues are an ongoing concern [1,2]. Vectors derived from adeno-associated viruses (AAVs) have gained increasing popularity as delivery systems for therapeutic gene transfer, primarily due to their non-pathogenic and broadly tropic nature [2,3]. Initially discovered as a contaminant in a simian adenovirus preparation [4], AAV is a non-enveloped, single-stranded DNA virus with a small, icosahedral capsid of approximately 25 nm. It is classified as a member of the Parvoviridae family, although is easily distinguished from its fellow family members by its incapacity to replicate in the absence of a helper virus such as adenovirus or herpes simplex viruses [5]. To date, thirteen naturally occurring AAV serotypes of human and simian origin have been described and are evaluated for in vivo transduction (AAV1-13) [6]. They show a wide range of tissue transduction preferences [7] and generally display low immunogenicity and sustained transgene expression. Taken together with their safety profile, the use of AAV vectors in early and late-stage clinical trials for monogenic diseases such as hemophilia [8], Leber’s congenital blindness [9] and muscular dystrophy [10] has been remarkably successful [2,11].
Despite applications for AAV vectors advancing fairly rapidly, poor transduction efficiency in certain tissues and low organ specificity in particular treatments restricts their usage. Gaining further insights into fundamental aspects of the AAV life cycle is imperative to begin to close gaps in our knowledge regarding how AAV interacts with the host cell, and potentially improve future therapeutic applications. This review focuses on our current understanding of AAV entry, a key step during infection. We discuss the identity and functions of AAV receptors and attachment factors, which likely contribute to AAV tissue tropism. In addition, we highlight the importance of host factors that act during the trafficking stage of AAV infection, as they also have the potential to contribute significantly to AAV tropism and transduction efficiency.
AAV receptors and attachment factors
Viruses employ a variety of molecular mechanisms to enter a host cell. Some utilize one host receptor to mediate different steps required for virus entry. These steps can include virus attachment to the host cell surface, triggering of endocytosis and, eventually, escape from the endocytic pathway. Others make use of more than one receptor to facilitate these steps, through simultaneous or sequential interactions [12–14]. AAVs interact with specific glycans or glycoconjugates displayed on the cell membrane to mediate surface attachment. These molecules allow AAV particles to accumulate on the cell surface and gain access to specific proteinaceous co-receptors [15]. This paradigm where initial attachment to the cells via glycan receptors is followed by engagement with proteinaceous receptors that direct post-attachment steps is not unique to AAV, but does underscore the complexity of the AAV entry mechanism and the dependence on multiple host factors for the determination of viral tropism. Even though AAV serotypes share approximately 60–99% identity in their capsids [15], they display distinct cell and tissue tropisms, and differ significantly in their transduction efficiencies. This is, in part, due to the differential glycan receptor usage for surface attachment.
Glycan attachment
The majority of studies on AAV biology have utilized the first infectious clone of AAV (AAV2) as a model, with AAV2 being the initial serotype whose surface attachment receptor was identified as heparan sulfate proteoglycan (HSPG) two decades ago [16]. This discovery was followed by the structural elucidation of AAV2 [17], and identification of the HSPG interacting sites on the AAV2 particle [18,19]. Other AAV serotypes were also identified from human and simian sources, and found to associate to different glycan moieties. Direct binding, transduction assays, mutagenesis and structural approaches were developed to identify the capsid amino acid residues involved in glycan receptor recognition for most AAVs, and this knowledge has contributed significantly to capsid modifications for the purpose of improving transduction specificity and/or efficiency [20]. Three glycan receptor groups have been categorized as primary AAV attachment receptors: HSPG for AAV2, AAV3 and AAV13, O- and N-linked sialic acid moieties for AAV1, AAV4, AAV5 and AAV6 and N-linked galactose for AAV9 [15]. The glycan usage of AAV7, AAV8 and AAV10-12 is currently unknown.
