Abstract
The developmental period of adolescence is characterized by increasing incidence of health risk behaviors (HRBs). Based on theoretical models that emphasize the moderating role of cognitive control (Carver, Johnson, & Joorman, 2008), we examine how neural correlates of cognitive control and risk sensitivity interact to predict HRBs among late adolescents (17-20 years). Neuroimaging data indicate that risk-related hemodynamic activity in the anterior insula (AI) during anticipation of uncertain outcomes predicts HRBs among late adolescents exhibiting greater dorsal anterior cingulate cortex (dACC) activity during a cognitive interference task but not among late adolescents requiring less dACC activity. These results present neural evidence for a significant moderating effect of cognitive control on the link between risk sensitivity and HRBs among late adolescents.
Keywords: health risk behaviors, cognitive control, risk sensitivity, adolescent risk taking, brain imaging
1. Introduction
The developmental period of adolescence is characterized by heightened vulnerability to health risk behaviors (HRBs), including experimenting with drugs and alcohol and engaging in unsafe sexual activity, that together are proximal causes of drug addiction and sexually transmitted diseases (Centers for Disease Control and Prevention, CDC, 2014). Risk-taking in adolescence is increasingly thought to be derived in part from distinct developmental trajectories of two neural systems: a network underlying the valuation of reward and risk associated with appetitive and aversive stimuli, and a network exerting control over the pursuit or avoidance of risky options (Casey, Getz, & Galván, 2008; Steinberg, 2010). One variation of this dual systems model, the triadic model, emphasizes three neural systems that are responsible for approach or motivation (striatum, orbitofrontal cortex), avoidance or emotion (insula, amygdala, hippocampus), and the regulatory system (dorsolateral prefrontal cortex, ventromedial cortex, orbital prefrontal cortex, anterior cingulate cortex). Similar to the dual systems model, the triadic model emphasizes the potential imbalance in maturation across these brain regions and highlights the importance of functional connectivity between these interrelated regions (Ernst & Fudge, 2009). Although such developmental imbalance describes ontogenetic trajectories of brain development, it does not explain why only a subset of adolescents is prone to engage in serious risky behaviors that confer negative health consequences, thus highlighting the significance of individual differences in brain development related to adolescent substance use (e.g., Bjork & Pardini, 2015). In addition, prior studies have primarily focused on examining independent contributions of these two neural systems to risk taking, particularly neural responses to reward. The role of neural responses to risk in the development of HRBs is not clearly understood, thus it is examined in the current study.
Work in decision neuroscience has revealed neural mechanisms that underlie decisions made in risky environments. Neuroimaging studies among adults have identified neural representations of (i) the appetitive or aversive value associated with the potential outcome of a decision and (ii) the probability or risk associated with potential outcomes (Levy & Glimcher, 2012). Although neuroimaging research to date has paid less attention to risk sensitivity compared to reward seeking related to adolescents' risky decision making, available studies have demonstrated that adolescents show greater corticostriatal recruitment than adults during the avoidance of risk (Barkley-Levenson, Van Leijenhorst, & Galván, 2013) and adolescents show lower amygdala activation in response to reward omission (Ernst et al., 2005). Prior neuroimaging work in adults has identified separable representations of value and risk, with processing of risk being most consistently associated with activity in the insular cortex (Mohr, Biele, & Heekeren, 2010). However, work in adolescents has yet to examine processing of risk separately from processing of value, and thus we focus on neural activity associated with differing levels of risk, while controlling for the expected value of decision options. The definition of risk for risk sensitivity in the current study is consistent with the behavioral economics views on risk, namely variance of potential outcomes. As reviewed by Schonberg and colleagues (2011), this definition of risk is different from the clinical definition of risk (i.e., potential for negative outcomes) that is implied by HRBs (i.e., behaviors that harm oneself or others).
Neural correlates of cognitive control have been posited to contribute to impulse control difficulties in adolescence (Casey et al., 2008). The ability to detect and respond to behavioral errors is a critical component of cognitive control and is supported by a network of prefrontal regions, including the anterior cingulate cortex (Luna, Padmanabhan, & O'Hearn, 2010). Indeed, brain activation in cognitive control related brain regions (orbitofrontal/ventrolateral prefrontal cortex and dorsal anterior cingulate cortex) is correlated with risk-taking performance and substance use behavior in adolescents (Eshel, Nelson, Blair, Pine, & Ernst, 2007; Feldstein Ewing, Houck, & Bryan, 2015; see Kim-Spoon, Kahn et al., 2016 for review).
