Abstract
Many proteins and peptides self-associate into highly ordered and structurally similar amyloid cross-β aggregates. This fibrillation is critically dependent on properties of the protein and the surrounding environment that alter kinetic and thermodynamic equilibria. Here, we report on dominating surface and solution effects on the fibrillogenic behavior and amyloid assembly of the C-36 peptide, a circulating bioactive peptide from the α1-antitrypsin serine protease inhibitor. C-36 converts from an unstructured peptide to mature amyloid twisted-ribbon fibrils over a few hours when incubated on polystyrene plates under physiological conditions through a pathway dominated by surface-enhanced nucleation. In contrast, in plates with nonbinding surfaces, slow bulk nucleation takes precedence over surface catalysis and leads to fibrillar polymorphism. Fibrillation is strongly ion-sensitive, underlining the interplay between hydrophilic and hydrophobic forces in molecular self-assembly. The addition of exogenous surfaces in the form of silica glass beads and polyanionic heparin molecules potently seeds the amyloid conversion process. In particular, heparin acts as an interacting template that rapidly forces β-sheet aggregation of C-36 to distinct amyloid species within minutes and leads to a more homogeneous fibril population according to solid-state NMR analysis. Heparin’s template effect highlights its role in amyloid seeding and homogeneous self-assembly, which applies both in vitro and in vivo, where glycosaminoglycans are strongly associated with amyloid deposits. Our study illustrates the versatile thermodynamic landscape of amyloid formation and highlights how different experimental conditions direct C-36 into distinct macromolecular structures.
Introduction
Amyloid protein deposits are highly ordered fibrillar aggregates with characteristic β-sheet structures associated with many well-known pathological disorders such as Parkinson’s, Alzheimer’s, and systemic amyloidosis (1, 2). However, coexisting amyloid formation pathways for a single protein and the presence of oligomeric intermediates and polymorphic arrangements complicate our comprehension of the amyloid structure in human disease. The ordered aggregation process is considered to be a nucleated polymerization event involving a fibrillation-competent nucleus (3, 4), but external conditions such as ionic strength, cosolutes, and surfaces all modulate the energy landscape of amyloid conversion and ultimately affect both the kinetics of formation and the intra- and interpeptide structural arrangement (5, 6, 7, 8, 9, 10, 11, 12, 13, 14). Electrostatic modulation can act on multiple levels by Debye-Hückel ionic screening of peptide charges, “salting-out” Hofmeister effects, and specific ion binding to promote amyloid nucleation, elongation, and conformational conversion from oligomers to amyloid nuclei (15, 16). Cosolutes may promote or retard fibrillation through specific binding or through general crowding phenomena (13, 17, 18, 19). Surfaces represent repetitive molecular arrays that can lower nucleation barriers and stimulate self-assembly through promotion of favorable hydrophobic and hydrophilic interactions (5, 20, 21, 22, 23). Biological surfaces in the form of lipid membranes and polymeric structural networks such as glycosaminoglycans (GAGs) are particularly relevant for understanding amyloid formation in vivo. The GAGs are constituents of amyloid plaques (24, 25, 26, 27, 28, 29, 30, 31) and can accelerate fibril formation and deposition (32, 33, 34), dependent on their functional groups (35, 36, 37), sulfation degree (38), and polymer chain length (39, 40, 41).
In this study, we provide a comprehensive kinetic description of surface and solution effects on a proteolytically generated amyloid peptide (C-36) from the C-terminal region of the α1-antitrypsin (α1AT) serine protease inhibitor. C-36 is intrinsically disordered in solution (42), and has been found in various bodily fluids, in atherosclerotic plaques, and in alveolar fibrillar deposits (43, 44). C-36 is bioactive (43) and is also represented in newly detected C-terminal transcripts of the SERPIN1A gene (45). We document how plate surface types (hydrophilic nonbinding surfaces or hydrophobic polystyrene surfaces) and negatively charged surfaces (silica glass beads and heparin polysaccharides) all modulate the amyloid formation of C-36. We demonstrate the surface modulation of microscopic nucleation rate constants by evaluation of global fits to experimental kinetic data of aggregate mass using the framework presented by Knowles and coworkers (46, 47, 48). These models have successfully been applied to illustrate the dominating nucleation mechanism of Aβ-40 and Aβ-42 aggregation (49) and allow us to distinguish between primary and secondary nucleation pathways (details in the Supporting Material). We show that surfaces alter the kinetic terms of fibrillation but also direct C-36 to distinct amyloid morphologies, observed by transmission electron microscopy (TEM) and solid-state NMR. In particular, a negatively charged polyanionic template such as heparin catalyzes a potent template-directed polymerization of C-36 that results in distinct heparin-containing amyloid aggregates. We discuss the effects of solution conditions and polyanionic scaffolds for potentiation and modulation of amyloid formation.
Materials and Methods
Materials
Unless stated otherwise, all chemicals were from Sigma-Aldrich (St. Louis, MO) and were of analytical grade. All solutions were prepared using deionized water (Milli-Q; Millipore, Billerica, MA). Synthetic C-36 (NH2-SIPPEVKFNKPFVFLMIEQNTKSPLFMGKVVNPTQK-NH2) was made on a solid-phase peptide synthesizer, purified by RP-HPLC and validated with MALDI-MS. Recombinant C-36 (unlabeled and U-[13C, 15N]-labeled was produced as described in Oktaviani et al. (42). Dry peptide powders were solubilized in H2O, the solution was filtered (0.22 μm), and the peptide concentration was determined using a 2D-Quant kit (GE Healthcare, Little Chalfont, UK). Thioflavin T (ThT) was dissolved to a 10 mM stock solution in 96% ethanol. 9-(2,2-dicyanovinyl) julolidine (DCVJ) was dissolved to a 5 mM stock solution in dimethyl sulfoxide. Heparin sodium salt from bovine intestinal mucosa (H0777; Sigma-Aldrich) was solubilized in H2O to 10 mg/mL. Silica glass beads (ø = 1.00–1.18 mm, GP1090; Whitehouse Scientific, Waverton, Cheshire, UK) were washed in 96% ethanol before use. Phosphate-buffered solution (PB) was 20 mM NaH2PO4/Na2HPO4, pH 7.42, and phosphate-buffered saline solution (PBS) was 20 mM NaH2PO4/Na2HPO4, 150 mM NaCl, pH 7.42. Tris-buffer (TB) was 20 mM Tris, pH 7.42. Tris-buffered-saline (TBS) was 20 mM Tris, 150 mM NaCl, pH 7.42. Ammonium bicarbonate buffer (ABC) was 20 mM NH4HCO3, pH 7.42. HEPES buffer, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), was 20 mM, pH 7.42. MOPS buffer, 3-(N-morpholino)propanesulfonic acid (MOPS), was 20 mM, pH 7.42.
ThT fibrillation assays
C-36 fibrillation reactions were set up with 40 μM ThT in a volume of 100 μL in half-area polystyrene surface plates (PS; Corning microplate #3880, Corning Life Sciences, Tewksbury, MA) and nonbinding surface plates (NBS; Corning microplate #3881) ThT fluorescence was monitored on an Optima FLUOstar plate reader (BMG Labtech, Baden-Württemberg, Germany) with 450 nm excitation and 485 nm emission filters. NBS have a nonionic hydrophilic surface (polyethylene-oxidelike) that minimizes molecular interactions and have been used extensively in several kinetic studies of Aβ “bulk nucleation” and amyloid formation (5, 48). PS have a nonpolar surface (owing to the styrene groups) that readily adsorbs protein and which may act as a surface catalyst for nucleation. Fibrillation was carried out at 37°C under quiescent conditions unless otherwise stated. Each condition was assayed in triplicate or quadruplicate and repeated at least twice.
Concentration-dependent C-36 fibrillation
We measured ThT traces of amyloid formation in PBS at various peptide concentrations, m0 = 2–16 μM, in PS and NBS plates. Kinetic traces of the fibrillation reactions on each plate were normalized to the maximal fluorescence intensity obtained in the experimental window (28 h for PS, 92 h for NBS) and we assumed sequestration of all monomers into aggregates under all conditions. Polynomial and exponential nucleation growth processes can captured by a global power law scaling behavior, t1/2 ∼ m0γ, where t1/2 is the time to reach half-completion and γ reports on the reaction order of the dominating growth process (46, 47) (details in Supporting Material). We plotted t1/2 as a function of the peptide concentration on a double-log plot and the linear range was analyzed by the R software package (https://www.r-project.org/) to extract the scaling exponent (γ) (i.e., the slope in the double-log plot) and SE.
Evaluation of nucleation pathways by global fitting
The derived scaling exponents for fibrillation in NBS and PS plates (γNBS and γPS) were used to calculate the experimental nucleus size (n) for primary and secondary nucleation-dominated fibrillation based on the mathematical description by Meisl et al. (4), Cohen et al. (46), and Knowles et al. (47). Global fits to average representative ThT curves were carried out with the fibrillation models “nucleation elongation, unseeded” (primary pathway model, PP) and “secondary nucleation dominated, unseeded” (secondary pathway model, SP) in the online program Amylofit (http://www.amylofit.ch.cam.ac.uk/login) (4). The models represent closed analytical solutions to monomer-dependent growth of fibrillar mass as a function of time, M(t). The values of nc and n2 were used as an empirical restraint for the primary and secondary pathway models, respectively. The model fits were qualitatively evaluated in their ability to represent the experimental curves as a function of the primary and secondary nucleation rate constant terms (knk+ and k2k+). The rate at which new aggregates are formed (dP/dt) in the presence of both primary and secondary nucleation (SP model) is given by
where [m] is the monomer concentration and [M] is the aggregate concentration. If we assume [m] = m0 at the early stages of aggregation, we can calculate the critical aggregate mass fraction (Fcrit = Mcrit/m0) at which the primary and secondary nucleation contribute equally to the rate of aggregate formation. The expression of Fcrit becomes (4)
The value of Fcrit is used to compare the SP model fits between surfaces for the C-36 data presented herein.
DCVJ fluorescence
DCVJ was added to a concentration of 20 μM to probe the presence of prefibrillar and oligomeric intermediate species during C-36 fibrillation (50). DCVJ fluorescence was followed at the same excitation and emission wavelengths used for ThT.
Heparin-stimulated C-36 fibrillation
We added different heparin amounts to C-36 fibrillation reactions (8 μM) in PBS in NBS plates and followed the ThT traces. The presence of heparin did not affect the pH of the solution at any of the experimental concentrations. For the purpose of stoichiometric calculations, we specified the polydisperse heparin concentration in disaccharide units (heparinDS). We calculated the average mass of the heparinDS unit from compositional data of several heparin fragments (dp10–dp30) published in 2006 in a mass spectrometric characterization of medium-weight heparin (51). Based on the 20 most intense fragments, the heparinDS unit mass was 557 Da with an average sulfate to carboxylate ratio of 2.7 per disaccharide. Accounting for counterion condensation (52) on a 24-disaccharide heparin chain, the sodium counterion fractions (1-fav) are θNa = 0.56 and 0.58 at ionic strengths of 10 and 200 mM (details in the Supporting Material). The average heaparinDS mass with counterions is 605 Da, which was used to determine the heparinDS concentration from the dry weight heparin sodium salt.
Circular Dichroism spectroscopy
Heparin’s effect on the C-36 secondary structure was monitored by wavelength spectra recorded in the far-UV area from 190 to 250 nm on a J-800 spectropolarimeter (Jasco, Oklahoma City, OK) using a quartz cuvette with a 0.1 cm path length and 25 μM C-36 in 160 μL PB at 25°C. Time-course profiles at 222 nm for heparinDS/peptide ranging from 1.2:1 to 18.5:1 were recorded with a 20 s dead-time that allowed for sample mixing and transfer to the 0.1 cm quartz cuvette. Concurrently, we also measured the ThT trace after a 5:1 heparinDS/peptide addition for a PS fibrillation of 25 μM C-36 in PB in 160 μL at 25°C. The normalized ThT trace was fitted to a monoexponential function.
FITC-heparin association with C-36 aggregates
Fluorescein isothiocyanate (FITC)-labeled heparin (50 μg/mL, 83 μM) was mixed with different C-36 monomer amounts in 50 μL PBS. The FITC-heparin disaccharide concentration was calculated by using the heparinDS molecular mass of 605 Da. Reactions were spun down at 15,000g after 2 h incubation at 37°C. The fluorescence signal from the residual FITC-heparin in solution was monitored on an Omega FLUOstar plate reader (BMG Labtech) with 485 nm excitation and 520 nm emission filters.