Proteinaceous receptors
In addition to interacting with glycan moieties, several surface proteinaceous receptors have been identified for the different AAV serotypes (see Table 1). For AAV2, several of the putative (co)-receptors (c-MET, FGFR1, CD9) are thought to facilitate the interaction of AAV2 with HSPG because these receptors or their ligands intimately associate with HSPG [21–23]. Other (co)-receptors for various serotypes were identified through correlation studies between gene expression data and permissiveness of cells to AAV (EGFR, PDGFR) [24,25], or yeast two-hybrid screens (LamR) [26]. The relevance of these receptors has been demonstrated using different techniques and in different cell types (Table 1), although molecular and structural details of the interaction interface between the (co)-receptors and AAV are largely lacking. Additionally, it is still unclear how these co-receptors specifically facilitate entry, and what other host factors might be involved in the AAV internalization process.
Table 1.
Proteinaceous AAV receptors and their validation
| Proteinaceous receptor |
AAV Serotype |
Validation | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Loss of function studies |
Overexpression studies in poorly permissive cell lines |
Ligand/ecto- domain inhibition assay |
Receptor antibody inhibition assay |
Binding assay | Internalization assay |
Biodistribution /infection correlation |
In vivo evaluation |
|||
| aVβ5 integrin | AAV2 | – | YES (CS-1 cells) | – | – | YES (virus overlay assay) | YES (immunofluorescence) | – | – | [66] |
| Fibroblast growth factor receptor-1 (FGFR1) | AAV2 | – | YES in conjunction with HSPG (Raji cells) | YES; ligand (NIH3T3 and 293 cells) | – | YES (enhanced binding to FGFR1/HSPG-overexpressing Raji cells) | – | – | – | [23] |
| AAV3H | – | – | YES; ectodomain (KB cells) | – | YES (dot blot) | – | – | – | [67] | |
| Hepatocyte growth factor receptor (c-MET) | AAV2 | – | YES (NIH3T3 cells) | YES; ligand (HeLa cells) | YES (NIH3T3 cells overexpressing c-MET) | YES (virus overlay assay) | YES (immunofluorescence) | – | – | [21, 68] |
| AAV3 | YES; siRNA (HuH7 cells) | – | YES; ligand (HuH7 cells) | YES (HeLa and HuH7 cells) | – | – | – | Poor murine transduction by AAV3 | [68] | |
| A5β1 integrin | AAV2 | – | YES (CHOB2 and CS1 cells) | YES; ectodomain (HEK293 cells) | YES (293 cells) | YES (Solid phase binding profiles) | YES (wt AAV2 vs R513A mutant) | – | YES (Comparison of wt AAV2 with R513A mutant) | [69] |
| CD9 tetraspanin | AAV2 | Yes; siRNA (MCF7 and T47D cells) | YES (T47D, BT8Ca, rat BT12Ca cells) | – | YES - only in low HSPG cells | – | – | YES (HSPG-low vs HSPG-high cell types) | – | [22] |
| Laminin receptor (LamR) | AAV8, AAV2, AAV3, AAV9 | Yes; siRNA (NIH3T3 cells) | YES (NIH3T3 cells) | YES; ligand for AAV8 (HeLa cells) | YES for AAV8 (HeLa cells) | YES - AAV2, AAV8 (yeast bait/prey assay) | – | – | YES for AAV2, AAV8 (antibody inhibition assay) | [26] |
| Platelet-derived growth factor receptor (PDGFR) | AAV5 | YES; siRNA (NIH3T3 cells) and inhibitor treatment (NCI60 cells) | YES (HeLa and 32D cells) | YES; both (32D (ligand) and Cos (ectodomain) cells) | YES (co-precipitation) | YES | YES (co-immunofluorescence studies in brain) | [24] | ||
| Epidermal growth factor receptor (EGFR) | AAV6 | YES; siRNA (HEK293, HN13 cells) | YES (32D cells) | – | – | YES (co-precipitation) | YES (inhibitor use) | YES | YES (tumor-specific reduction) | [25] |
