Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder. Missense mutations of DCTN1 have been identified as a possible genetic risk factor for ALS. Here, we tested the DCTN1 protein-coding exons in 510 sporadic ALS patients in whom SOD1, TARDBP, FUS, and C9orf72 genes were screened before. Polymerase chain reaction and Sanger sequencing were used for mutation discovery. The results revealed two rare heterozygous missense variants, c.1867C>T (p.R623W) and c.2798C>T (p.A933V). These two patients exhibited spinal disease onset without cognitive impairment, and their onset age and diagnosis delay was within the average range of Chinese ALS patients. Our results suggested that variants in DCTN1 are not common risk factors for Chinese sporadic ALS and that the frequency of variants of unknown significance in the cohort study was 0.39%.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder characterized by the selective impairment of the motor cortex and motor neurons of the brainstem and the spinal cord. Approximately 90% of ALS cases are sporadic, and the remaining 10% are inherited[1, 2]. A recent study of 1624 Chinese ALS patients substantially advanced the understanding of the clinical features and epidemiology of this rare disease[3]. Compared with other nationalities, Chinese patients exhibited an earlier age of onset, a lower percentage of bulbar-onset ALS and better prognosis. It is increasingly recognized that ALS represents a heterogeneous group of diseases that share clinical features rather than a single entity [4]. Genetic studies are important for shedding light on the potential mechanisms of heterogeneity associated with ALS, and such studies have been performed worldwide. The human dynactin subunit 1 gene (DCTN1) was first identified in a family co-segregating with ALS in 2004[5]. Although DCTN1 is not a common genetic cause in familial cases, the mutation frequencies in sporadic ALS vary from 0.51% to 2.87% in different populations [6–9]
DCTN1 is located on chromosome 2p13 and encodes six different isoforms in human. The isoform 1 (known as P150) is a component of the dynactin complex that is expressed in almost every cell[10]. Dynactin subunit 1 includes a CAP-Gly domain, a dynein-binding domain, an Arp1 and Hap1 binding site and they are connected with 3 coiled coil structures (CC1a,CC1b,CC2)[11, 12]. The dynactin complex acts as a connector of cargos with microtubules and cytoplasmic dynein in the process of retrograde vesicle transport, which is involved in centripetal movement of lysosomes and endosomes, ER-to-Golgi transport, spindle formation, chromosome movement, nuclear positioning, and axonogenesis[5, 13]. Disturbing this interaction influences fast axonal transport and consequently disrupts physiologic neuronal function[11]. Mutations of DCTN1 are associated with distal hereditary motor neuropathy VIIIB (OMIM 607641), Perry syndrome (OMIM 168605) and ALS (OMIM 105400)[5, 12, 14]
At least 14 missense mutations and a splicing change of DCTN1 were reported in familial and sporadic ALS patients, but the clinical manifestations of ALS caused by mutant DCTN1 were different among families[5, 7, 15–18]. However, most of the cases were identified in Caucasians. It has been described that the frequencies of mutations of some genes (especially C9orf72) in Chinese sporadic ALS was inconsistent with Caucasians[19–21]. In our study, DCTN1 was first sequenced in a large Chinese population with sporadic ALS, and we wanted to investigate the mutation frequencies and spectrum in DCTN1.
Material and methods
Ethics statement
This study was approved by the institutional ethics committee of Peking University Third Hospital (PUTH) (IRB00006761). The study group obtained written informed consent from each patient before they participated in the study.
Participants
This study included 510 sporadic ALS patients (males: females ratio was 1.78; mean age at diagnose ± standard deviation (SD) = 53±11 years) registered with the Neurological Department of Peking University Third Hospital from 2013 to 2014 and 210 non-neurologic control subjects (117 males, 93 females; Males: Females ratio was 1.26; mean age ± SD = 55±13 years). Additional 280 non-neurologic controls (490 controls in all) were used to verify the suspicious pathogenic variants found in patient cohort. All subjects were of Han ethnicity, and the ALS patients met the revised El Escorial criteria of clinically definite, probable, or laboratory-supported probable ALS[22]. Diagnosis delay means the time from symptom onset to a confirmed diagnosis of ALS. All included patients were participating in long-term follow-up and the revised ALS functional rating score (ALSFRS-R) was evaluated every three months prior to study enrollment. We screened SOD1, TARDBP, FUS and C9orf72 genes for all patients before our research.