Based on theories proposing that cognitive control system is a “regulator” that modulates the operation of reactive responses to the environment to serve goal-directed behavior (Carver, Johnson, & Joormann, 2008; Ernst & Fudge, 2009), we hypothesized that cognitive control would statistically moderate the link between risk sensitivity and HRBs. Specifically, we tested whether prefrontal functioning moderates the link between neural sensitivity to risky outcomes and HRBs. We assessed hemodynamic correlates of cognitive conflict within a multi-source interference task, a task developed and validated to reliably elicit interference-related activity in the dorsal anterior cingulate cortex (Bush, Shin, Holmes, Rosen, & Vogt, 2003; Fitzerald et al., 2010; Perkins, Welsh, Stern, Taylor, & Fitzerald, 2013). Hemodynamic correlates of risk sensitivity were assessed using a lottery task in which the variance of outcomes differed across trials, controlling for the expected value of decision options. In similar lottery tasks, risk-related activity has most consistently and specifically been identified in the anterior insular cortex (Platt & Huettel, 2008; see Mohr et al., 2010 for review).
2. Methods
2.1. Participants
The current sample included 24 late adolescents (18 males and 6 females) ages 17 to 20 years (M = 18.56, SD = 1.08), who were 100% non-Hispanic White from Southwestern Virginia. Participants for the current study were recruited from a community sample of adolescents who participated in an existing study. As a part of data collection for the existing study, adolescents reported HRBs. Based on these HRB data, we recruited adolescents whose HRBs were relatively high (n = 12; e.g., using a drug “a few times a week” to “everyday” and/or having 6 or more sexual partners in lifetime) and demographically matched adolescents whose HRB levels were relatively low (n = 12; e.g., “never” to “used a drug 3-5 times in lifetime”), following the oversampling extreme observations strategy to maximize individual differences in HRBs in a small sample (e.g., McClelland & Judd, 1993). The items that determined high vs. low HRB levels were chosen because they are widely used to assess severe levels of substance use or key elements of sexual risk for exposure to HIV/STDs among late adolescents (e.g., Johnston, O'Malley, Bachman, Schulenberg, & Miech, 2014; Saewyc et al., 2006).
This community sample's ethnic and socioeconomic demographics closely matched those of southwest Virginia in the most recent U.S. Census at the time of data collection (United States Census Bureau, 2012). In terms of demographic characteristics, the high and low HRB adolescents were matched on age (M = 18.8 years for the high HRB adolescents and M = 18.3 years for the low HRB adolescents), gender (3 females and 9 males for both), ethnicity (100% White for both), and parents' highest education degree (M = 5.8 for high HRB adolescents and M = 5.7 for low HRB adolescents with 5 indicating “Associate Degree”). The sample showed a typical range of intellectual functioning for community samples, based on performance on the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001), with the range of the IQ scores = 83 to 134 (M = 114.33, SD = 13.34). All participants provided written consent or assent (along with parent's consent) for a protocol approved by the university's institutional review board.
2.2. Measures
2.2.1. Health Risk Behaviors (HRBs; CDC, 2012)
Participants were asked to indicate typical frequency and age of initiation of cigarette, alcohol, and marijuana use. Standardized scores of substance use severity (based on frequency) and onset were averaged across cigarette, alcohol, and marijuana use to obtain a substance use severity composite score (α = .79) and a substance use onset composite score (α =.75). To assess risky sexual behaviors, adolescents completed questions regarding: age at sexual debut, number of sex partners in lifetime, condom use at last intercourse, and alcohol/drug use before/during sexual intercourse. A composite score of risky sexual behavior severity was generated by averaging standardized scores of the number of sex partners, condom use, and alcohol/drug use before/during sexual intercourse (α = .91). A composite HRB variable was generated by averaging standardized scores of (i) substance use severity, (ii) substance use onset, (iii) risky sexual behavior severity, and (iv) sexual behavior onset.
2.2.2. fMRI Data of Risk Sensitivity and Cognitive Control
Imaging acquisition and analysis
For both tasks, functional images were acquired using a 3.0T Siemens Tim Trio with the following parameters: echo-planar images hyperangulated 30 degrees from the anterior commissure-posterior commissure line, gradient recalled echo; repetition time (TR) = 2 s; echo time (TE) = 30 ms; flip angle = 90°; 34 axial slices, 4.0 mm slice thickness, 220×220 mm field of view (FOV), 64×64 grid. The structural scan was acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence (TR = 1200 ms, TE = 2.66 ms, FoV = 245×245 mm, 1 mm slice thickness, 192 slices with spatial resolution of 1×1×1 mm).