| Adeno-associated virus receptor (AAVR) | AAV1, AAV2, AAV3B, AAV5, AAV6, AAV8, AAV9 | YES; CRISPR/Cas9 (HAP1, HeLa, HEK293, U2OS, HuH7, K562, MEF, A549 cells) | YES (Caco-2, NIH3T3, Raji, HT29 cells) | YES; ectodomain (HeLa cells) | YES (HeLa cells) | YES (ELISA, SPR) | – | – | YES (knock-out studies) | [27] |
Recently, a genome-scale genetic screening approach was used to systematically identify genes required for AAV2 entry [27]. Genes necessary for heparan biosynthesis were amongst the 46 significant genes identified but none of the previously identified (co)-receptors. Instead, a poorly characterized transmembrane-containing protein, KIAA0319L, was the single highest scoring gene and was found, upon further examination, to be an essential host receptor for AAV2 as well as a broad range of other AAV serotypes. KIAA0319L was a key requirement for AAV2 transduction in human transformed cell lines derived from diverse tissues including the liver, kidney, and lung. Additionally, mice with a genetic knockout in this gene demonstrated a robust resistance to AAV9 infection. KIAA0319L was thus renamed AAV receptor (AAVR).
Notably, the ectodomain of AAVR consists of five polycystic kidney disease (PKD) domains, which are immunoglobulin (Ig)-like domains [28]. Members of the Ig-like superfamily comprise of Ig-like domains, and include several cell surface proteins such as coxsackie and adenovirus receptor (CAR) and the poliovirus receptor (PVR). These proteins are commonly exploited by viruses for cellular entry [29], possibly because of their adhesive nature and their capacity for rapid endocytosis which can be triggered by disruption of receptor homodimers after viral ligand binding [30]. AAV2 may thus utilize AAVR in a similar fashion given that it binds to AAVR in the PKD domain region of the ectodomain [27], although AAVR’s specific function in AAV entry is still unknown.
Possible functions of AAVR in AAV infection
It is surprising that one receptor is able to mediate cellular uptake of several serotypes, given the repertoire of glycan receptors used by AAVs, and the observation that AAVs appear to make use of a variety of mechanisms to enter the cell. Evidence indicates that AAV particles can be internalized via clathrin-mediated endocytosis [31], caveolar endocytosis, macropinocytosis [32] and the clathrin-independent carriers and GPI-enriched endocytic compartment (CLIC/GEEC) pathway [33]. However, not all routes contribute equally to productive transduction: the majority of AAV particles that enter cells are being trafficked through unproductive paths that do not lead to transduction. The trafficking path and required host factors can be a cell-type dependent [34] but pathways for diverse serotypes (AAV2 and AAV5) converge in the Golgi before proceeding to the nucleus [35–37]. Given that AAVR is rapidly endocytosed from the plasma membrane to the trans-Golgi network, a route that is strikingly similar to what we know of AAV’s trafficking, and that AAVR binds directly to AAV particles via its ectodomain, we propose three possible ways in which AAVR could be facilitating AAV infectivity (Figure 1): (i) AAVR interacts with AAV at the surface and aids in AAV cellular uptake into an endosomal pathway, (ii) AAVR interacts with AAV in the early endosomal system and facilitates trafficking to the trans-Golgi, (iii) AAVR interacts with AAV once it reaches the Golgi and facilitates escape from the trans-Golgi network into the cytoplasm. Further investigation is necessary to distinguish between these possible roles bearing in mind that they are not mutually exclusive.
Figure 1.