Transmission Electron Microscopy Fibril samples for TEM were spun down, washed once in H2O, and deposited (5 μL) on a carbon-coated copper grid. The grid was washed once in H2O, stained with 1% uranyl acetate in water, and blotted dry. TEM images were collected using a G2 Tecnai spirit electron microscope (FEI, Hillsboro, OR) operated at 90 KeV with a LaB6 filament. The conditions were identical for all TEM analyses, minimizing any postformation effect on fibril morphology (53).
Solid-state NMR experiments
Solid-state NMR spectroscopy was carried out on ∼5 mg of recombinant U-[13C, 15N]-labeled fibril material produced in the absence or presence of heparin (denoted “N-fibrils” and “H-fibrils”, respectively). The N-fibrils were formed by incubation of monomeric C-36 at 50 μM for five days in PBS under slight agitation. H-fibrils were formed by incubation of monomeric C-36 at 40 μM in PBS for 8 h at a heparinDS/peptide ∼16.5:1 (400 μg/mL heparin). The fibrils were loaded into 4.0 mm MAS rotors and solid-state NMR (ssNMR) spectra were recorded at 1°C at 12 kHz magic angle spinning (MAS) on a 700-MHz spectrometer equipped with a standard triple-resonance 4 mm MAS probe (Bruker BioSpin, Rheinstetten, Germany). 13C-13C correlation spectra were acquired using dipolar assisted rotational resonance (DARR) recoupling (54) with a mixing time of 20 ms. The collected spectra were processed identically (details in the Supporting Material).
Results
Differential C-36 fibrillation kinetics on hydrophobic and hydrophilic surfaces
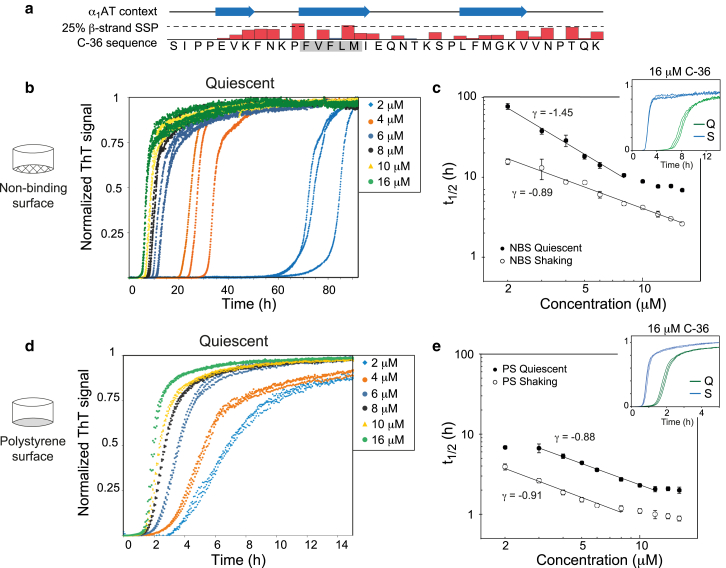
The C-36 peptide forms three β-strands in its structural context of α1AT but only has a weak β-strand secondary structural propensity in solution (Fig. 1 a). However, a centrally located FVFLM hydrophobic stretch is aggregation prone and ThT fluorescence confirmed potent fibrillation of the C-36 peptide that scaled with neutralization of the peptide’s overall charge (Fig. S1 a). The fibrillation resulted in monomer depletion (Fig. S1 b) and displayed a classic sigmoidal shape for pH > 4, which was indicative of a nucleation-dependent polymerization reaction and a hallmark of most amyloid systems (55). We explored surface modulation of C-36 fibrillation by measuring ThT traces of C-36 at monomer concentrations (m0) from 2 to 16 μM in nonbinding surface plates (NBS) and polystyrene surface plates (PS). Under quiescent conditions, NBS resulted in C-36 fibrillation half-times ranging from 77 h (2 μM C-36) to 7 h (16 μM), indicating a relatively slow nucleation process on the nonbinding hydrophilic surface (Fig. 1 b). In contrast, PS led to fast C-36 fibrillation with half-times from 7 h (2 μM) to 2 h (16 μM) (Fig. 1 d). Gel filtration of the peptide before assay start did not diminish the large variability in lag times for low m0 in NBS, suggesting that small aggregates were not the cause of this variation. The ThT plateau level correlated monotonically with m0, although there were small deviations from linearity, particularly at <5 μM C-36 (Fig. S2).
Figure 1.
Surface type profoundly affects C-36 fibrillation kinetics and alters the balance between primary and secondary nucleation. (a) Given here is an overview of the C-36 peptide with indication of the secondary structure in the context of α1AT and the weak β-strand secondary structural propensity (SSP) in solution determined by liquid-state NMR (adapted from (42)). The aggregation-prone FVFLM is boxed in gray. (b) Kinetic traces are displayed in triplicates for C-36 under quiescent conditions carried out in NBS. (c) Double logarithmic plots show the time to half-completion (t1/2) ± SD under quiescent or shaking conditions (300 RPM) as a function of C-36 concentration. The scaling relationship for indicated linear fits with SE is γ = −1.45 ± 0.09 for quiescent conditions and γ = −0.89 ± 0.04 for shaking. (d) Kinetic traces are displayed in triplicates for C-36 under quiescent conditions carried out in polystyrene surface plates (PS). (e) Double logarithmic plots of the time to half completion (t1/2) ± SD under quiescent or shaking conditions (300 RPM) are given as a function of C-36 concentration. The scaling relationship for indicated linear fits with SE is γ = −0.88 ± 0.03 for quiescent conditions and γ = −0.91 ± 0.03 for shaking. Insets in (c) and (e) compare kinetic traces for 16 μM C-36 under shaking (S) and quiescent (Q) conditions. To see this figure in color, go online.
We employed double logarithmic plots of the fibrillation half-times (t1/2) versus m0 to perform a global analysis of the protein aggregation process (4, 46, 47) (see the Supporting Material for details). The data displayed a global power law scaling behavior (t1/2 ∼ m0γ) expected for nucleation growth processes (Fig. 2 c) (46, 47). The scaling exponent, γ, varied with the plate surface type (γPS = −0.88, γNBS = −1.45) and was reduced with shaking in NBS but not in PS (γPS-S = −0.91, γNBS-S = −0.89). Shaking clearly accelerated fibrillation for both surface types (Fig. 2, c and d, insets) and for NBS, the concomitant change in γ suggested an alteration of the microscopic pathways to a more fibril-mass accelerated process. This was also evident from the more steplike curve profile under shaking, all in support of a strong secondary nucleation term enhanced by processes such as filament breakage (48). For PS, the underlying pathways, probed by γ and the curve profiles, changed only negligibly with the introduction of shaking. It is important to note that the concentration-dependent fibrillation for NBS and PS under quiescent conditions and for PS under shaking conditions were subject to a small saturation effect for m0 > 8–10 μM, i.e., a flattening of the plot of t1/2 versus m0. This positive curvature at higher concentrations indicates a shift in the microscopic pathways as a function of m0, which can relate to saturation of secondary pathways (49). A full treatment of such saturation effects is beyond the scope of the work presented here and we focus primarily on the kinetic data within the linear range of γ.
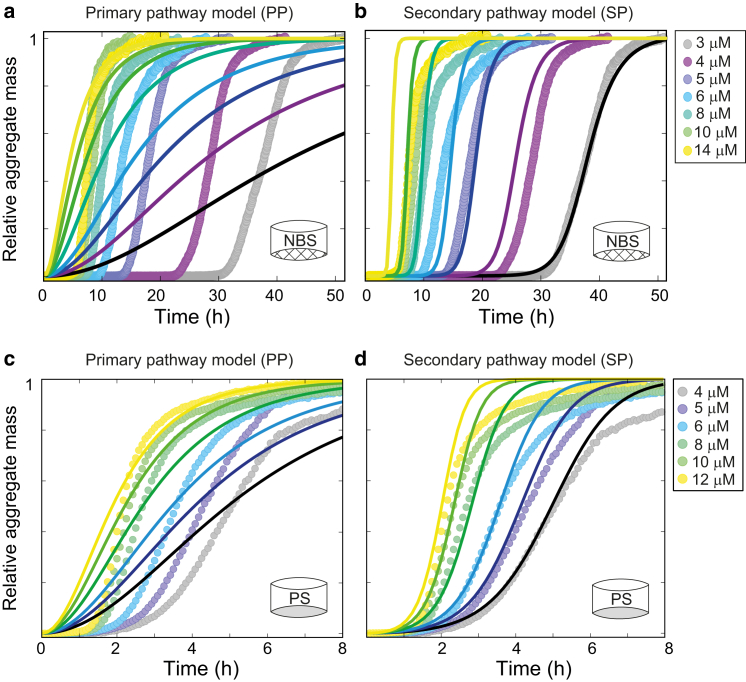
Figure 2.
Secondary nucleation pathway models describe C-36 fibrillation behavior. Global fits to averaged C-36 time-course profiles were performed with the Amylofit online tool (4). (a) The PP model with primary nucleation alone failed to represent the C-36 NBS quiescent data. The nucleus size (nc) was set to 2.9, derived from the γNBS-Q scaling exponent of −1.45 (γ = nc/2). (b) The SP model with primary and secondary nucleation terms fit the data adequately for lower m0. The nucleus size for secondary nucleation (n2) was set to 1.9 (γNBS-Q = (n2−1)/2 = −1.45) and nc was fitted to 1.85. The extracted values for the secondary and primary nucleation contribution were k+k2 = 1.0⋅1015 and k+kn = 93, respectively. Fcrit at which the two nucleation pathways contributed equally to new aggregate formation was 10−8 (for m0 = 8 μM). (c) The PP model failed to represent the C-36 PS quiescent data with nc = 1.76, derived from γPS−Q scaling exponent of −0.88. (d) The SP model with n2 set to 0.76 and nc = 1.425 (fitted) represented the data up to t1/2 adequately. The fit estimates for the secondary and primary nucleation contribution were k+k2 = 2.0⋅109 and k+kn = 2.1⋅105, respectively. Fcrit at which the two nucleation pathways contributed equally to new aggregate formation was 0.005 (for m0 = 8 μM). To see this figure in color, go online.
Primary nucleation is enhanced on a polystyrene surface
Global fitting to ThT traces was carried out with the Amylofit analysis platform that contains full analytical solutions to models of amyloid growth (4). Fits of NBS reactions under quiescent conditions (γNBS = −1.45) strongly suggested a dominating secondary pathway. The secondary pathway (SP) model, which includes contributions from primary and secondary nucleation, reached satisfactory agreement with experimental results whereas the PP model, which is restricted to primary nucleation alone, failed to do so (Fig. 2, a and b). The SP model did not adequately account for the observed lag-times at higher concentrations that we attribute to the saturation effect and consequent change in γ (monomer-dependence). Under shaking conditions with a γ-value valid for the entire concentration range, the SP model agreed well with experimental results (Fig. S3 a).
Global fitting to ThT traces under quiescent conditions in PS (γPS = −0.88) failed to produce a satisfactory fit for the PP model alone (Fig. 2 c). Early stages of aggregation up to the midpoint of fibrillation were adequately captured by the SP model with a dominant secondary nucleation term (Fig. 2 d). However, the SP model fit for PS required a much stronger primary nucleation term compared to the NBS quiescent condition, which was evident from both the rate constants (NBS, k2/kn ∼1013; PS, k2/kn ∼104) and the increase in the fractional aggregate concentration at which new aggregate formation by the secondary nucleation matched the primary nucleation (NBS, Fcrit = 10−8; PS, Fcrit = 0.005). The stronger monomer-dependent primary nucleation term in PS plates was consistent with the reduction in lag-times and a reduced exponential shape of the ThT traces compared to the NBS counterparts. Under shaking conditions the SP model reproduced experimental curves for low m0 up to t1/2, but also failed to describe the slow decay at later stages of the fibrillation process (Fig. S3 b). We observed a slight improvement of the global fit to PS data with the inclusion of a secondary pathway saturation term (Fig. S4), but a full representation of the curve profile could only be obtained by aborting global fitting and allowing a secondary nucleation rate decrease with concentration (Fig. S4). However, the qualitative evaluation of dominant nucleation mechanisms by global fitting to the simplistic PP and SP models captured surface-induced changes in the microscopic pathways reasonably well and clearly illustrated strong surface effects on aggregation kinetics.