Risk processing task parameters
To identify neural regions associated with risk sensitivity, participants engaged in a modified economic lottery choice task (Holt & Laury, 2002) in which they made choices to accept or reject uncertain gambles while their blood-oxygen-level-dependent (BOLD) response was recorded. As illustrated in Figure 1a, participants were given the option to either accept the gamble at a cost that ranged from one to seven dollars or reject the gamble at no cost. Participants understood that each of their choices in the task was equally likely to be picked as five of their choices would be selected at random ex post to determine their earnings (real money). Participants received a base pay for their participation, and were told that the earnings in the task would be added or subtracted from their base pay. This was done to provide monetary incentive for our economic risk task (Smith, 1976). In actuality, no money was deducted from the participant's compensation, and any gains from the task were added to their total at the end of the study.
Figure 1.
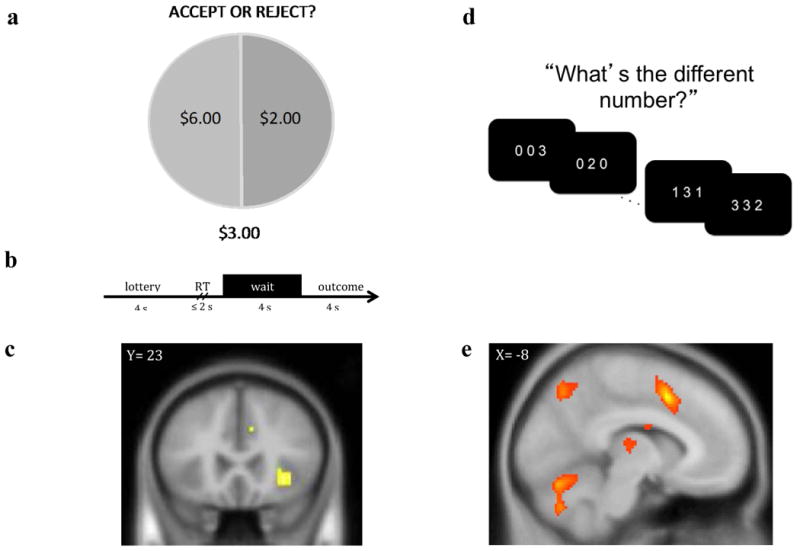
a) Participants performed a lottery choice task in which they were asked to make a series of decisions to either (i) accept the lottery at a cost that ranged between $1 and $7 or (ii) reject the lottery at no cost. Lotteries varied by risk level determined by the variance of possible outcomes (low = $4:$4, medium = $2;$6, high = $0:$8). b) Wait phase activity was modeled across the 4 s prior to the display of the outcome and was parametrically modulated by the risk level of the gamble. c) Participants exhibited greater right anterior insular activation for riskier gambles prior to receiving the results of their choice, t(22)=3.67, p < .001 uncorrected (displayed at p < .005 uncorrected). d) Participants performed the multi-source interference task (MSIT), in which they were asked to identify the value of the number that differed from two other concurrently presented numbers regardless of the position of the number in the sequence. e) Participants exhibited greater dorsal anterior cingulate cortex activation for interference relative to congruent trials, t(23) = 9.38, p(FDR) < .05 (displayed at p < .001, FDR corrected).
Options were displayed for 4 s, after which participants were able to enter a decision using two buttons of an MR-compatible response pad. Participants were able to enter a response for up to 2 s. Following participants' button press responses, a 4 second wait period elapsed before the outcome of the lottery was displayed for 4 s (see Figure 1b). Lotteries varied by risk level, defined as the variance of potential outcomes (low variance outcomes = $4:$4, mid = $2:$6, high = $0:$8). The probability associated with each outcome was held constant at 50%, and the expected value of lottery outcomes was held constant (i.e., $4). The outcomes and costs varied for each of 54 trials and were matched across level of risk (low, mid, high), and the order of lotteries was randomized. The task took approximately 13 minutes to complete. After completing the task, participants randomly selected five numbers between 1 and 54, and these five numbers were used to identify five gambles according to a list in a sealed envelope given to participants. The earnings for each of the participant's five selected gambles were summed, and the participant received that amount or $20, whichever was higher.