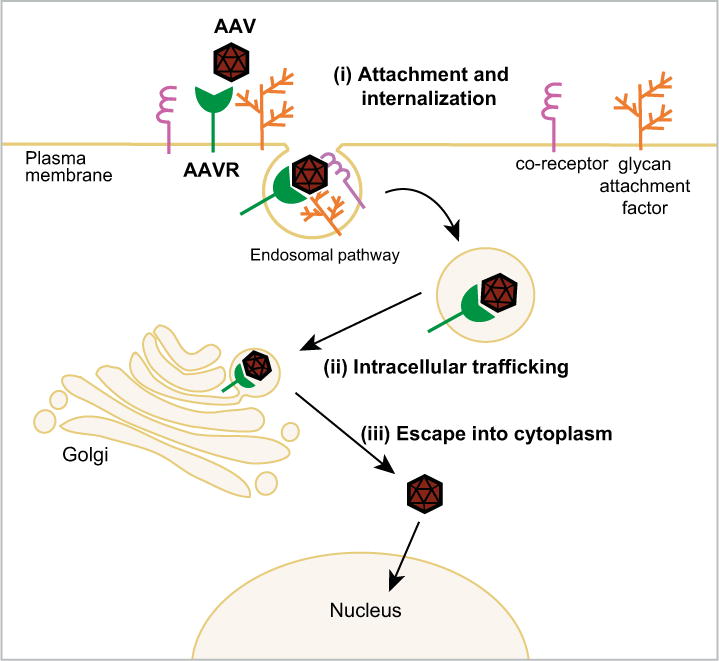
Schematic representation of the possible roles of AAVR in the AAV life cycle - (i) AAV binds to HSPG, allowing subsequent interaction with AAVR at the cell surface, which facilitates entry into the endosomal network; (ii) AAVR interacts with AAV in the endosomal system and facilitates trafficking to the trans-Golgi. (iii) Once in the trans-Golgi network, AAVR facilitates AAV escape into the cytoplasm.
Host factors facilitating AAV trafficking
While AAV receptors are likely an important determinant of cellular tropism, post-attachment steps within the AAV life cycle (eg. trafficking through the endosomal system [36] and nuclear import [38]), also significantly contribute to AAV transduction efficiency. In particular, AAV trafficking after internalization is a major stage during the infection process where the virus encounters obstacles that affect functional infection. As AAV journeys through the cell, the capsid remains intact and must rely on host factors to travel to the nucleus [31,39]. If these factors are expressed at insufficient levels, infection can be affected. Analogous to other parvoviruses, AAV is believed to enter early endosomal compartments associated with Rab5 [40–42], although its route through the rest of the endosomal network is currently poorly understood. There is evidence to support AAV2 trafficking through late endosomes and recycling endosomes [43] but this is likely to be cell-type specific, possibly determined by the host factors available. The cell’s extensive microtubule network is used by AAV2 to facilitate its transport while still contained within endosomal vesicles [44], and the majority of AAV serotypes utilize syntaxin-5-mediated retrograde transport to facilitate trafficking directly to the trans-Golgi network [36]. Identification of other cellular proteins that contribute to AAV trafficking has been a challenging task, particularly because productive AAV entry is an inefficient process; high ratios of non-infectious particles and the existence of dead-end endocytic routes confound the study of AAV cellular transport. However, evaluation of the effect of small drug and genetic inhibitors and enhancers on functional transduction has greatly contributed to our understanding of AAV trafficking [33,36,44]. Furthermore, the unbiased, genome-wide screen by Pillay et al [27] identified multiple genes with potential roles in AAV trafficking including proteins in the retromer complex, Golgi-associated retrograde protein (GARP) complex and Wiskott-Aldrich syndrome protein and scar homolog (WASH) complex. These complexes are primarily involved in cellular trafficking between endosomes and the trans-Golgi network, and interestingly, have been implicated in the trafficking of bacterial toxins [45,46] and other DNA viruses [47,48]. In particular, human papilloma virus (HPV), another non-enveloped single-stranded DNA virus, has been shown to arrive at the trans-Golgi network in a retromer-dependent manner, via direct interaction with components of the retromer complex [48]. This interaction not only mediates virus trafficking to the Golgi, but also endosomal escape [49]. Given the similarity in trafficking routes of HPV and AAV, it is possible that the retromer plays a comparable role in AAV infection [37,50–53]. However, if AAVR is in fact responsible for transporting AAV particles to the Golgi, it is also possible that the retromer might be interacting with AAVR, and thus indirectly mediating AAV trafficking. As with HPV, AAV undergoes a conformational change in capsid conformation as it travels through the endosomal system [37,50], which is potentially induced by acidic changes [51] and cathepsins [52]. This change is a strict requirement for functional transduction, as without this modification in the capsid, no infection is observed [53]. It is therefore likely that endosomal escape may only occur once this change occurs, and may be dependent on host factors. Further studies on the role of specific host factors in AAV trafficking and/or endosomal escape could prove important in identifying rate-limiting events in AAV transduction, and provide insights into intracellular factors that act as tissue tropism determinants.