Polystyrene surface bypasses a pre-ThT hydrophobic species
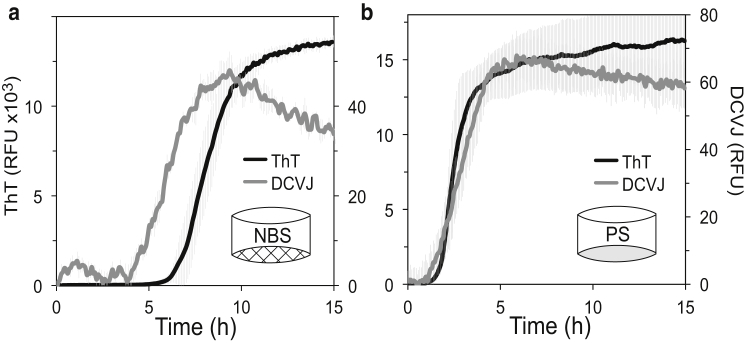
Amyloid pathways have been shown to involve the formation of oligomeric clusters that can be both on- or off-pathway species, some with distinct structures and cellular toxicity (56, 57, 58, 59, 60, 61). To link the distinct differences in kinetics of C-36 fibrillation on NBS or PS to the nature of their primary fibrillation steps and prefibrillar characteristics, we traced C-36 fibrillation with ThT and the DCVJ molecular rotor that reports on hydrophobic prefibrillar oligomers (50, 62). For C-36 fibrillation in NBS, a pre-ThT increase in the DCVJ signal was observed (Fig. 3 a), suggesting the existence of DCVJ-positive hydrophobic oligomeric clusters preceding the ThT-positive fibrillar species. In contrast, the PS surface produced almost identical time-resolved traces for DCVJ and ThT (Fig. 3 b). Bypassing the pre-ThT hydrophobic species on the PS surface was consistent with a strong surface-catalyzed primary nucleation. We interpret the NBS scenario as C-36 nucleation in bulk, which presents low intrinsic primary nucleation and extended lag times in the absence of a stimulatory hydrophobic surface. We speculate that the formation of hydrophobic oligomeric clusters or the transition of these clusters into more fibrillar species is rate limiting for C-36 amyloid formation under these circumstances.
Figure 3.
The PS surface bypasses a pre-ThT hydrophobic species observed by the DCVJ tracer. ThT and DCVJ fluorescence was measured for 16 μM C-36 fibrillation reactions in PS and NBS. (a) The DCVJ signal (gray) preceded the ThT signal (black) in NBS plates, potentially probing a pre-ThT oligomeric population. (b) The DCVJ and ThT signals displayed highly similar traces in PS plates with no apparent DCVJ increase before the ThT signal. The decrease in the DCVJ signal after reaching its maximal value is interpreted as consolidation of the mature fibrils. Gray error bars indicate the triplicate SD for each point.
Anions potentiate C-36 fibrillation on hydrophobic and hydrophilic surfaces
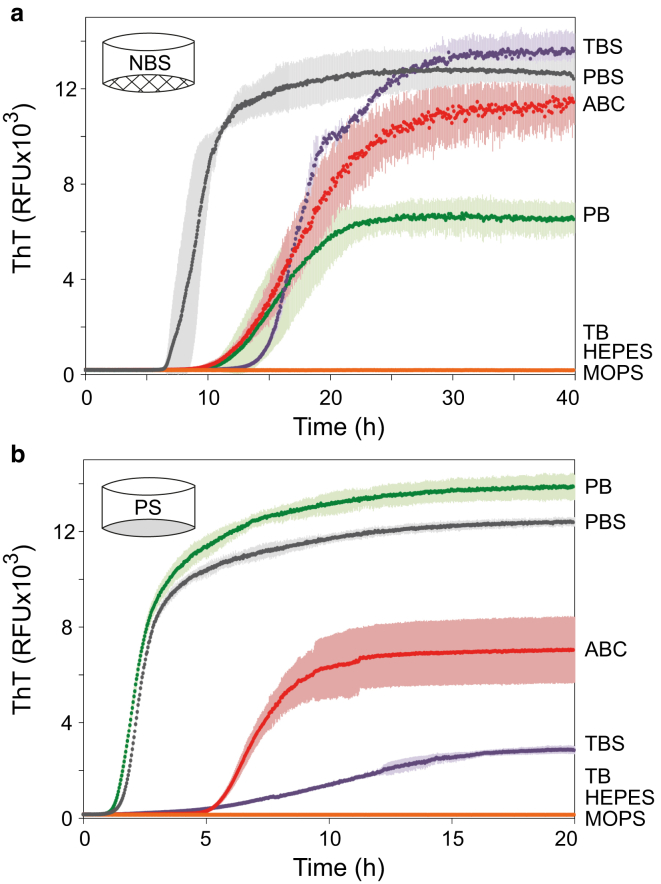
We used a selection of buffers to evaluate the effect of solvent composition on the C-36 aggregation kinetics in NBS and PS (Fig. 4). The highest aggregation potential was observed in buffers containing phosphate or bicarbonate anions, whereas no ThT-positive fibrillation took place in Tris-buffer or in HEPES and MOPS zwitterionic buffers. These fibrillation-inert buffers did not result in any monomer depletion or other aggregates (data not shown). Monomeric C-36 gave rise to largely identical 1H-15N chemical shift correlations (Fig. S5) in PBS and HEPES. This suggested that the differential aggregation behavior did not relate to any buffer-induced changes in backbone configurations. There was no change in resonance peak shape or relative intensity, suggesting no chemical exchange with other monomer configurations or oligomers (63, 64). Rather, potentiation of fibrillation must be associated with promotion of intermolecular contacts by relevant buffer or salt ions via specific ion binding or charge screening (65, 66). In evidence of this, C-36 fibrillates in TBS but not TB (which lacks NaCl) and C-36 was also induced to fibrillate in HEPES if sodium chloride (Fig. S6) or phosphate ions were added. C-36 did not aggregate in HEPES buffer even in the presence of the stimulating PS surface or glass beads (data not shown), underlining that transition from a soluble peptide in solution to a highly ordered insoluble β-sheet aggregate is an environmentally sensitive phase-transition event.
Figure 4.
Fibrillation is highly affected by the solution composition. C-36 fibrillation kinetics (8 μM) and ThT endpoint signal showed a large effect of the buffer ion identity and the ionic strength for reactions carried out in NBS (a) or PS (b). All buffers were prepared to pH 7.42 and had approximate ionic strengths of IPB = 49 mM, IPBS = 200 mM, IABC = 20 mM, ITB = 16 mM, ITBS = 166 mM, IHEPES = 7 mM, and IMOPS = 11 mM. Colored error bars indicate the triplicate SD for each point. TBS is Tris-buffered saline, TB is Tris buffer, PBS is phosphate-buffered saline, PB is phosphate buffer, and ABC is ammonium bicarbonate buffer. See Materials and Methods for exact buffer compositions. To see this figure in color, go online.
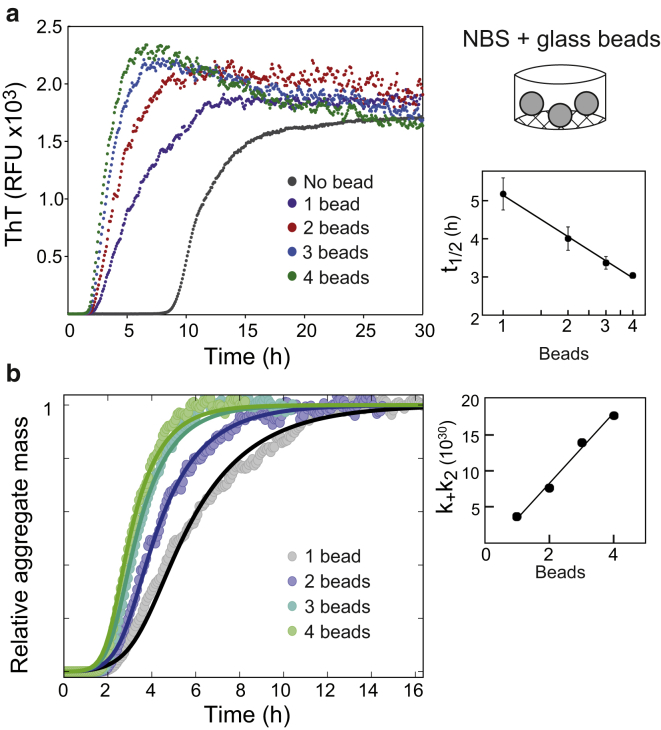
Glass beads stimulate surface-catalyzed amyloid formation
The strong template-directed surface-catalyzed nucleation of C-36 was further illustrated by fibrillation in NBS in the presence of silica glass beads. The bead surface provided new nucleation terms that entirely overruled the much smaller bulk nucleation rates seen in the absence of beads and caused a profound decrease in the fibrillation lag phase. Increasing the glass surface area (i.e., number of beads) clearly accelerated the fibrillation in a seedlike manner with a semilog relationship between the number of beads and t1/2 (Fig. 5 a). The presence of one glass bead resulted in a more rounded and less steep ThT trace compared to the control NBS reaction without beads. This suggested a decrease in the aggregate mass-catalyzed secondary growth. The apparent lag time reduction was therefore ascribed to an increased primary nucleation component, similar to the situation on the PS surface. From the ThT traces, we noticed that increasing the number of beads only marginally decreased the growth’s infliction point, indicative of a low dependency of primary nucleation on the bead surface area. Instead, the accelerated growth could be explained through global fitting by the secondary nucleation term (k+k2) that correlated linearly with the number of beads (Fig. 5 b). The nucleus sizes resulting in the best fit were nc = 2 and n2 = 5, which reflected the involvement of each pathway in the glass surface-induced effects and was different compared to the situation without beads (nc = 1.85 and n2 = 1.9; Fig. 2 d). Based on these findings, we propose that the presence of a glass surface stimulates primary nucleation of C-36 but the largest contribution of the surface is to catalyze seedlike surface-based fibril elongation and secondary nucleation. The DCVJ trace correlated directly with the amyloid mass probed by ThT (Fig. S7), bypassing the preamyloid species detected in the absence of glass beads (Fig. 3 a). This is consistent with a surface-nucleated reaction, like the polystyrene surface, where DCVJ and ThT time profiles change in parallel (Fig. 3 b).
Figure 5.
An exogenous glass bead surface stimulates nucleation pathways of C-36. (a) Shown here are ThT traces of 8 μM C-36 in PBS with the addition of the indicated number of silica glass beads. A semilog relationship between the number of glass beads and t1/2 is observed (right). (b) Given here are normalized aggregate mass curves using the highest ThT level for each reaction. Global fits to the SP model are displayed with best fit values: nc = 2, n2 = 5, k+kn = 5.7⋅107. The secondary nucleation term, k+k2, was individually fit to each curve and displayed a linear relationship to the number of glass beads (right). Fcrit at which the two nucleation pathways contributed equally to new aggregate formation in the presence of one glass bead was 0.0038 (for m0 = 8 μM). To see this figure in color, go online.