At the first level of the GLM, two events were modeled: the onset of the lottery and the 4 s wait period prior to the decision outcome. Two parametric modulators of the wait period representing the (i) risk level (low, mid, high) and (ii) expected outcome minus cost of each lottery were included as regressors, in addition to six motion-parameter covariates. Separate analyses for the first, second, and third blocks of trials were carried out (in each block of 18 trials, the % of risk level was equal, comprising of 6 low, 6 mid, and 6 high risk levels), and revealed that risk-related activity in anterior insula (AI) decreased with increasing experience with the task. Thus, the risk-related region of interest (ROI) analysis reported below was derived from the first third of trials (regardless of whether participants chose to accept or reject the lottery). Second-level pooled analysis, illustrated in Figure 1c, revealed that hemodynamic activity was maximally related to increasing level of risk in right AI (coordinates = 33, 23, -5; p < .001, uncorrected), consistent with prior reports (for review, see Mohr et al., 2010). The value of the first eigenvariate for each subject surrounding the peak voxel of activation within the anatomically defined anterior cingulate was extracted using a spherical mask of 6 mm thresholded at p < .005. One participant's risk processing data were not usable due to recording errors, but the SEM analyses included this participant using full information maximum likelihood estimation (FIML) that permits partial data cases and maximizes the likelihood of the model given the observed data available (Arbuckle, 1996).
For the behavioral measure, constant relative risk aversion (CRRA) was calculated based on the first third of trials (regardless of whether participants chose to accept or reject the lottery) using the following utility function, u (x) = x 1 − r where u = utility function for money x, implying risk preference for r < 0, risk neutrality for r = 0, and risk aversion for r > 0 (Holt & Laury, 2002). Using maximum likelihood estimation, choices were fit to a logistic function P(chosen) = 1/1+e γ (EUrisky− EUsafe), where P(chosen) corresponds to the predicted probability of the chosen option, γ corresponds to inverse temperature (i.e., a measure of relative consistency in choice selection), and EU represents the expected utility of the option.
Cognitive control task parameters
To identify neural regions involved in cognitive control, participants engaged in the multi-source interference task (MSIT; Bush et al., 2003) illustrated in Figure 1c, while BOLD signal was recorded. The MSIT requires participants to indicate which of three numbers is different from the other two. In congruent trials, target numbers were presented among zeros, and numbers were congruent with presented locations. In incongruent trials, target numbers were presented among non-zero integers and were incongruent with the target locations (e.g., 2 was in the third position). Following Bush et al.'s (2003) study, we used difference scores for reaction time (RT)—i.e., incongruent RT minus congruent RT. Consistent with previous reports (Bush et al., 2003), we found a significant interference effect in RT: t(23) = 21.05, p < .001. For imaging data, events for congruent block and incongruent block trials were modeled with block durations of 42 s and convolved with a canonical hemodynamic response function. In addition to the congruent and incongruent block regressors of interest, six motion-parameter covariates were included. For each participant, incongruent and congruent conditions were contrasted, as illustrated in Figure 1d/e, and individual-level ROI values were extracted from these contrasts using dACC coordinates reported by Bush et al. (2003). Specifically, the first eigenvariate value of the contrast image was extracted using a spherical mask of 6 mm surrounding coordinates -8, 19, 44, representing MNI conversion of coordinates in dACC (24c'/32′), thresholded at p < .005.
2.3 Data analysis
We used two-group structural equation models (SEM) to test our hypothesis regarding the moderating effects of cognitive control in the link between risk sensitivity and HRBs; moderation effects could be tested by the difference in model fits using the change in the Comparative Fit Index (CFI) that is not affected by sample size (i.e., ΔCFI > .01 reflects a meaningful difference in model fit; Cheung & Rensvold, 2002). We formed high dACC activation (above median) vs. low dACC activation (below median) groups using dACC brain activity scores and compared two nested models: the Configural Invariance model in which all parameters were freely estimated across the two groups and the Equal Effect model in which an equality constraint was imposed to test numeric invariance between low and high cognitive control groups with respect to the effect of risk sensitivity on HRBs. If the strength of the association between risk sensitivity and HRBs significantly differs between the two cognitive control groups, model fit should become significantly worse by imposing an equality constraint.