Sequencing analysis of DCTN1
Genomic DNA was extracted from whole blood using standard protocols (QIAGEN, Valencia, California, USA). A total of 24 pairs of primers were designed for PCR amplification of the 32 protein-coding exons, including intron-exon boundaries, of DCTN1 (GenBank NM_004082.4). PCR was performed in a total volume of 25 μl, which consisted of 20 ng of genomic DNA, 10 pmol of each primer, 12.5μl of 2×Taq PCR Master Mix (Tsingke Biotechnology Co., Ltd., Beijing, China) and 9.5μl of deionized water. The thermocycling conditions were as follows: initial denaturation at 98°C for 2 min, 30 cycles at 98°C for 10 s, 57–65°C for 15 s (the annealing temperature ranged from 57°C to 65°C depending on the primer pair, see supplementary material), and extension at 72°C for 15 s, and a final extension at 72°C for 5 min. We used agarose gel electrophoresis and magnetic beads cleanup method for isolating and purifying PCR products. BigDye Terminator v3.1 Cycle Sequencing Kit and 3730XL DNA Analyzer was used for the amplification of PCR products and Sanger sequencing. All products were sequenced at Tsingke Biotechnology Co., Ltd. Each identified mutation was confirmed by both forward and reverse sequencing, and all mutated sequences were re-amplified and re-sequenced.
Software used to design primers and analyze data
The primers were designed using the Primer3Plus online tool (http://www.primer3plus.com; the primer sequences are available in S1 Table). The sequencing results were analyzed using DNAStarLasergene 7.1 software. The PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), MutationTaster (http://www.mutationtaster.org), SIFT (http://sift.jcvi.org/) programs were used to predict the effects of each mutation on protein function. The Short Genetic Variations Database (dbSNP 142), the NHLBI Exome Variant Server (EVS), the 1000 Genomes Project (TGP), and the Exome Aggregation Consortium (ExAC) databases were checked for the presence of the variants identified in the cohort. The PhyloP and PhastCons (http://compgen.bscb.cornell.edu/phast/) were used to determine whether the variants were evolutionary conserved. The UniProt Web site (http://www.uniprot.org) was used for the alignment of the mutation sites.
Results
Genetic analyses
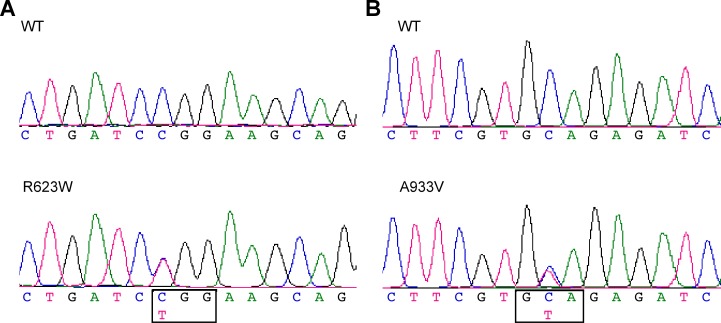
Sequence analysis of DCTN1 in the patients’ cohort revealed 16 single nucleotide variants (listed in S2 Table). Among them, 14 variants were considered benign polymorphisms, because they were synonymous or have been identified in EVS, TGP or ExAC database (more than 4,300 exomes from East Asian were documented in ExAC). The remaining two variants are heterozygous single base-pair substitutions, c.1867C>T (p.R623W) and c.2798C>T (p.A933V) (GenBank NM_004082.4 and NP_004073.2, see Fig 1 and Table 1). These sites were wild type in all 490 controls. The variants did not affect the splicing site. Moreover, we confirmed the lack of mutations in the SOD1, TARDBP, FUS and C9orf72 genes in these two patients.
Fig 1. Novel missense mutations in DCTN1 identified in sporadic ALS patients.
(a), (b): Partial chromatograms of two novel mutations of DCTN1.