Heparin potently modulates C-36 amyloid formation
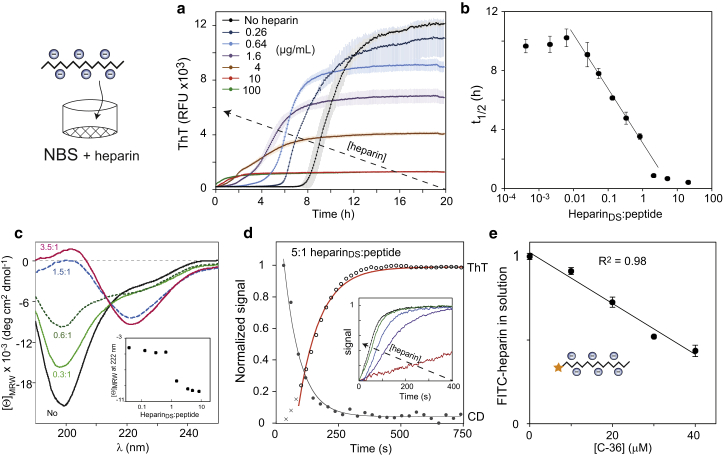
Heparin is abundant in amyloid deposits and the addition of heparin to C-36 fibrillation reactions caused a potent and concentration-dependent lag phase reduction (Fig. 6 a). At heparin disaccharide (heparinDS) to peptide ratios > 1:1, the fibrillation acceleration was accompanied by a much smaller fluorescence endpoint signal and the curve profile changed from a sigmoidal shape to a monoexponential inverted decay. The reaction exhibited a seedlike semilog relationship over a concentration range corresponding to 0.02–1 heparinDS per C-36 peptide (Fig. 6 b). Within this range, fitting with the SP model could be achieved by a heparin-dependent contribution to the primary nucleation term (Fig. S8) that supports heparin’s role in new aggregate formation. At heparinDS/peptide > 2:1, both final ThT levels and t1/2 values approached plateau values. The residual monomeric C-36 decreased to almost zero at a high heparin ratio, suggesting that the decrease in ThT endpoint fluorescence was not the result of reduced aggregation (Fig. S9 a). In fact, heparin was able to bind preformed C-36 fibrils (Fig. S9 b) and caused a concentration-dependent ThT fluorescence attenuation (Fig. S9 c). The ThT was not displaced from the fibrillar surface and heparin’s effect on the ThT emission could be through shielding of fibril-bound ThT or alteration of ThT’s exact binding mode (Fig. S9 d). The aggregation process induced by heparin resulted in a clear concentration-dependent β-sheet conversion by circular dichroism (CD) spectroscopy (Fig. 6 c) and mirrored the ThT signal with a unimolecular association pattern at high heparinDS/peptide (Fig. 6 d). The association rate was dependent on the amount of heparin (Fig. 6 d, inset) with a saturation value of ∼0.42 μM/s (Fig. S9 e). The active participation of heparin in the aggregation process was confirmed by the gradual disappearance of FITC-labeled heparin from solution with increasing amounts of C-36 peptide, with an apparent stoichiometry of 1.26 heparinDS per peptide (Fig. 6 e). Notably, heparin induced amyloid formation of C-36 also in HEPES and H2O (where heparin-free C-36 did not fibrillate), underpinning its highly potent action as an amyloid-inducing biological agent (data not shown).
Figure 6.
Heparin induces rapid C-36 fibrillation and β-sheet aggregates with lower ThT fluorescence. (a) ThT traces of 8 μM C-36 in PBS show potent alteration of C-36 fibrillation by additions of heparin with lag phase and endpoint ThT reductions. Colored error bars indicate triplicate SD for each point. (b) A semilogarithmic plot of the of t1/2 as a function of heparinDS/peptide reveals a scaling relationship for ratios ranging from 0.02:1 to 1:1, correlating with the heparin-induced ThT fluorescence reduction. (c) A clear conversion to β-sheet is observed by CD (25 μM peptide) as a function of the heparinDS/peptide. Inset shows the mean residue ellipticity change at 222 nm. (d) Time-resolved heparin-induced C-36 aggregation is followed by ThT and CD at 222 nm with 25 μM C-36 and heparin addition to ∼5:1 heparinDS/peptide. Single exponential fits are shown for each curve with exclusion of the first three data points for the ThT signal (crosses). Inset illustrates increasing aggregation speed (normalized 222 nm CD signal) at higher heparin concentrations (29, 58, 115, 231, and 461 μM). The maximal extrapolated C-36 aggregation rate was 0.42 ± μM/s (Fig. S9e). (e) FITC-labeled heparin binds C-36 and is removed from solution. FITC-heparin concentration was 50 μg/mL, corresponding to 83 μM heparinDS. The mean value for heparinDS per C-36 peptide was 1.26 ± 0.04 (SE) based on the linear regression of two combined independent experiments. To see this figure in color, go online.
Fibril morphology is directed by surface type
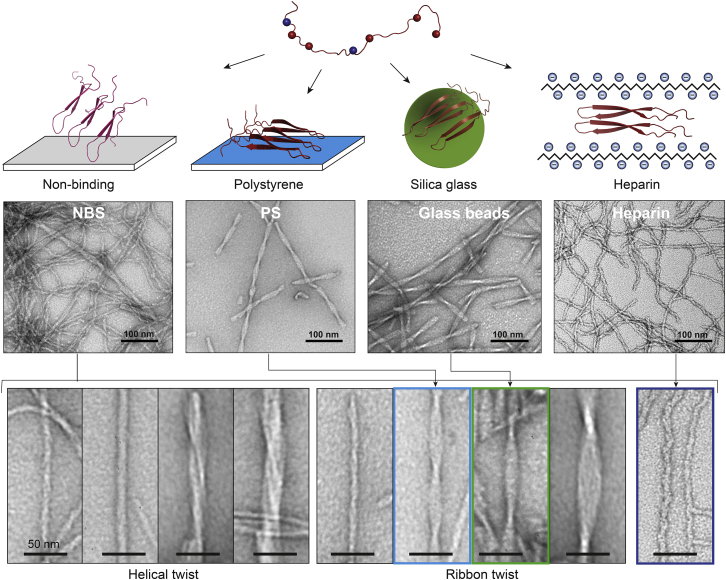
We analyzed the morphological species formed by C-36 as a function of surface type to gain a more complete description of surface-guided fibrillation pathways and the underlying energy landscape (53, 67, 68, 69). TEM imaging of C-36 fibrils formed in PS and NBS revealed clear surface-dependent shifts in fibrillar morphology. The nonbinding surface led to a heterogeneous population of fibrillar species, ranging from abundant small curvilinear fibrils with widths from 5.7 ± 0.8 nm to large twisted ribbons with widths of 23.4 ± 3.4 nm (Fig. 7). In contrast, the polystyrene surface-catalyzed fibrils were a homogeneous population of twisted ribbons with average widths of 15 ± 1 nm and a major periodicity of 56 ± 3 nm (Fig. 7). These fibril types were also formed in NBS but in much smaller numbers. The morphological appearance of the C-36 fibrils on either surface was reproducible and did not change with m0 or with prolonged incubation or storage. We corroborated the template effect of the surface type by seeding experiments in NBS using either preformed NBS (mixed morphology) or PS seeds (twisted ribbons). In the absence of the polystyrene surface, the PS seeds did not propagate the twisted ribbon morphology but ended up with a mixed morphology characteristic of NBS (Fig. S10 a). Both NBS and PS seeds effectively by-passed primary nucleation, had similar elongation rates, and demonstrated the concentration-dependent scaling behavior predicted by the semilog scaling theory (Fig. S10 b) (47, 70).
Figure 7.
Surface-guided fibrillation of C-36 to distinct amyloid arrangements is shown. C-36 is intrinsically disordered in solution and has an overall positive charge. The distribution of charged residues is shown by blue (negative) and red (positive) balls. On nonbinding surfaces, several pathways coexist and lead to a large array of fibrillar structures (bottom) with protofibrillar organization into helical and ribbon twist fibrils of various sizes. Polystyrene (blue), silica glass (green), and heparin (dark blue) all bypass a slow primary nucleation to enhance fibrillation rates, likely through a favorable charge compensation by their negative surface. The presence of these stimulating surfaces selectively enhances the formation of distinct fibril morphologies. To see this figure in color, go online.
The addition of external surfaces, glass beads, and heparin, caused clear alterations to the fibrillar morphology that correlated with the aggregation kinetics. The presence of glass beads in NBS resulted in a shift from the mixed NBS morphology to a homogenous twisted ribbon morphology (Fig. 7), which resembled fibrils formed in PS. However, the glass bead ribbon fibrils had a larger twist periodicity (89 ± 10 nm) and a marginally increased width (16 ± 1 nm). A closer examination of the PS and glass-induced ribbon fibrils revealed that PS fibrils consisted of three protofibrils, whereas the glass-induced fibrils consisted of four (Fig. S11). Heparin, despite its fast induction of C-36 aggregation, did not result in amorphous structures but also caused an ordered amyloid conversion process. The resulting homogenous fibrillar species had a curvilinear appearance and a width of 6.3 ± 1.2 nm (heparinDS/peptide > 10:1; Fig. 7). This heparin-induced morphology was caused by heparin’s interaction with the C-36 monomer because existing fibrils could not be remodeled by addition of heparin during the course of C-36 fibrillation (Fig. S12). In fact, TEM analysis demonstrated partitioning between ribbon twist or curvilinear fibrils, proportional to the ThT level at the time of heparin addition, which was particularly evident at the t1/2 heparin addition (t = 2 h) when the population of the two fibril types was nearly equal (Fig. S12).
Solid-state NMR probes C-36 fibril diversity and the structural steering by heparin
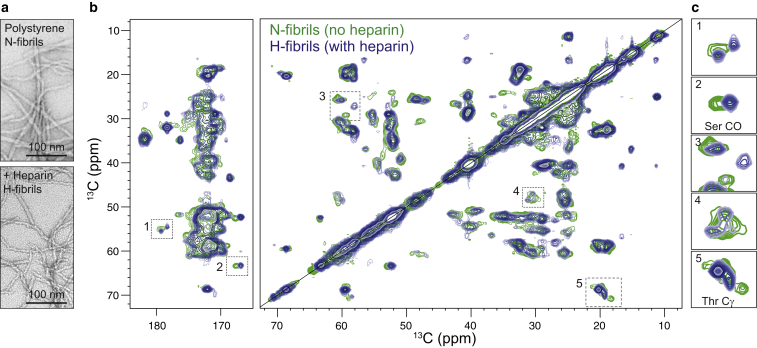
We prepared recombinant uniformly 13C,15N-labeled C-36 to study fibrillar morphologies in detail by ssNMR. Recombinant C-36 displayed a ThT trace similar to the synthetic version when fibrillated in PS plates (Fig. S13 a). The dominant fibril species was twisted ribbons but a fraction of small curvilinear fibrils was also present (Fig. 8 a). Heparin forced the production of homogenous curvilinear fibrils for the recombinant C-36, as observed with the synthetic version (Fig. 8 a). Nonheparin fibrils (N-fibrils) and heparin-induced fibrils (H-fibrils) had identical β-strand signatures by Fourier transform infrared spectroscopy (Fig. S13 b). Distinctly different spectral 13C-13C correlation signatures were obtained from N- and H-fibrils by DARR ssNMR experiments (Fig. 8 b). Firstly, the spectral resolution was improved for H-fibrils compared to N-fibrils, which was evident by the appearance of the two spectra. The improvement for the H-fibrils was likely due to well-defined chemical shifts associated with a more homogeneous structural arrangement. Secondly, unique chemical shifts were observed with H-fibrils in addition to those shared with N-fibrils (Fig. 8, b and c). Finally, N-fibrils featured at least three distinct Thr-Cγ and two Ser-CO resonance crosspeaks, but only two major Thr-Cγ peaks and one Ser-CO peak were visible in the H-fibrils spectrum. C-36 contains two Thr residues (Thr21 and Thr34) and two Ser residues (Ser1 and Ser23) (Fig. 1 a). The presence of more than two Thr crosspeaks for N-fibrils likely indicates fibril heterogeneity with various structural arrangements represented in the same spectrum. The missing Ser1 signal for H-fibrils could be an indication of some flexibility around the N-terminus in these fibrils.
Figure 8.
Nonheparin and heparin-induced fibrils display atomic level differences by solid-state NMR. (a) Given here is the fibril morphology of recombinant C-36 fibrils formed on polystyrene in the absence (N-fibrils) or in the presence (H-fibrils) of heparin. (b) Shown here is an overlay of 13C-13C correlation DARR spectra of N-fibrils (green) and H-fibrils (blue) recorded by MAS ssNMR on a 700 MHz magnet. (c) Excerpts from selected chemical shift regions illustrate the clear differences between N- and H-fibrils. To see this figure in color, go online.