3. Results
ROI extracted dACC values of the MSIT were significantly correlated with behavioral RT data: r (24) = .37 (one-tailed p = .05). The result is consistent with prior research demonstrating the association between increased RT and higher fMRI signal during the MSIT (Bush et al., 2003) as well as significant condition (congruent vs. incongruent) effects (Bush et al., 2003; Fitzgerald et al., 2010). Furthermore, medial prefrontal cortex activation during MSIT has been found to decrease with age (Perkins et al., 2013). Taken together, findings suggest that participants who were more challenged by the incongruent condition exhibited greater differentiation in hemodynamic activity between conditions (see plots in Appendix). Thus higher dACC scores indicate poorer cognitive control. There was a trend showing that high AI values of the risk processing task were related to high CRRA values: r (16)1 = .27, p = .31. Additionally, high AI activation was significantly correlated with low HRBs: r (23) = -.41, p = .03 for the whole sample. The correlation was higher in the high dACC group [r (12) = -.64, p = .03], compared to the low dACC group [r (11) = -.17, p = .62]. Research has shown that risk aversion and insula activation during decision making under risk show robust increases with age (Paulsen, Carter, Platt, Huettel, & Brannon, 2012). Taken together, findings suggest that greater AI activation implies more mature risk processing.
The results indicated significant differences between the low and high dACC groups with respect to the association between AI activation during risk processing and HRBs, with ΔCFI = .24 [χ2(0) = 0, p = .00, CFI = 1.00 for Configural Invariance model, and χ2(1) = 2.02, p = .15, CFI = .76 for Equal Effects model]. As shown in Figure 2, AI was a significant predictor of HRBs for those showing high dACC activation, explaining 41% of the variance (b = -1.53, SE = .53, p = .004; b* = -.64, 95% CI [-0.97; -0.30]). However, AI was not a significant predictor for those showing low dACC activation, explaining 1% of the variance (b = -.23, SE = .71, p = .75; b* = -.09, 95% CI [-0.65; 0.47]). The findings were consistent when examined separately for four individual variables of the HRBs composite (i.e., severity and onset of substance use and risky sex), showing that AI explained 22 to 52% of the variance in HRBs (b* = .47 to .72) for those with high dACC activation, compared to 1 to 6 % of the variance (b* = .10 to .24) for those with low dACC activation.
Figure 2.
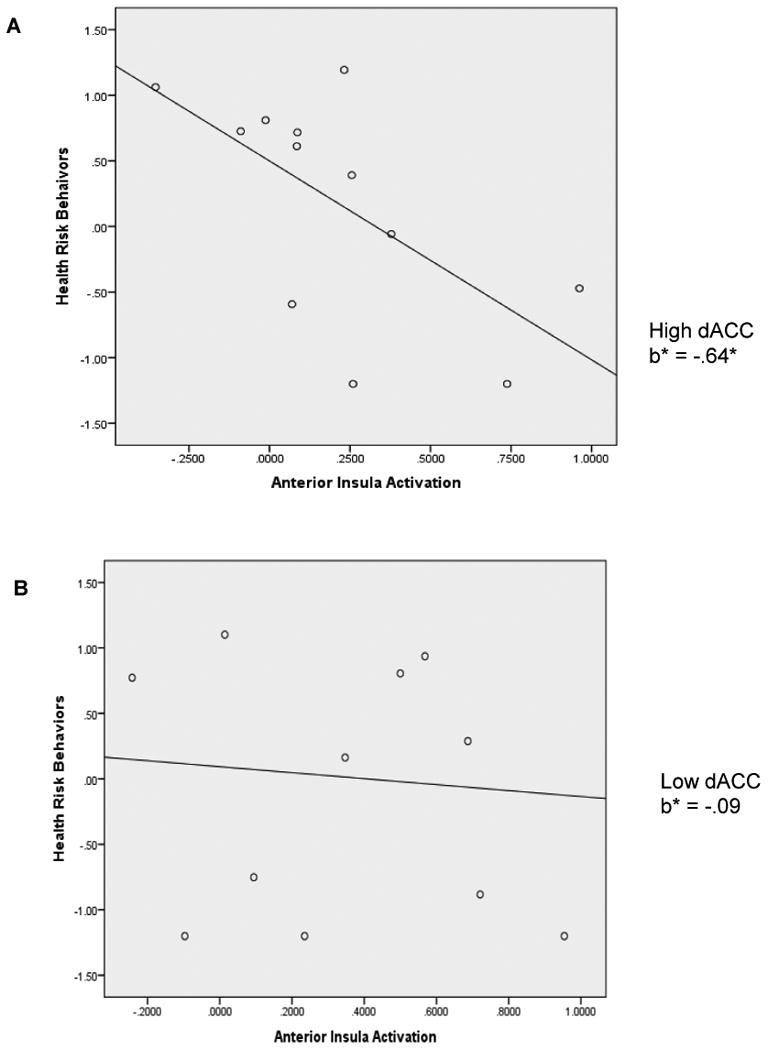
Regression lines for relations between anterior insula activation during risk processing and health risk behaviors as moderated by dorsal anterior cingulate cortex (dACC) activation during Multi Source Interference Task. A = adolescents with high dACC activity; B = adolescents with low dACC activity; b* = standardized regression coefficient (simple slope). *p < .05.