Concluding remarks
AAV entry mechanisms are complex involving multiple receptors and specific host factors facilitating intracellular trafficking of AAV. The discovery of the multi-serotype receptor AAVR adds another component that may influence tissue tropism and could potentially be harnessed to improve transduction specificity and efficiency of AAV vectors. The study of virus-host interactions has accelerated in the past decade, in part due to technological advances that facilitate loss-of-function genetic screens in human cells. Such screens can be performed in an unbiased, high-throughput manner, and as a consequence of the functional nature of the approach, can lead to the identification of cellular proteins that viruses require to propagate. These technologies include RNA interference [54] and, more recently, haploid genetic screens [55] and CRISPR-based screens [56,57] that allow complete knockout of gene expression improving the signal-to-noise ratio. They have allowed others and us to re-visit fundamental aspects of the AAV life cycle with a greater depth of understanding as to how host-encoded proteins facilitate infection [27,58–60]. The ability to create isogenic knockout cell lines in multiple different cell types using CRISPR genome editing may be used in future studies to systematically asses the relative contributions of the different (co)receptors and pathways reported allowing better separation between major and minor contributors to AAV entry. Furthermore, therapeutic application of CRISPR to edit the genome using AAV vectors holds promise as novel modality in gene therapy [61–65].
Highlights.
AAV primary attachment occurs via glycans in a serotype specific manner
AAVR is a proteinaceous receptor that binds AAV through Ig-like PKD domains
AAVR is essential for transduction by multiple, diverse serotypes
AAV transduction requires retrograde transport to the trans-Golgi network
Acknowledgments
We thank Juliana Idoyaga and members of the Carette lab for discussions, and Michael Chapman for critical reading of the manuscript and valuable advice. This work was supported by the National Institutes of Health (DP2AI104557) and the David and Lucile Packard Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Sirika Pillay and Jan E. Carette are inventors on a patent owned by Stanford University on the uses of AAVR in gene therapy.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 2.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 3.Hastie E, Samulski RJ. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success–a personal perspective. Hum Gene Ther. 2015;26:257–265. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 5.Geoffroy MC, Salvetti A. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr Gene Ther. 2005;5:265–271. doi: 10.2174/1566523054064977. [DOI] [PubMed] [Google Scholar]
- 6.Zinn E, Vandenberghe LH. Adeno-associated virus: fit to serve. Curr Opin Virol. 2014;8:90–97. doi: 10.1016/j.coviro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. This paper describes long-term safety and efficacy of factor IX gene therapy in hemophilia B patients injected with vectors based on AAV8. A transient immune reaction to the vector was successfully managed with corticosteroid treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samulski RJ, Muzyczka N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu Rev Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 12.Pillay S, Carette JE. Hunting Viral Receptors Using Haploid Cells. Annu Rev Virol. 2015;2:219–239. doi: 10.1146/annurev-virology-100114-055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolly CL, Sattentau QJ. Attachment factors. Adv Exp Med Biol. 2013;790:1–23. doi: 10.1007/978-1-4614-7651-1_1. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmi KM, Johnson EM, Stehle T, Dermody TS. Attachment and cell entry of mammalian orthoreovirus. Curr Top Microbiol Immunol. 2006;309:1–38. doi: 10.1007/3-540-30773-7_1. [DOI] [PubMed] [Google Scholar]