The ssNMR data are consistent with the hypothesis that heparin steers C-36 into a homogeneous fibril structure, of which only a small fraction is present in a fibril population formed without heparin. We did not observe any carbonyl, aromatic, or aliphatic C-36 signals in the mobile phase by INEPT-like J-coupling-based experiments (71, 72), indicating a lack of residues with high degree of mobility outside the C-36 fibril arrangement (Fig. S14 a). The presence of crosspeaks from Ser1 and Thr34 in N-fibrils corroborates a fibrillar arrangement that spans the entire C-36 peptide chain, which is in agreement with a low proteolytic susceptibility of the C-36 fibrils (Fig. S14 b).
Discussion
Involvement of surfaces in C-36 amyloid nucleation
Our results suggest that the nucleation of C-36 and its incorporation into distinct amyloid fibrillar structures involves not only intermolecular hydrophobic forces but also surface-based interactions and electrostatic forces that potentiate the hydrophobic clustering and hydrogen bonding leading to a β-sheet structure. Through our kinetic analysis of C-36 fibrillation, we clearly demonstrated surface-induced enhancement of primary nucleation. The critical aggregate mass fraction, Fcrit, was 10−8 in NBS and effectively increased to 0.005 in PS and to 0.004 with glass beads. It also increased in the presence of heparin to 5⋅10−8 for a heparinDS/peptide ratio of 0.02, and to 0.001 for a ratio of 2.0. The increase in Fcrit implies a more dominant role of primary nucleation in the early phases of fibrillation. This more than thousandfold enhancement of primary nucleation compared to bulk nucleation in NBS plates indicates that C-36 has a clear adsorption preference to negatively charged (silica, heparin) and hydrophobic (PS) surfaces versus an NBS at pH 7.4.
The presence of stimulating surfaces (PS, glass beads, and heparin) abrogated a pre-ThT species recognized by DCVJ, suggesting acceleration or by-passing of a rate-limiting step, such as oligomer formation. Surface-induced nucleation likely involves adsorption of the aggregation-prone peptides that may stimulate nucleation through at least three mechanisms. These are: crowding through weak surface-attractive forces resulting in an apparent increase in the local peptide concentration, a situation similar to other crowding phenomena (12, 17, 73); a surface-induced conformational transition to a β-prone aggregate state that thermodynamically favors subsequent peptide self-assembly (74, 75); and change in the peptide’s hydration shell or electrostatic profile that lowers the desolvation barrier and the repulsive Coulombic forces to facilitate nucleation through hydrophobic clustering (5, 76, 77, 78).
Such hydrophobic clustering has been exemplified in the dynamic adsorption of multiple layers of β2M on various nanoparticles that stimulated amyloid formation (79) and in the formation of protein coronas on gold nanoparticles that acted as catalytic seeds (80). For C-36, we found support for a surface-induced conformational transition because PS, silica glass beads, and heparin propagated a homogenous amyloid template whereas the bulk nucleation in NBS did not. The surface enhancement of a particular amyloid arrangement may relate to the exact peptide configuration upon adsorption from which the amyloid seed grows; however, it may also involve an initial condensation followed by structural ordering into a more favorable confirmation (81). Our C-36 results on silica glass beads may be an example of this kind of surface-dependent condensation-ordering event that is less sensitive to the solution concentration of peptide and to the surface area.
Modulation of amyloid formation by the solution conditions
Protein self-association and adsorption on surfaces is governed by the interplay between electrostatic and van der Waals forces. As a consequence, electrolytes are strong modulators of such interactions through the pH, ionic strength, and specific solute properties. In general, protein aggregation and amyloid formation is affected by salts in several ways. At low ionic strengths (I < 0.1 M), salts primarily reduce repulsive electrostatic interactions through Debye-Hückel screening which in turn stimulates aggregation rates, where the slope of log(k/k0) versus is proportional to the product of the effective charges of the two reacting species (15, 82). At intermediate to high ionic strengths, salt ions can promote or delay aggregation by affecting the interfacial hydration layer at surfaces (83, 84), empirically ranked in the Hofmeister series (85) (effects reviewed in (86)). Weakly hydrated ions (chaotropes) are readily adsorbed at (protein) surfaces, decrease the interfacial tension, and enhance solubility whereas more strongly hydrated ions (kosmotropes) promote aggregation (87, 88). However, many studies of amyloid formation have reported anion specific ion binding effects that scale with the affinity of the ion to an anion-exchange resin, known as the electroselectivity series (66, 89, 90). In addition, it has also become increasingly clear that weak electrolytes (buffer ions) can play a major role in protein stability, aggregation, and surface adsorption (91, 92, 93, 94).
Our broad characterization of C-36 fibrillation in different biological and laboratory buffers demonstrated strong ion-specific effects. The biologically relevant phosphate and bicarbonate systems allowed for C-36 fibrillation to take place without the need for additional salt (I ∼20–50 mM), whereas Tris, HEPES, and MOPS did not. Interestingly, buffer ions can specifically modify a protein’s overall effective charge, as shown in a case study of lysozyme electrophoretic mobility. The study found that the positively charged protein displayed highest mobility in Tris, whereas it was reduced to ∼70% in carbonate and to ∼15% in phosphate buffers at pH 7.1 (95). We observed that the maximal C-36 fibrillation rate in PBS was reached around pH 8—well below C-36’s isoelectric point of 10.8 (Fig. S1), which supports a potential charge shielding by phosphate. The failure of C-36 to fibrillate in Tris, HEPES, and MOPS could therefore be a consequence of the peptide’s charge of +4e at neutral pH that is not efficiently reduced by the solutes to allow self-association. For the zwitterionic HEPES and MOPS buffer ions, a more active role in enhancing the electrostatic repulsion and decreasing attractive van der Waals forces through specific association to positively charged groups is also possible (96). If intrinsic electrostatic repulsions were inhibitory for C-36 self-association, efficient Debye screening using salts would mitigate this effect. Indeed, C-36 fibrillation took place in Tris buffers in the presence of 150 mM NaCl and we observed a clear enhancement of fibrillation in HEPES with increasing ionic strength. HEPES also results in changes of Aβ and hCT fibrillation compared to phosphate-based buffers (91, 97); Aβ forms fibrils with delayed kinetics compared to PBS and hCT forms spherical oligomers, supporting that buffer ions have a dramatic effect on peptide self-assembly pathways.
Several studies have described selective ion effects on protein self-association with particular anion binding at the protein surface and anion-mediated acceleration of aggregation (66, 98, 99, 100, 101). Phosphate and bicarbonate have a kosmotropic nature and also display an ability to specifically bind Lys/Arg-rich sites on proteins and polypeptides (102, 103, 104, 105). We speculate that C-36 self-association may be guided by specific anion binding that neutralizes Coulombic electrostatic repulsions between intrinsic lysyl amines in the sequence to facilitate primary nucleation. Site-specific reduction of both intra- and interpeptide repulsions may potentiate the initial contacts through the aggregation-prone pentapeptide-stretch, FVFLM, where π-stacking of the aromatic rings may play an instrumental role, as seen with other amyloids (106, 107, 108).
The surfaces on which fibrillation takes place represent an interface for both solutes and proteins that must also be considered in amyloid studies. We observed great differential solute effects as a function of surface type. On the hydrophobic polystyrene surface, sodium chloride did not accelerate fibrillation in the presence of phosphate, whereas it had a clear catalyzing role on bulk fibrillation (NBS). Conversely, fibrillation in TBS was as potent as in carbonate in bulk, but was markedly reduced on polystyrene and reached a far lower endpoint ThT level. Surfaces have properties related to their chemical nature but their interactions and adsorption properties are also modified by solution conditions through hydration, ion binding, and Hofmeister effects (94). We have shown that the complex solute interplay involves protein-surface as well as protein-protein interactions and is a critical determinant of the amyloidogenic potential and pathway.
Heparin plays a major role in accelerating amyloid formation
Heparin and heparan sulfate (HS) are negatively charged polyelectrolytes that stimulate amyloidogenesis of several amyloid precursors relevant to human disease such as α-synuclein (37), serum amyloid A (109), Aβ (35, 110), Tau (111), β2-macroglobilin (34), IAPP (112), and immunoglobulin light-chain protein (113, 114). HS proteoglycans are ubiquitously associated with pathologic amyloid deposits in diseased tissues along with hypersulfated heparin-like forms (115, 116, 117, 118). The inhibition of HS biosynthesis and the upregulation of heparanase reduce amyloid load, suggesting that the polyanions play an active role in promoting amyloid deposition in vivo (39, 119). Heparin has in recent years been shown to form an active part of amyloid hormone deposits, further highlighting the importance of polyanions in amyloid formation and as biologically active surfaces (120).
The strong acceleration of C-36 fibrillation by heparin and its binding to C-36 with approximately one disaccharide unit per peptide for both monomeric C-36 (1.3:1 binding) and mature fibrils (1:1 binding), demonstrated the active role of heparin in guiding amyloid deposition. Heparin dominated kinetics as well as morphology through direct modulation of the positively charged C-36 monomer, as shown by fluorescence, CD spectroscopy, and TEM. We observed a clear fibrillation rate increase with heparin concentration that saturated at high heparinDS/peptide. A monomorphic fibril appearance was induced by heparin that did not turn into amorphous aggregates at excess GAGs, as seen in some amyloid systems (40, 41, 121).
The highly favorable C-36/heparin interaction occurred on a min-to-s timescale and the morphologically distinct heparin aggregates acted as potent seeds for elongation and secondary nucleation at low heparinDS/peptide. A similar rapid β-sheet conversion has been observed for amyloid-β peptides (41), GAPDH (122), muscle acylphosphatase (123), and calcitonin (124). However, the HS-induced muscle acylphosphatase aggregation also bears a resemblance to C-36 in regard to its fast aggregation at subsaturation followed by a seeded slower aggregation phase (125). Such heterogeneous seeding by HS and heparin is of particular interest because GAG-peptide β-sheet aggregates may, even in small numbers, drastically affect local amyloid formation in vivo by acting as pathological chaperones that seed various amyloid pathways. In contrast, the complete kinetic and morphological steering by heparin in excess represents sequestration that could, as a general principle, both bypass formation of cytotoxic oligomeric species in amyloid systems and present a less toxic aggregate mass in itself, as demonstrated for apomyoglobin and IAPP (112, 121).
Multiple amyloid peptides and proteins interact with heparin based on electrostatic contacts with surface-exposed basic motifs or association to a sufficiently dense positively charged surface (11, 121, 126, 127). In a number of cases, direct contacts have a pH-switch of interaction regulated by protonation of one or several central His residues, such as H36 in Serum amyloid A, H18 in hIAPP, H31 in transthyretin, and H13 in Aβ (11, 112, 127, 128). C-36 lacks His and Arg residues and charge complementarity must be provided by one or several of the five Lys residues (K7, K10, K22, K29, and K36). Heparin interactions are largely driven by entropy through the polyelectrolyte effect, where most of the favorable free energy change comes from the displacement of heparin-bound sodium counterions upon protein binding (129, 130). This has been illustrated for the heparin-interacting Aβ (12, 13, 14, 15, 16, 17, 18) segment VHHQKL (+2e, pH 6.0) and the KWK-CO2 oligopeptide (+2e, pH 6.0), both containing consensus BXB motifs and binding one disaccharide unit per peptide (129, 131). C-36 (amidated) has a net nominal charge of +4e at a neutral pH and its contact to the heparin disaccharide could be mediated by a favorable entropic interaction through one of the basic motifs, BXXB and BX6B. Native heparin consists of ∼24 disaccharides, each with a structural mean charge of −3.7e, and approximately two monovalent counterions are bound per disaccharide according to counterion condensation theory (see the Supporting Material). Theoretically, C-36 could therefore bind two disaccharides by counterion substitution and our result of ∼1.3 is within this limit of an entropy-dominated binding. By ssNMR and TEM, we have shown that heparin steers C-36 into morphologically distinct curvilinear fibrils and remains associated with the fibrillar aggregate. It would be interesting to examine the details of the heparin/C-36 binding, which will require an accurate structural model of the C-36 amyloid fibrils. Such information is accessible through detailed ssNMR studies, as recently demonstrated for heparin’s specific interaction with the 3Q morphology of Aβ fibrils (132).