Table 1. Novel mutations of DCTN1 in Chinese patients with sporadic amyotrophic lateral sclerosis.
| Mutations | cDNA | Exon | Patients | Controls | MutationTaster | PolyPhen-2 (score) | SIFT (score) | PhyloP (score) | PhastCons (score) |
|---|---|---|---|---|---|---|---|---|---|
| p.R623W | c.1867C>T | 17 | 1/510 | 0/490 | Disease causing | Probably damaging (0.994) | Damaging (0.016) | Conserved (2.479) | Conserved (1) |
| p.A933V | c.2798C>T | 24 | 1/510 | 0/490 | Disease causing | Probably damaging (0.993) | Damaging (0.018) | Conserved (5.8) | Conserved (1) |
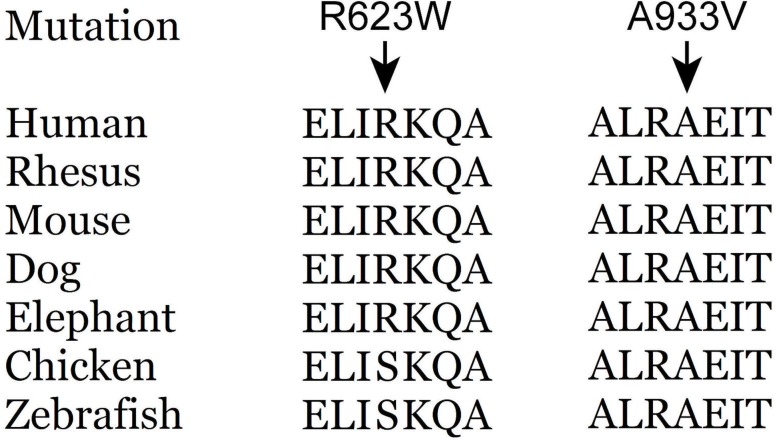
Supported by PhyloP and PhastCons software (see Table 1), both p.R623W and p.A933V locate in a conserved dynein-binding site, between two coiled coil structures. The alignment of conservation of these sites is shown in Fig 2. According to 3 different programs, p.R623W and p.A933V were considered likely pathogenic (summarized in Table 1).
Fig 2. Alignment of human dynactin subunit 1 (isoform 1) amino acid sequences from different species.
The p.R623W variant was conserved in mammals, p.A933V variant was conserved in vertebrata.
Clinical features
The two patients carrying the two variants denied symptoms of dementia or static tremor, they also denied family history of ALS or limb weakness. The cognitive screens on the first visit were insignificant. The follow-up examinations did not show any cognitive impairment or parkinsonian symptoms.
The patient with p.R623W was a female with an ALS onset age of 61 years. The first symptom was weakness of the left hand, then the weakness spread to right hand. 4 months later, both legs were involved. Neither dysarthria nor other bulbar symptoms were observed when she visited the clinic. She had no family history of ALS or other neuromuscular diseases. An EMG study revealed diffuse neurogenic changes, a pathologically brisk deep tendon reflex was observed upon neurologic examination, and Babinski sign was positive for both feet. The diagnosis delay was 6 months, and the FRS-R score at the first visit was 34/48, 2 years after the onset of disease reveals a 23/48 of FRS-R score, the progression rate was 0.46 score per month.
The patient with p.A933V was a 41-year-old female. She complained of right leg weakness without an evident cause. After 8 additional months, she presented with contralateral leg weakness. She developed dysphagia in 9 months after the first symptom. Her symptoms had not fluctuated. She had no family history of neuromuscular disease. On examination, she exhibited weakness and muscle atrophy in all four limbs with occasional spasticity, although the sensory, autonomic, and extrapyramidal systems were normal. EMG showed diffuse neurogenic changes in the sternocleidomastoid, the first dorsal interosseous, the rectus abdominis and the anterior tibia. The diagnosis delay was 18 months, and the first examined FRS-R score was 38/48, 2 years’ follow-up reveals a 30/48 of FRS-R score, the progression rate was 0.33 score per month.
Discussion
This was the first study assessing DCTN1 gene in a large sporadic ALS cohort of Chinese mainland. The present study distinguished two novel variants in patients’ cohort, p.R623W and p.A933V, the pathogenicity is supported by the following facts. These two variants were not present in the 490 race-matched control or population based databases (EVS, dbSNP, 1000 Genomes Project, and ExAC, including more than 65,000 individuals, >4,300 East Asian population) and both located in a highly-conserved region. The pathogenicity is also supported by in silico prediction, all 3 software suggest a deleterious effect of the mutant gene. Although we lack the parental confirmation for these two variants but they denied familial history, so these variants could be potential de novo mutations. According to the ACMG guideline[23], the pathogenicity for these two variants were supported by evidence PM2 and PP3, so these two would be judged as variants of uncertain significance (VOUS). Another variants p.G1062D (c.3185G>A) discovered in our patient cohort was also reported in a Japanese ALS cohort[9]. But the variant was observed in East Asian population with a low frequency (MAF = 0.0001156) from the ExAC database. Further functional study will be contributive.