Additionally, we tested the moderation effects of cognitive control using behavioral performance on MSIT (reaction time). SEM results indicated non-significant moderating effects, with ΔCFI = 0 between the Configural Invariance model and the Equal Effect model [χ2(0) = 0, p = .00, CFI = 1.00 for Configural Invariance model, and χ2(1) = 0.09, p = .77, CFI = 1.00 for Equal Effects model]. Regardless of the reaction time levels, high levels of risk related insula activity were related to low levels of self-reported HRBs: b = -1.29, SE = .44, b* = -.52, p = .003. Overall, the findings suggest that neural indicators of cognitive control—even in the absence of effects by behavioral indicators of cognitive control—have moderating effects that distinguish a subgroup of individuals for whom insula activation during risk processing is linked to HRBs.
4. Discussion
Based on theoretical models that emphasize the moderating role of cognitive control over reactivity (Carver et al., 2008; Ernst & Fudge, 2009), we tested whether neural correlates of cognitive control and risk sensitivity may interact to produce differential vulnerability to HRBs. We found evidence of the hypothesized interaction between AI activation during risk processing and dACC activation during cognitive interference processing. Lower levels of AI activation were substantially related to higher levels and earlier onset of HRBs among late adolescents with high dACC activation (greater neural responses associated with poorer cognitive control) but not among late adolescents with low dACC activation (diminished neural responses associated with stronger cognitive control). Some theories of adolescent risk taking, including the triadic model, have suggested the potential role of AI in harm avoidance (Richards, Plate, & Ernst, 2013). Similarly, a recent review emphasizes the importance of including AI in developmental models of risk taking and decision making (Smith, Steinberg, & Chein, 2014); however, no empirical study has yet examined its role related to adolescent risk-taking behavior. Our finding illustrates how AI and dACC interact with each other to predict individual differences in adolescent risk-taking behaviors in real-world contexts.
The present study presents preliminary evidence for the moderating role of cognitive control in the link between risk sensitivity and HRBs at the level of neural correlates. Previous studies have shown weak main effects of risk processing (such as punishment sensitivity) and cognitive control. For example, self-reported or behavioral performance of punishment sensitivity is not significantly associated with substance use among adolescents (Colder et al., 2013; van Leeuwen, Creemers, Verhulst, Ormel, & Huizink, 2011). Further, a recent longitudinal study involving a community sample of adolescents reported that poor inhibition performance was significantly yet weakly related to later alcohol and marijuana use (Squeglia, Jacobus, Nguyen-Louie, & Tapert, 2014). In contrast, a couple of recent studies found significant moderation effects of cognitive control on risk sensitivity (shown as punishment sensitivity and fear reactivity), showing that low risk sensitivity was related to high risk-taking and substance use behaviors among young adults with weak cognitive control but not among those with strong cognitive control (Jonker, Ostafin, Glashouwer, van Hemel-Ruiter, & de Jong, 2014; Kim-Spoon, Holmes, & Deater-Deckard, 2015). In light of previous work, our results make theoretical contributions to the research on adolescent brain development and risk taking by suggesting the neural interaction between cognitive control and risky decision processes that explain individual differences in HRBs. Notably, our data demonstrate that the moderating effect of cognitive control was evident when using the neural activation of cognitive control but not the behavioral measure.
Research has shown a significant interaction between reward seeking (as opposed to risk sensitivity) and cognitive control such that among early adolescents with weak cognitive control (indicated by poor performance and heightened prefrontal activity during an interference control task), those with high reward seeking (indicated by self-reported behavioral activation system) are more likely to initiate substance use at an early age. However, reward seeking did not promote substance use among early adolescents with strong cognitive control (Kim-Spoon, Deater-Deckard et al., 2016). Mid-adolescents show heightened reward sensitivity relative to adults (Ernst et al., 2005; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Van Lijenhorst et al., 2010). During early to mid-adolescence, regulating effects of cognitive control on reward seeking may be particularly important for delaying initiation of substance use. In contrast, the findings from the current study and Jonker et al. (2014)'s study suggest that regulating effects of cognitive control on risk sensitivity (indicated by heightened insula activity and self-reported punishment sensitivity) may be particularly important for demoting progression to severe HRBs among late adolescents and young adults. Future research should consider possible differential pathways by which reward seeking and risk sensitivity may interface with cognitive control to contribute to the development—onset, course, and trajectory—of HRBs.