External factors guide amyloid assembly pathways
Amyloid fibrils represent a thermodynamically favorable state on the protein folding landscape. However, their formation is preceded by a multitude of structural intermediates, ranging from hydrophobic oligomeric assemblies to protofibrils or even amorphous aggregates. An increase in protein concentration can lead to the occurrence of multiple species with less thermodynamic stability, depending on the exact energetics associated with each state (133, 134, 135), but extrinsic factors also play a major role in modulating both rate constants and transition energies of various self-assembly pathways. The clear distinction among dominant fibril morphologies on PS, NBS, glass beads, and heparin surfaces demonstrates template-guided morphological steering and strong catalytic enhancements. Because all individual morphological forms were present under bulk nucleation (NBS) but individually enhanced under strong surface-induced nucleation, including heparin, we ascribe the surface effects to distinct alterations of the thermodynamic landscape. However, the selection of one fibrillation pathway over another required sustained surface stimulation: 1) PS seeds failed to propagate a uniform morphology in the absence of the PS surface and 2) the fraction of H-fibrils in the endpoint fibril population scaled directly to the heparinDS/peptide, despite seeding effects. The strong surface effects clearly reflect alterations of protofibrillar organization, and the spectral properties of N- and H-fibrils by ssNMR support atomic level differences in the β-strand building block as well.
Conclusions
Amyloid formation is involved in numerous human disorders and there is a need to further understand the factors that influence such protein self-assembly processes. In this study, we have shown that both hydrophobic (polystyrene) and negatively charged surfaces (silica glass beads and heparin) lead to accelerated primary nucleation for the positively charged C-36 peptide from α1-antitrypsin. The C-36 peptide contains a serpin-conserved aggregation-prone region, circulates in bodily fluids, and has been found in atherosclerotic plaques. The surface-mediated kinetic enhancement by several orders of magnitude and the bypassing of early oligomeric states led to a select subset of amyloid endpoint structures, which highlights the role of scaffolds for macromolecular assembly. We also demonstrated how the surrounding ionic composition strongly modulated C-36 fibrillation, presumably by increasing or lowering the free energy barrier of nucleation through charge screening and selective ion binding. The charge compensation effect was also evident by the potent stimulation of C-36 fibrillation by polyanionic heparin molecules. Heparin was directly implicated in the formation of amyloid aggregates and guided C-36 to a distinct morphological appearance. Such template-driven conversion is highly relevant for understanding the intricate relationship between biological polyelectrolytes in the context of amyloidosis. Imaging amyloid-GAG deposits in vivo by targeting specific amyloid-associated polyanionic structures has expanded the relevance of this entangled relationship (117, 118, 136). Therefore, future research into GAG-mediated β-sheet aggregation and fibrillation should focus on the structural properties of GAG/protein coassemblies. This would not only provide useful information for the modulation of disease amyloid deposition in the complex in vivo environment, but also for understanding the functional roles of GAG-containing secretory granules containing reversible peptide hormone aggregate structures (120, 137). Such knowledge is highly relevant to the development of self-regulatory peptide deposits and therapeutic drug functionalization.
Author Contributions
M.W.R., N.C.N., and D.E.O. designed the study. M.W.R. and D.W.J. carried out the experiments. M.B. assisted with ssNMR. J.M. assisted with fibrillation models. M.W.R., D.W.J., D.E.O., N.C.N., and J.J.E. analyzed the results. M.W.R. and D.E.O. wrote the manuscript.
Acknowledgments
We are grateful to Erik Holm Nielsen for the chemical synthesis of C-36, Aslan Hüsnu for preliminary AFM screens, and Sharon Preece for manuscript editing and proofreading.
This work was supported by grants from the Danish National Research Foundation (DNRF59), the Program Commission on Strategic Growth Technologies, Innovation Fund Denmark (0603-00439B), and the Carlsberg Foundation.
Editor: Elizabeth Rhoades.
Footnotes
Michael W. Risør’s present address is Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037.
Supporting Materials and Methods and fourteen figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30680-X.
Contributor Information
Michael W. Risør, Email: mwrisoer@gmail.com.
Daniel E. Otzen, Email: dao@inano.au.dk.
Supporting Citations
References (138, 139, 140, 141, 142, 143, 144, 145) appear in the Supporting Material.
Supporting Material
References
- 1.Bourdenx M., Koulakiotis N.S., Tsarbopoulos A. Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: examples of amyloidopathies, tauopathies and synucleinopathies. Prog. Neurobiol. 2015;155:171–193. doi: 10.1016/j.pneurobio.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Wechalekar A.D., Gillmore J.D., Hawkins P.N. Systemic amyloidosis. Lancet. 2016;387:2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 3.Morris A.M., Watzky M.A., Finke R.G. Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim. Biophys. Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Meisl G., Kirkegaard J.B., Knowles T.P. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc. 2016;11:252–272. doi: 10.1038/nprot.2016.010. [DOI] [PubMed] [Google Scholar]
- 5.Vácha R., Linse S., Lund M. Surface effects on aggregation kinetics of amyloidogenic peptides. J. Am. Chem. Soc. 2014;136:11776–11782. doi: 10.1021/ja505502e. [DOI] [PubMed] [Google Scholar]
- 6.Goers J., Uversky V.N., Fink A.L. Polycation-induced oligomerization and accelerated fibrillation of human α-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Necula M., Chirita C.N., Kuret J. Rapid anionic micelle-mediated α-synuclein fibrillization in vitro. J. Biol. Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary N., Nagaraj R. Self-assembly of short amyloidogenic peptides at the air-water interface. J. Colloid Interface Sci. 2011;360:139–147. doi: 10.1016/j.jcis.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 9.Moores B., Drolle E., Leonenko Z. Effect of surfaces on amyloid fibril formation. PLoS One. 2011;6:e25954. doi: 10.1371/journal.pone.0025954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonifácio M.J., Sakaki Y., Saraiva M.J. ‘In vitro’ amyloid fibril formation from transthyretin: the influence of ions and the amyloidogenicity of TTR variants. Biochim. Biophys. Acta. 1996;1316:35–42. doi: 10.1016/0925-4439(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 11.Noborn F., O’Callaghan P., Li J.P. Heparan sulfate/heparin promotes transthyretin fibrillization through selective binding to a basic motif in the protein. Proc. Natl. Acad. Sci. USA. 2011;108:5584–5589. doi: 10.1073/pnas.1101194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C.F., Bird S., Vaux D.J. Combined effects of agitation, macromolecular crowding, and interfaces on amyloidogenesis. J. Biol. Chem. 2012;287:38006–38019. doi: 10.1074/jbc.M112.400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao M., Estel K., Ebbinghaus S. Modulation of human IAPP fibrillation: cosolutes, crowders and chaperones. Phys. Chem. Chem. Phys. 2015;17:8338–8348. doi: 10.1039/c4cp04682j. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen L., Khurana R., Fink A.L. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–6046. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 15.Buell A.K., Hung P., Knowles T.P. Electrostatic effects in filamentous protein aggregation. Biophys. J. 2013;104:1116–1126. doi: 10.1016/j.bpj.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruzafa D., Conejero-Lara F., Morel B. Modulation of the stability of amyloidogenic precursors by anion binding strongly influences the rate of amyloid nucleation. Phys. Chem. Chem. Phys. 2013;15:15508–15517. doi: 10.1039/c3cp52313f. [DOI] [PubMed] [Google Scholar]
- 17.Uversky V.N., M Cooper E., Fink A.L. Accelerated α-synuclein fibrillation in crowded milieu. FEBS Lett. 2002;515:99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 18.Sukenik S., Harries D. Insights into the disparate action of osmolytes and macromolecular crowders on amyloid formation. Prion. 2012;6:26–31. doi: 10.4161/pri.6.1.18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syme C.D., Nadal R.C., Viles J.H. Copper binding to the amyloid-β (Aβ) peptide associated with Alzheimer’s disease: folding, coordination geometry, pH dependence, stoichiometry, and affinity of Aβ-(1–28): insights from a range of complementary spectroscopic techniques. J. Biol. Chem. 2004;279:18169–18177. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- 20.Cabaleiro-Lago C., Quinlan-Pluck F., Linse S. Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS Chem. Neurosci. 2010;1:279–287. doi: 10.1021/cn900027u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahse K., Garvey M., Fahr A. DHPC strongly affects the structure and oligomerization propensity of Alzheimer’s Aβ(1–40) peptide. J. Mol. Biol. 2010;403:643–659. doi: 10.1016/j.jmb.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Galvagnion C., Buell A.K., Dobson C.M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015;11:229–234. doi: 10.1038/nchembio.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giehm L., Otzen D.E. Strategies to increase the reproducibility of protein fibrillization in plate reader assays. Anal. Biochem. 2010;400:270–281. doi: 10.1016/j.ab.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Calamai M., Kumita J.R., Dobson C.M. Nature and significance of the interactions between amyloid fibrils and biological polyelectrolytes. Biochemistry. 2006;45:12806–12815. doi: 10.1021/bi0610653. [DOI] [PubMed] [Google Scholar]
- 25.Oláh J., Vincze O., Ovádi J. Interactions of pathological hallmark proteins: tubulin polymerization promoting protein/p25, β-amyloid, and α-synuclein. J. Biol. Chem. 2011;286:34088–34100. doi: 10.1074/jbc.M111.243907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veerhuis R., Boshuizen R.S., Familian A. Amyloid associated proteins in Alzheimer’s and prion disease. Curr. Drug Targets CNS Neurol. Disord. 2005;4:235–248. doi: 10.2174/1568007054038184. [DOI] [PubMed] [Google Scholar]
- 27.Morrison J.C., L’Hernault N.L., Quigley H.A. Ultrastructural location of extracellular matrix components in the optic nerve head. Arch. Ophthalmol. 1989;107:123–129. doi: 10.1001/archopht.1989.01070010125040. [DOI] [PubMed] [Google Scholar]
- 28.Snow A.D., Kisilevsky R., DeArmond S.J. Sulfated glycosaminoglycans in amyloid plaques of prion diseases. Acta Neuropathol. 1989;77:337–342. doi: 10.1007/BF00687367. [DOI] [PubMed] [Google Scholar]
- 29.Snow A.D., Willmer J.P., Kisilevsky R. Sulfated glycosaminoglycans in Alzheimer’s disease. Hum. Pathol. 1987;18:506–510. doi: 10.1016/s0046-8177(87)80036-9. [DOI] [PubMed] [Google Scholar]
- 30.van Horssen J., Otte-Höller I., Verbeek M.M. Heparan sulfate proteoglycan expression in cerebrovascular amyloid β deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol. 2001;102:604–614. doi: 10.1007/s004010100414. [DOI] [PubMed] [Google Scholar]
- 31.Su J.H., Cummings B.J., Cotman C.W. Localization of heparan sulfate glycosaminoglycan and proteoglycan core protein in aged brain and Alzheimer’s disease. Neuroscience. 1992;51:801–813. doi: 10.1016/0306-4522(92)90521-3. [DOI] [PubMed] [Google Scholar]
- 32.Snow A.D., Sekiguchi R., Morgan D.G. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A β-amyloid in rat brain. Neuron. 1994;12:219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 33.Ariga T., Miyatake T., Yu R.K. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer’s disease and related disorders: amyloidogenesis and therapeutic strategies—a review. J. Neurosci. Res. 2010;88:2303–2315. doi: 10.1002/jnr.22393. [DOI] [PubMed] [Google Scholar]