- 15.Huang LY, Halder S, Agbandje-McKenna M. Parvovirus glycan interactions. Curr Opin Virol. 2014;7:108–118. doi: 10.1016/j.coviro.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. This paper demonstrates that heparan sulfate proteoglycan is a receptor for AAV2 virions establishing the importance of glycans in membrane attachment for AAV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. This paper reports the atomic structure of AAV2 at 3-A resolution determined by x-ray crystallography. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, Von der Lieth CW, King JA, Kleinschmidt JA. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77:11072–11081. doi: 10.1128/JVI.77.20.11072-11081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell J, Taylor KA, Chapman MS. Adeno-associated virus-2 and its primary cellular receptor–Cryo-EM structure of a heparin complex. Virology. 2009;385:434–443. doi: 10.1016/j.virol.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Madigan VJ, Asokan A. Engineering AAV receptor footprints for gene therapy. Curr Opin Virol. 2016;18:89–96. doi: 10.1016/j.coviro.2016.05.001. This review provides a structural perspective of capsid-glycan interactions and describes how this can be exploited to engineering new, lab-derived AAV capsids with favorable transduction profiles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurzeder C, Koppold B, Sauer G, Pabst S, Kreienberg R, Deissler H. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparan sulphate proteoglycans. Int J Mol Med. 2007;19:325–333. [PubMed] [Google Scholar]
- 23.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 24.Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- 25.Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16:662–664. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, et al. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. This paper describes the discovery of a multi-serotype receptor for AAV, which binds AAV particles and is essential for transduction of a wide range of human cell types in culture and in an in vivo mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM. Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum Mol Genet. 2000;9:1641–1649. doi: 10.1093/hmg/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 29.Bhella D. The role of cellular adhesion molecules in virus attachment and entry. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140035. doi: 10.1098/rstb.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas S, Zussy C, Loustalot F, Henaff D, Menendez G, Morton PE, Parsons M, Schiavo G, Kremer EJ. Disruption of the coxsackievirus and adenovirus receptor-homodimeric interaction triggers lipid microdomain- and dynamin-dependent endocytosis and lysosomal targeting. J Biol Chem. 2014;289:680–695. doi: 10.1074/jbc.M113.518365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–576. doi: 10.1016/j.chom.2011.10.014. This paper describes the CLIC/GEEC pathway as an important endocytic route for productive infection of AAV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg MS, Nicolson S, Bhatt AP, McLendon M, Li C, Samulski RJ. Recombinant adeno-associated virus utilizes cell-specific infectious entry mechanisms. J Virol. 2014;88:12472–12484. doi: 10.1128/JVI.01971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–1687. doi: 10.1128/JVI.02520-14. This paper describes that AAV transduction requires retrograde trafficking trough the trans-Golgi network through a syntaxin-5 dependent mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19:649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolson SC, Samulski RJ. Recombinant adeno-associated virus utilizes host cell nuclear import machinery to enter the nucleus. J Virol. 2014;88:4132–4144. doi: 10.1128/JVI.02660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson JS, Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbison CE, Lyi SM, Weichert WS, Parrish CR. Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking. J Virol. 2009;83:10504–10514. doi: 10.1128/JVI.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Joo KI, Wang P. Endocytic processing of adeno-associated virus type 8 vectors for transduction of target cells. Gene Ther. 2013;20:308–317. doi: 10.1038/gt.2012.41. [DOI] [PubMed] [Google Scholar]
- 42.Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- 43.Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao PJ, Samulski RJ. Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J Virol. 2012;86:10462–10473. doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bujny MV, Popoff V, Johannes L, Cullen PJ. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J Cell Sci. 2007;120:2010–2021. doi: 10.1242/jcs.003111. [DOI] [PubMed] [Google Scholar]