Findings from the current study should be interpreted in the context of study limitations. First, the cross-sectional design and analyses do not allow us to infer causality in the identified relationships, and such inferences should be examined in future longitudinal studies. Second, although findings with sufficient effect sizes (that are not affected by sample size) that adhere to our theoretically based model help to alleviate the issue of small sample size, generalizability of this work would benefit from future replications with a larger, more ethnically diverse and gender balanced sample. Third, focusing on AI and dACC represents a simplistic, limited approach to testing the dual systems models. Future researchers should consider ways to simultaneously consider multiple, carefully chosen ROIs such as using latent factor modeling (e.g., Nees et al., 2012). Finally, future research should explore factors that may determine the directions of the regulating effects of cognitive control to predict adolescent HRBs. In their Fuzzy Trace theory, Reyna and Farley (2006) proposed two distinct routes to risk taking: a reactive route resulting in impulsive risk taking driven by a failure to inhibit behavior versus a reasoned route resulting in intentional risk taking. Future research would benefit from examining whether cognitive modulation demotes impulsive risk taking or promotes intentional risk taking depending on internal and external factors.
Our innovative approach using neural variables illustrates how cognitive control regulates the contribution of risk sensitivity to real-life risk-taking behaviors among late adolescents. Our findings present preliminary evidence of the moderation effects of cognitive control at neural levels, illustrating how individual differences in adolescent HRBs can be explained by joint contributions of two neural systems theorized to influence the development of risk-taking—valuation and cognitive control neural systems. In supporting the theoretically hypothesized moderating role of prefrontal functioning, our results imply that the interaction between the valuation and control systems may be crucial to identifying subgroups of adolescents most vulnerable to developing maladaptive HRBs. Adolescent HRBs can result from diminished avoidance of potential negative consequences associated with a risky behavior, but if enough cognitive control capacity is available, the biased processing of risk can be regulated, resulting in a reduction of HRBs.
Acknowledgments
Funding Statement: This work was supported in part by grants from the National Institutes of Health (DA036017 to Jungmeen Kim-Spoon and Brooks King-Casas; MH 99437 to Kirby Deater-Deckard; MH087692 and MH091872 to Pearl Chiu) and from Virginia Tech Institute for Society, Culture, and Environment
Appendix
Figure A.
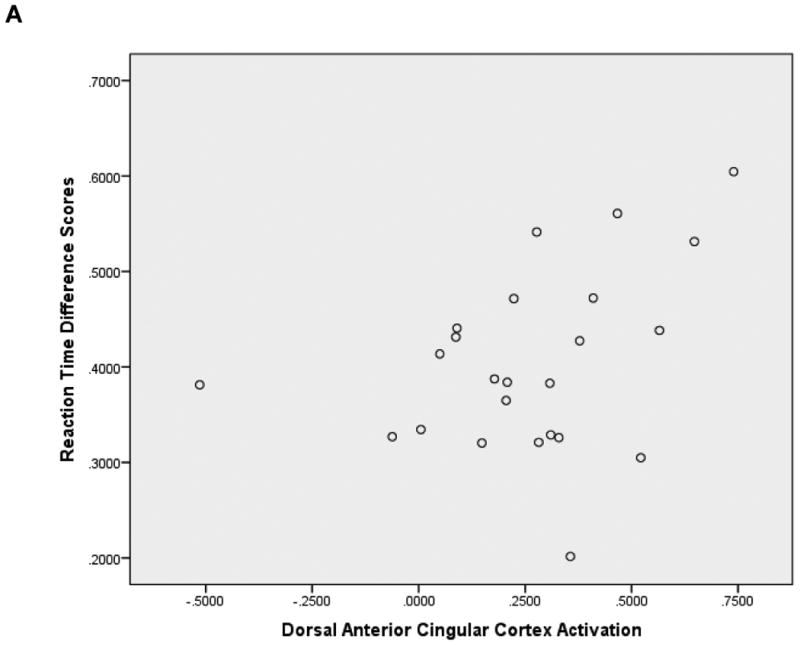
Associations of dorsal anterior cingulate cortex activation with reaction time difference (interference minus congruent conditions) scores. N = 24.
Footnotes
There were seven participants whose behavioral performance data during the first third block did not converge on a value according to the logistic function calculating CRRA. The results of our main moderation analyses were consistent between including and excluding AI scores of those seven participants, therefore they were included in the analyses.