- 34.Borysik A.J., Morten I.J., Hewitt E.W. Specific glycosaminoglycans promote unseeded amyloid formation from β2-microglobulin under physiological conditions. Kidney Int. 2007;72:174–181. doi: 10.1038/sj.ki.5002270. [DOI] [PubMed] [Google Scholar]
- 35.Castillo G.M., Lukito W., Snow A.D. The sulfate moieties of glycosaminoglycans are critical for the enhancement of β-amyloid protein fibril formation. J. Neurochem. 1999;72:1681–1687. doi: 10.1046/j.1471-4159.1999.721681.x. [DOI] [PubMed] [Google Scholar]
- 36.Castillo G.M., Cummings J.A., Snow A.D. Sulfate content and specific glycosaminoglycan backbone of perlecan are critical for perlecan’s enhancement of islet amyloid polypeptide (amylin) fibril formation. Diabetes. 1998;47:612–620. doi: 10.2337/diabetes.47.4.612. [DOI] [PubMed] [Google Scholar]
- 37.Cohlberg J.A., Li J., Fink A.L. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 38.Malmos K.G., Otzen D.E. Glycosaminoglycans and fibrillar polymorphism. In: Lyubchenko Y., Uversky V.N., editors. Bionanoimaging: Insights into Protein Misfolding and Aggregation. Elsevier/Academic Press; New York: 2013. pp. 281–290. [Google Scholar]
- 39.Li J.P., Galvis M.L., Lindahl U. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proc. Natl. Acad. Sci. USA. 2005;102:6473–6477. doi: 10.1073/pnas.0502287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen S.B., Yde P., Otzen D.E. Multiple roles of heparin in the aggregation of p25α. J. Mol. Biol. 2012;421:601–615. doi: 10.1016/j.jmb.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 41.McLaurin J., Franklin T., Zhang X., Deng J., Fraser P.E. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans effects on fibril nucleation and growth. Eur. J. Biochem. 1999;266:1101–1110. doi: 10.1046/j.1432-1327.1999.00957.x. [DOI] [PubMed] [Google Scholar]
- 42.Oktaviani N.A., Risør M.W., Mulder F.A. Optimized co-solute paramagnetic relaxation enhancement for the rapid NMR analysis of a highly fibrillogenic peptide. J. Biomol. NMR. 2015;62:129–142. doi: 10.1007/s10858-015-9925-8. [DOI] [PubMed] [Google Scholar]
- 43.Subramaniyam D., Glader P., Janciauskiene S. C-36 peptide, a degradation product of α1-antitrypsin, modulates human monocyte activation through LPS signaling pathways. Int. J. Biochem. Cell Biol. 2006;38:563–575. doi: 10.1016/j.biocel.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Dichtl W., Moraga F., Janciauskiene S. The carboxyl-terminal fragment of α1-antitrypsin is present in atherosclerotic plaques and regulates inflammatory transcription factors in primary human monocytes. Mol. Cell Biol. Res. Commun. 2000;4:50–61. doi: 10.1006/mcbr.2000.0256. [DOI] [PubMed] [Google Scholar]
- 45.Matamala N., Aggarwal N., Martinez-Delgado B. Identification of novel short C-terminal transcripts of human SERPINA1 gene. PLoS One. 2017;12:e0170533. doi: 10.1371/journal.pone.0170533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen S.I., Vendruscolo M., Knowles T.J. The kinetics and mechanisms of amyloid formation. In: Otzen D.E., editor. Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties. Wiley; Weinheim, Germany: 2013. [Google Scholar]
- 47.Knowles T.P.J., Waudby C.A., Dobson C.M. An analytical solution to the kinetics of breakable filament assembly. Science. 2009;326:1533–1537. doi: 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- 48.Cohen S.I.A., Linse S., Knowles T.P.J. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meisl G., Yang X., Knowles T.P.J. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc. Natl. Acad. Sci. USA. 2014;111:9384–9389. doi: 10.1073/pnas.1401564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindgren M., Sörgjerd K., Hammarström P. Detection and characterization of aggregates, prefibrillar amyloidogenic oligomers, and protofibrils using fluorescence spectroscopy. Biophys. J. 2005;88:4200–4212. doi: 10.1529/biophysj.104.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henriksen J., Roepstorff P., Ringborg L.H. Ion-pairing reversed-phased chromatography/mass spectrometry of heparin. Carbohydr. Res. 2006;341:382–387. doi: 10.1016/j.carres.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 52.Manning G.S. Approximate solutions to some problems in polyelectrolyte theory involving nonuniform charge distributions. Macromolecules. 2008;41:6217–6227. [Google Scholar]
- 53.Adamcik J., Mezzenga R. Adjustable twisting periodic pitch of amyloid fibrils. Soft Matter. 2011;7:5437. [Google Scholar]
- 54.Takegoshi K., Nakamura S., Terao T. 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- 55.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzen N., Nielsen S.B., Otzen D.E. The role of stable α-synuclein oligomers in the molecular events underlying amyloid formation. J. Am. Chem. Soc. 2014;136:3859–3868. doi: 10.1021/ja411577t. [DOI] [PubMed] [Google Scholar]
- 57.Chen S.W., Drakulic S., Cremades N. Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation. Proc. Natl. Acad. Sci. USA. 2015;112:E1994–E2003. doi: 10.1073/pnas.1421204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stroud J.C., Liu C., Eisenberg D. Toxic fibrillar oligomers of amyloid-β have cross-β structure. Proc. Natl. Acad. Sci. USA. 2012;109:7717–7722. doi: 10.1073/pnas.1203193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fändrich M. Oligomeric intermediates in amyloid formation: structure determination and mechanisms of toxicity. J. Mol. Biol. 2012;421:427–440. doi: 10.1016/j.jmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Stefani M. Structural features and cytotoxicity of amyloid oligomers: implications in Alzheimer’s disease and other diseases with amyloid deposits. Prog. Neurobiol. 2012;99:226–245. doi: 10.1016/j.pneurobio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Laganowsky A., Liu C., Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paslawski W., Andreasen M., Otzen D.E. High stability and cooperative unfolding of α-synuclein oligomers. Biochemistry. 2014;53:6252–6263. doi: 10.1021/bi5007833. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi T., Matsuzaki K., Hoshino M. Transient formation of intermediate conformational states of amyloid-β peptide revealed by heteronuclear magnetic resonance spectroscopy. FEBS Lett. 2011;585:1097–1102. doi: 10.1016/j.febslet.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Fawzi N.L., Ying J., Clore G.M. Kinetics of amyloid β monomer-to-oligomer exchange by NMR relaxation. J. Am. Chem. Soc. 2010;132:9948–9951. doi: 10.1021/ja1048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owczarz M., Motta A.C., Arosio P. A colloidal description of intermolecular interactions driving fibril-fibril aggregation of a model amphiphilic peptide. Langmuir. 2015;31:7590–7600. doi: 10.1021/acs.langmuir.5b01110. [DOI] [PubMed] [Google Scholar]
- 66.Marek P.J., Patsalo V., Raleigh D.P. Ionic strength effects on amyloid formation by amylin are a complicated interplay among Debye screening, ion selectivity, and Hofmeister effects. Biochemistry. 2012;51:8478–8490. doi: 10.1021/bi300574r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paravastu A.K., Petkova A.T., Tycko R. Polymorphic fibril formation by residues 10–40 of the Alzheimer’s β-amyloid peptide. Biophys. J. 2006;90:4618–4629. doi: 10.1529/biophysj.105.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petkova A.T., Leapman R.D., Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 69.Adamcik J., Castelletto V., Mezzenga R. Direct observation of time-resolved polymorphic states in the self-assembly of end-capped heptapeptides. Angew. Chem. Int. Ed. Engl. 2011;50:5495–5498. doi: 10.1002/anie.201100807. [DOI] [PubMed] [Google Scholar]
- 70.Lorenzen N., Cohen S.I., Otzen D. Role of elongation and secondary pathways in S6 amyloid fibril growth. Biophys. J. 2012;102:2167–2175. doi: 10.1016/j.bpj.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu K.-N., McGlinchey R.P., Tycko R. Segmental polymorphism in a functional amyloid. Biophys. J. 2011;101:2242–2250. doi: 10.1016/j.bpj.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kryndushkin D.S., Wickner R.B., Tycko R. The core of Ure2p prion fibrils is formed by the N-terminal segment in a parallel cross-β structure: evidence from solid-state NMR. J. Mol. Biol. 2011;409:263–277. doi: 10.1016/j.jmb.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Z., Fan J.-B., Liang Y. Crowded cell-like environment accelerates the nucleation step of amyloidogenic protein misfolding. J. Biol. Chem. 2009;284:30148–30158. doi: 10.1074/jbc.M109.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu M., Souillac P.O., Fink A.L. Surface-catalyzed amyloid fibril formation. J. Biol. Chem. 2002;277:50914–50922. doi: 10.1074/jbc.M207225200. [DOI] [PubMed] [Google Scholar]
- 75.Nault L., Vendrely C., Weidenhaupt M. Peptides that form β-sheets on hydrophobic surfaces accelerate surface-induced insulin amyloidal aggregation. FEBS Lett. 2013;587:1281–1286. doi: 10.1016/j.febslet.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 76.Ravikumar K.M., Hwang W. Role of hydration force in the self-assembly of collagens and amyloid steric zipper filaments. J. Am. Chem. Soc. 2011;133:11766–11773. doi: 10.1021/ja204377y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thirumalai D., Reddy G., Straub J.E. Role of water in protein aggregation and amyloid polymorphism. Acc. Chem. Res. 2012;45:83–92. doi: 10.1021/ar2000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assarsson A., Hellstrand E., Linse S. Charge dependent retardation of amyloid β aggregation by hydrophilic proteins. ACS Chem. Neurosci. 2014;5:266–274. doi: 10.1021/cn400124r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linse S., Cabaleiro-Lago C., Dawson K.A. Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. USA. 2007;104:8691–8696. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gladytz A., Abel B., Risselada H.J. Gold-induced fibril growth: the mechanism of surface-facilitated amyloid aggregation. Angew. Chem. Int. Ed. Engl. 2016;55:11242–11246. doi: 10.1002/anie.201605151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Auer S., Trovato A., Vendruscolo M. A condensation-ordering mechanism in nanoparticle-catalyzed peptide aggregation. PLOS Comput. Biol. 2009;5:e1000458. doi: 10.1371/journal.pcbi.1000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kutsch M., Hortmann P., Weingärtner H. Dissecting ion-specific from electrostatic salt effects on amyloid fibrillation: a case study of insulin. Biointerphases. 2016;11:019008. doi: 10.1116/1.4941008. [DOI] [PubMed] [Google Scholar]
- 83.Nihonyanagi S., Yamaguchi S., Tahara T. Counterion effect on interfacial water at charged interfaces and its relevance to the Hofmeister series. J. Am. Chem. Soc. 2014;136:6155–6158. doi: 10.1021/ja412952y. [DOI] [PubMed] [Google Scholar]
- 84.Flores S.C., Kherb J., Cremer P.S. Direct and reverse Hofmeister effects on interfacial water structure. J. Phys. Chem. C. 2012;116:14408–14413. [Google Scholar]
- 85.Kunz W., Henle J., Ninham B.W. “Zur lehre von der wirkung der salze” (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid. Int. Sci. 2004;9:19–37. [Google Scholar]
- 86.Salis A., Ninham B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014;43:7358–7377. doi: 10.1039/c4cs00144c. [DOI] [PubMed] [Google Scholar]
- 87.Bogár F., Bartha F., Dér A. On the Hofmeister effect: fluctuations at the protein-water interface and the surface tension. J. Phys. Chem. B. 2014;118:8496–8504. doi: 10.1021/jp502505c. [DOI] [PubMed] [Google Scholar]
- 88.Collins K.D. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods. 2004;34:300–311. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 89.Pedersen J.S., Flink J.M., Otzen D.E. Sulfates dramatically stabilize a salt-dependent type of glucagon fibrils. Biophys. J. 2006;90:4181–4194. doi: 10.1529/biophysj.105.070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jain S., Udgaonkar J.B. Salt-induced modulation of the pathway of amyloid fibril formation by the mouse prion protein. Biochemistry. 2010;49:7615–7624. doi: 10.1021/bi100745j. [DOI] [PubMed] [Google Scholar]
- 91.Garvey M., Tepper K., Fändrich M. Phosphate and HEPES buffers potently affect the fibrillation and oligomerization mechanism of Alzheimer’s Aβ peptide. Biochem. Biophys. Res. Commun. 2011;409:385–388. doi: 10.1016/j.bbrc.2011.04.141. [DOI] [PubMed] [Google Scholar]