- 46.Tafesse FG, Guimaraes CP, Maruyama T, Carette JE, Lory S, Brummelkamp TR, Ploegh HL. GPR107, a G-protein-coupled receptor essential for intoxication by Pseudomonas aeruginosa exotoxin A, localizes to the Golgi and is cleaved by furin. J Biol Chem. 2014;289:24005–24018. doi: 10.1074/jbc.M114.589275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao JC, Chu LW, Lo YT, Lee SP, Chen TJ, Huang CY, Ping YH, Chang W. Intracellular Transport of Vaccinia Virus in HeLa Cells Requires WASH-VPEF/FAM21-Retromer Complexes and Recycling Molecules Rab11 and Rab22. J Virol. 2015;89:8365–8382. doi: 10.1128/JVI.00209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, Guie MA, Poffenberger AC, Nelson CD, Atwood WJ, et al. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci U S A. 2013;110:7452–7457. doi: 10.1073/pnas.1302164110. This paper uses a genome-scale RNAi approach to identify the retromer as a cellular factor required for entry by human papillomavirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popa A, Zhang W, Harrison MS, Goodner K, Kazakov T, Goodwin EC, Lipovsky A, Burd CG, DiMaio D. Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection. PLoS Pathog. 2015;11:e1004699. doi: 10.1371/journal.ppat.1004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, Salganik M, Muzyczka N, Agbandje-McKenna M. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol. 2011;85:11791–11799. doi: 10.1128/JVI.05305-11. This paper provides evidence for structural changes in the capsid of AAV8 induced by changes in the pH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salganik M, Venkatakrishnan B, Bennett A, Lins B, Yarbrough J, Muzyczka N, Agbandje-McKenna M, McKenna R. Evidence for pH-dependent protease activity in the adeno-associated virus capsid. J Virol. 2012;86:11877–11885. doi: 10.1128/JVI.01717-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akache B, Grimm D, Shen X, Fuess S, Yant SR, Glazer DS, Park J, Kay MA. A two-hybrid screen identifies cathepsins B and L as uncoating factors for adeno-associated virus 2 and 8. Mol Ther. 2007;15:330–339. doi: 10.1038/sj.mt.6300053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. This paper describes that AAV2 undergoes a structural change in vivo during infection that exposes its VP1/VP2 N termini. This change likely occurs in the endosome and is required for successful transduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 55.Carette JE, Guimaraes CP, Varadarajan M, Park AS, Wuethrich I, Godarova A, Kotecki M, Cochran BH, Spooner E, Ploegh HL, et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science. 2009;326:1231–1235. doi: 10.1126/science.1178955. [DOI] [PubMed] [Google Scholar]
- 56.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holscher C, Sonntag F, Henrich K, Chen Q, Beneke J, Matula P, Rohr K, Kaderali L, Beil N, Erfle H, et al. The SUMOylation Pathway Restricts Gene Transduction by Adeno-Associated Viruses. PLoS Pathog. 2015;11:e1005281. doi: 10.1371/journal.ppat.1005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Schreiber CA, Sakuma T, Izumiya Y, Holditch SJ, Hickey RD, Bressin RK, Basu U, Koide K, Asokan A, Ikeda Y. An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses. PLoS Pathog. 2015;11:e1005082. doi: 10.1371/journal.ppat.1005082. This paper uses a genome-scale RNAi approach to identify the U2 snRNP spliceosome as AAV restriction factor and show that pharmaceutical inhibition can significantly enhance AAV transduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallen AJ, Barker GA, Fein DE, Jing H, Diamond SL. Enhancers of adeno-associated virus AAV2 transduction via high throughput siRNA screening. Mol Ther. 2011;19:1152–1160. doi: 10.1038/mt.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. This paper and the following four papers combined show proof-of-principle in mouse models of muscular dystrophy that CRISP can be used in vivo to edit the genome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351:403–407. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, Olson EN. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, Zhu K, Wagers AJ, Church GM. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 67.Blackburn SD, Steadman RA, Johnson FB. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch Virol. 2006;151:617–623. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- 68.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, Cheng B, Gee SW, McGoogan KE, Govindasamy L, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther. 2010;21:1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