References
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 243–277. [Google Scholar]
- Barkley-Levenson EE, Van Leijenhorst L, Galván A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Developmental Cognitive Neuroscience. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. doi:http://dx.doi.org/10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Pardini DA. Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Developmental Cognitive Neuroscience. 2015;11:56–64. doi: 10.1016/j.dcn.2014.07.008. doi:http://dx.doi.org/10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Molecular Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galván A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Survey. 2012 Retrieved from: http://www.cdc.gov/Features/YRBS/
- Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance – United States, 2013. 2014 Retrieved from: http://www.cdc.gov/mmwr/pdf/ss/ss6304.pdf.
- Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling. 2002;9:233–255. doi: 10.1207/S15328007SEM0902_5. [DOI] [Google Scholar]
- Colder CR, Hawk LW, Lengua LJ, Wiezcorek W, Eiden RD, Read JP. Trajectories of reinforcement sensitivity during adolescence and risk for substance use. Journal of Research on Adolescence. 2013;23:345–356. doi: 10.1111/jora.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair J, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychological. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity, and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents' frequency of risky sex and substance use. Addictive Behaviors. 2015;44:80–87. doi: 10.1016/j.addbeh.2014.12.007. doi:http://dx.doi.org/10.1016/j.addbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Perkins SC, Angstadt M, Johnson T, Stern ER, Welsh RC, Taylor SF. The development of performance-monitoring function in the posterior medial frontal cortex. NeuroImage. 2010;49:3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CA, Laury SK. Risk aversion and incentive effects. American Economic Review. 2002;92:1644–1655. doi: 10.1257/000282802762024700. [DOI] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Miech RA. Monitoring the Future national survey results on drug use, 1975-2013: Volume 2, College students and adults ages19-55. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Jonker NC, Ostafin BD, Glashouwer KA, van Hemel-Ruiter ME, de Jong PJ. Reward and punishment sensitivity and alcohol use: The moderating role of executive control. Addictive Behaviors. 2014;39:945–948. doi: 10.1016/j.addbeh.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Holmes C, Deater-Deckard K. Anger, fear, and attention control as precursors of adolescents' risk-taking behaviors. Journal of Child Psychology and Psychiatry. 2015;56:756–765. doi: 10.1111/jcpp.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes CJ, Lee JI, Chiu PH, King-Casas B. Behavioral and neural cognitive control moderates the effects of reward sensitivity on adolescent substance use. 2016 doi: 10.1016/j.neuropsychologia.2016.08.028. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Kahn RE, Lauharatanahirun N, Deater-Deckard K, Bickel WK, Chiu PH, King-Casas B. Substance use and self-regulation in adolescence: Neurobehavioral determinants of executive functioning. 2016 Manuscript under review. [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Current Opinion in Neurobiology. 2012;22:1027–38. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR. Neural processing of risk. The Journal of Neuroscience. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L the IMAGEN Consortium. Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Carter RM, Platt ML, Huettel SA, Brannon EM. Neurocognitive development of risk aversion from early childhood to adulthood. Frontiers in Human Neuroscience. 2012;5:178. doi: 10.3389/fnhum.2011.00178. doi:dx.doi.org/10.3389/fnhum.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SC, Welsh RC, Stern ER, Taylor SF, Fitzgerald KD. Topographic analysis of the development of individual activation patterns during performance monitoring in medial frontal cortex. Developmental Cognitive Neuroscience. 2013;6:137–148. doi: 10.1016/j.dcn.2013.09.001. doi:http://dx.doi.org/10.1016/j.dcn.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nature Neuroscience. 2008;11:398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neurosurgery. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Poldrack RA. Mind the gap: Bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends in Cognitive Sciences. 2011;15:11–19. doi: 10.1016/j.tics.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VL. Experimental economics: Induced value theory. American Economic Review. 1976:274–279. [Google Scholar]
- Smith AR, Steinberg L, Chein J. The role of the anterior insula in adolescent decision making. Developmental Neuroscience. 2014;36:196–209. doi: 10.1159/000358918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. doi: 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Saewyc E, Skay C, Richens K, Reis E, Poon C, Murphy A. Sexual orientation, sexual abuse, and HIV-risk behaviors among adolescents in the Pacific Northwest. American Journal of Public Health. 2006;96:1104–1110. doi: 10.2105/AJPH.2005.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. American Community Survey. 2012 Retrieved April 3, 2013. from http://factfinder2.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t.
- Van Leeuwen AP, Creemers HE, Verhulst FC, Ormel J, Huizink AC. Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance abuse: The TRAILS study. Journal of Studies on Alcohol and Drugs. 2011;72:70–78. doi: 10.15288/jsad.2011.72.70. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]