- 92.Zhu L., Zhang X.J., Perrett S. Relationship between stability of folding intermediates and amyloid formation for the yeast prion Ure2p: a quantitative analysis of the effects of pH and buffer system. J. Mol. Biol. 2003;328:235–254. doi: 10.1016/s0022-2836(03)00249-3. [DOI] [PubMed] [Google Scholar]
- 93.Barnett G.V., Razinkov V.I., Roberts C.J. Specific-ion effects on the aggregation mechanisms and protein-protein interactions for anti-streptavidin immunoglobulin γ-1. J. Phys. Chem. B. 2015;119:5793–5804. doi: 10.1021/acs.jpcb.5b01881. [DOI] [PubMed] [Google Scholar]
- 94.Cugia F., Sedda S., Salis A. Are specific buffer effects the new frontier of Hofmeister phenomena? Insights from lysozyme adsorption on ordered mesoporous silica. RSC Advances. 2016;6:94617–94621. [Google Scholar]
- 95.Cugia F., Monduzzi M., Salis A. Interplay of ion specificity, pH and buffers: insights from electrophoretic mobility and pH measurements of lysozyme solutions. RSC Advances. 2013;3:5882. [Google Scholar]
- 96.Koerner M.M., Palacio L.A., Petrache H.I. Electrodynamics of lipid membrane interactions in the presence of zwitterionic buffers. Biophys. J. 2011;101:362–369. doi: 10.1016/j.bpj.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itoh-Watanabe H., Kamihira-Ishijima M., Naito A. Characterization of the spherical intermediates and fibril formation of hCT in HEPES solution using solid-state 13C-NMR and transmission electron microscopy. Phys. Chem. Chem. Phys. 2013;15:16956–16964. doi: 10.1039/c3cp52810c. [DOI] [PubMed] [Google Scholar]
- 98.Gokarn Y.R., Fesinmeyer R.M., Brems D.N. Effective charge measurements reveal selective and preferential accumulation of anions, but not cations, at the protein surface in dilute salt solutions. Protein Sci. 2011;20:580–587. doi: 10.1002/pro.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronga L., Palladino P., Rossi F. Effect of salts on the structural behavior of hPrP α2-helix-derived analogues: the counterion perspective. J. Pept. Sci. 2006;12:790–795. doi: 10.1002/psc.818. [DOI] [PubMed] [Google Scholar]
- 100.Munishkina L.A., Henriques J., Fink A.L. Role of protein-water interactions and electrostatics in α-synuclein fibril formation. Biochemistry. 2004;43:3289–3300. doi: 10.1021/bi034938r. [DOI] [PubMed] [Google Scholar]
- 101.Poniková S., Antošová A., Sedlák E. Lysozyme stability and amyloid fibrillization dependence on Hofmeister anions in acidic pH. J. Biol. Inorg. Chem. 2015;20:921–933. doi: 10.1007/s00775-015-1276-0. [DOI] [PubMed] [Google Scholar]
- 102.Copley R.R., Barton G.J. A structural analysis of phosphate and sulphate binding sites in proteins. Estimation of propensities for binding and conservation of phosphate binding sites. J. Mol. Biol. 1994;242:321–329. doi: 10.1006/jmbi.1994.1583. [DOI] [PubMed] [Google Scholar]
- 103.Osheroff N., Brautigan D.L., Margoliash E. Mapping of anion binding sites on cytochrome c by differential chemical modification of lysine residues. Proc. Natl. Acad. Sci. USA. 1980;77:4439–4443. doi: 10.1073/pnas.77.8.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sträter N., Sun L., Lipscomb W.N. A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by dizinc leucine aminopeptidase. Proc. Natl. Acad. Sci. USA. 1999;96:11151–11155. doi: 10.1073/pnas.96.20.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin X.Y., Leclercq L., Cottet H. Investigating the influence of phosphate ions on Poly(L-lysine) conformations by Taylor dispersion analysis. Macromolecules. 2014;47:5320–5327. [Google Scholar]
- 106.Gazit E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 107.Levy M., Garmy N., Fantini J. The minimal amyloid-forming fragment of the islet amyloid polypeptide is a glycolipid-binding domain. FEBS J. 2006;273:5724–5735. doi: 10.1111/j.1742-4658.2006.05562.x. [DOI] [PubMed] [Google Scholar]
- 108.Marshall K.E., Morris K.L., Serpell L.C. Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry. 2011;50:2061–2071. doi: 10.1021/bi101936c. [DOI] [PubMed] [Google Scholar]
- 109.Aguilera J.J., Zhang F., Colón W. Divergent effect of glycosaminoglycans on the in vitro aggregation of serum amyloid A. Biochimie. 2014;104:70–80. doi: 10.1016/j.biochi.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McLaurin J., Franklin T., Fraser P.E. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans effects on fibril nucleation and growth. Eur. J. Biochem. 1999;266:1101–1110. doi: 10.1046/j.1432-1327.1999.00957.x. [DOI] [PubMed] [Google Scholar]
- 111.Goedert M., Jakes R., Crowther R.A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 112.De Carufel C.A., Nguyen P.T., Bourgault S. New insights into the roles of sulfated glycosaminoglycans in islet amyloid polypeptide amyloidogenesis and cytotoxicity. Biopolymers. 2013;100:645–655. doi: 10.1002/bip.22243. [DOI] [PubMed] [Google Scholar]
- 113.Ren R., Hong Z., Trinkaus-Randall V. Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils. J. Biol. Chem. 2010;285:37672–37682. doi: 10.1074/jbc.M110.149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McLaughlin R.W., De Stigter J.K., Ramirez-Alvarado M. The effects of sodium sulfate, glycosaminoglycans, and Congo Red on the structure, stability, and amyloid formation of an immunoglobulin light-chain protein. Protein Sci. 2006;15:1710–1722. doi: 10.1110/ps.051997606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Snow A.D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab. Invest. 1987;56:120–123. [PubMed] [Google Scholar]
- 116.Zhang X., Li J.P. Heparan sulfate proteoglycans in amyloidosis. Prog. Mol. Biol. Transl. Sci. 2010;93:309–334. doi: 10.1016/S1877-1173(10)93013-5. [DOI] [PubMed] [Google Scholar]
- 117.Smits N.C., Kurup S., van Kuppevelt T.H. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J. Biol. Chem. 2010;285:41143–41151. doi: 10.1074/jbc.M110.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wall J.S., Richey T., Kennel S.J. In vivo molecular imaging of peripheral amyloidosis using heparin-binding peptides. Proc. Natl. Acad. Sci. USA. 2011;108:E586–E594. doi: 10.1073/pnas.1103247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kisilevsky R., Szarek W.A., Berkin A. Inhibition of amyloid A amyloidogenesis in vivo and in tissue culture by 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-α- and β-d-glucose: implications for the treatment of various amyloidoses. Am. J. Pathol. 2004;164:2127–2137. doi: 10.1016/s0002-9440(10)63771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maji S.K., Perrin M.H., Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vilasi S., Sarcina R., Sirangelo I. Heparin induces harmless fibril formation in amyloidogenic W7FW14F apomyoglobin and amyloid aggregation in wild-type protein in vitro. PLoS One. 2011;6:e22076. doi: 10.1371/journal.pone.0022076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Torres-Bugeau C.M., Ávila C.L., Chehín R.N. Characterization of heparin-induced glyceraldehyde-3-phosphate dehydrogenase early amyloid-like oligomers and their implication in α-synuclein aggregation. J. Biol. Chem. 2012;287:2398–2409. doi: 10.1074/jbc.M111.303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Motamedi-Shad N., Monsellier E., Chiti F. Amyloid formation by the model protein muscle acylphosphatase is accelerated by heparin and heparan sulphate through a scaffolding-based mechanism. J. Biochem. 2009;146:805–814. doi: 10.1093/jb/mvp128. [DOI] [PubMed] [Google Scholar]
- 124.Malmos K.G., Bjerring M., Otzen D.E. How glycosaminoglycans promote fibrillation of salmon calcitonin. J. Biol. Chem. 2016;291:16849–16862. doi: 10.1074/jbc.M116.715466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Motamedi-Shad N., Garfagnini T., Chiti F. Rapid oligomer formation of human muscle acylphosphatase induced by heparan sulfate. Nat. Struct. Mol. Biol. 2012;19:547–554. doi: 10.1038/nsmb.2286. S1–S2. [DOI] [PubMed] [Google Scholar]
- 126.Torrent M., Nogués M.V., Boix E. The “CPC clip motif”: a conserved structural signature for heparin-binding proteins. PLoS One. 2012;7:e42692. doi: 10.1371/journal.pone.0042692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McLaurin J., Fraser P.E. Effect of amino-acid substitutions on Alzheimer's amyloid-β peptide-glycosaminoglycan interactions. Eur. J. Biochem. 2000;267:6353–6361. doi: 10.1046/j.1432-1327.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- 128.Elimova E., Kisilevsky R., Ancsin J.B. Heparan sulfate promotes the aggregation of HDL-associated serum amyloid A: evidence for a proamyloidogenic histidine molecular switch. FASEB J. 2009;23:3436–3448. doi: 10.1096/fj.09-134981. [DOI] [PubMed] [Google Scholar]
- 129.Mascotti D.P., Lohman T.M. Thermodynamics of charged oligopeptide-heparin interactions. Biochemistry. 1995;34:2908–2915. doi: 10.1021/bi00009a022. [DOI] [PubMed] [Google Scholar]
- 130.Minsky B.B., Nguyen T.V., Dubin P.L. Heparin decamer bridges a growth factor and an oligolysine by different charge-driven interactions. Biomacromolecules. 2013;14:4091–4098. doi: 10.1021/bm401227p. [DOI] [PubMed] [Google Scholar]
- 131.Nguyen K., Rabenstein D.L. Interaction of the heparin-binding consensus sequence of β-amyloid peptides with heparin and heparin-derived oligosaccharides. J. Phys. Chem. B. 2016;120:2187–2197. doi: 10.1021/acs.jpcb.5b12235. [DOI] [PubMed] [Google Scholar]
- 132.Stewart K.L., Hughes E., Middleton D.A. Atomic details of the interactions of glycosaminoglycans with amyloid-β fibrils. J. Am. Chem. Soc. 2016;138:8328–8331. doi: 10.1021/jacs.6b02816. [DOI] [PubMed] [Google Scholar]
- 133.Ricchiuto P., Brukhno A.V., Auer S. Protein aggregation: kinetics versus thermodynamics. J. Phys. Chem. B. 2012;116:5384–5390. doi: 10.1021/jp302797c. [DOI] [PubMed] [Google Scholar]
- 134.Auer S., Meersman F., Vendruscolo M. A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates. PLOS Comput. Biol. 2008;4:e1000222. doi: 10.1371/journal.pcbi.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pellarin R., Caflisch A. Interpreting the aggregation kinetics of amyloid peptides. J. Mol. Biol. 2006;360:882–892. doi: 10.1016/j.jmb.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 136.Wall J.S., Richey T., Chopra A. (125)I-Labeled single-chain monoclonal antibody, NS4F5, that targets the GlcNS6S-IdoA2S motif of heparan sulfate proteoglycans for the in vivo imaging of peripheral amyloidosis. Nucl. Med. Biol. 2012;39:65–75. [PubMed] [Google Scholar]
- 137.Dannies P.S. Prolactin and growth hormone aggregates in secretory granules: the need to understand the structure of the aggregate. Endocr. Rev. 2012;33:254–270. doi: 10.1210/er.2011-1002. [DOI] [PubMed] [Google Scholar]
- 138.Cohen S.I.A., Vendruscolo M., Knowles T.P.J. From macroscopic measurements to microscopic mechanisms of protein aggregation. J. Mol. Biol. 2012;421:160–171. doi: 10.1016/j.jmb.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 139.Cohen S.I.A., Vendruscolo M., Knowles T.P.J. Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J. Chem. Phys. 2011;135:065105. doi: 10.1063/1.3608916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Manning G.S. Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J. Chem. Phys. 1969;51:924–933. [Google Scholar]
- 141.Diakun G.P., Edwards H.E., Phillips G.O. The relationship between counterion activity coefficients and the anticoagulant activity of heparin. Macromolecules. 1978;11:1110–1114. [Google Scholar]
- 142.Rabenstein D.L., Robert J.M., Peng J. Multinuclear magnetic resonance studies of the interaction of inorganic cations with heparin. Carbohydr. Res. 1995;278:239–256. doi: 10.1016/0008-6215(95)00263-4. [DOI] [PubMed] [Google Scholar]
- 143.Beirne J., Truchan H., Rao L. Development and qualification of a size exclusion chromatography coupled with multiangle light scattering method for molecular weight determination of unfractionated heparin. Anal. Bioanal. Chem. 2011;399:717–725. doi: 10.1007/s00216-010-4187-5. [DOI] [PubMed] [Google Scholar]
- 144.Fung B.M., Khitrin A.K., Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 145.Zandomeneghi G., Krebs M.R., Fändrich M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004;13:3314–3321. doi: 10.1110/ps.041024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.