The present study distinguished two novel VOUS. The frequency of DCTN1 rare variants in sporadic ALS was 0.39% (2 of 510), lower than the data previously reported in sporadic ALS in Japanese (1.07%, 5 of 469)[9], American (2.07%-2.87%, 5 of 242, 10 of 349)[6, 7]and Irish (0.51%, 2 of 394)[8]. Although the size of control cohort and reference genetic databases used were different between studies, our result could also be a reflection of different frequency or penetrance of DCTN1 gene between populations. It is reported that rare variants of DCTN1 gene may coexist with variants of other ALS causing genes (SETX, ANG, SOD1, FIG4)[6]. So, there was also a possibility that oligogenic mutations may be a factor for our variant carriers. This observation suggests genetic diversity and provides an opportunity to explore the mechanisms of rare variants related to ALS. Our patients carrying DCTN1 VOUS did not reveal a uniform clinical manifestation, partly because number of ALS patients carrying such variants was too few to establish any characteristic.
p150 protein contains a shoulder and 3 coiled-coil structures (CC1a, CC1b, CC2) of the dynactin complex[24]. The highly-conserved GAP-Gly domain of p150 connects the microtubules via residues 29–95 near the N-terminus[11]. Mutations in CAP-Gly domain of the dynactin may cause Parkinson’s disease with hypoventilation and depression (Perry syndrome)[14]. The p.R623W and p.A933V variants located in the dynein-binding domain, several ALS-causative mutations (p.M571T, p.H668I and p.R785W) are in this domain[5, 7]. A defective ICD domain may disrupt the dynein-dynactin complex to transport cargos along microtubules and this will affect the axonal transport, which plays a vital role in motor neuron diseases[25, 26]. Interestingly, mutations of other dynactin related protein such as protein bicaudal D homolog 2(gene BICD2), and cytoplasmic dynein heavy chain (gene DYNC1H1) may cause lower extremity-predominant spinal muscular atrophy (SMALED1, OMIM 158600, and SMALED2, OMIM615290),which affects the lower motor neuron in spinal cord. It indicates that the dynein-dynactin complex is important for motor neuron survival.
In conclusion, we assess the frequency of DCTN1 variants in a Chinese cohort of sporadic ALS cases and controls. We identified two novel missense VOUSs, p.R623W and p.A933V, the frequency was 0.39% for Chinese sporadic ALS. Our results suggest variants in DCTN1 are not common risk factors for Chinese sporadic ALS patients.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank patients, family members and volunteer controls for their participation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochimica et biophysica acta. 2015;1852(4):679–84. doi: 10.1016/j.bbadis.2014.08.010 . [DOI] [PubMed] [Google Scholar]
- 2.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23. doi: 10.1038/nn.3584 ; PubMed Central PMCID: PMCPMC4544832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Zhang B, Chen R, Tang L, Liu R, Yang Y, et al. Natural history and clinical features of sporadic amyotrophic lateral sclerosis in China. J Neurol Neurosurg Psychiatry. 2015;86(10):1075–81. doi: 10.1136/jnnp-2015-310471 . [DOI] [PubMed] [Google Scholar]
- 4.Marangi G, Traynor BJ. Genetic causes of amyotrophic lateral sclerosis: New genetic analysis methodologies entailing new opportunities and challenges. Brain research. 2015;1607:75–93. doi: 10.1016/j.brainres.2014.10.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munch C, R S, T M, V H, AD S, A K, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63(4):724–6. [DOI] [PubMed] [Google Scholar]
- 6.Cady J, Allred P, Bali T, Pestronk A, Goate A, Miller TM, et al. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Annals of neurology. 2015;77(1):100–13. doi: 10.1002/ana.24306 ; PubMed Central PMCID: PMCPMC4293318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couthouis J, Raphael AR, Daneshjou R, Gitler AD. Targeted exon capture and sequencing in sporadic amyotrophic lateral sclerosis. PLoS genetics. 2014;10(10):e1004704 doi: 10.1371/journal.pgen.1004704 ; PubMed Central PMCID: PMCPMC4191946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenna KP, McLaughlin RL, Byrne S, Elamin M, Heverin M, Kenny EM, et al. Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. Journal of medical genetics. 2013;50(11):776–83. doi: 10.1136/jmedgenet-2013-101795 ; PubMed Central PMCID: PMC3812897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura R, Sone J, Atsuta N, Tohnai G, Watanabe H, Yokoi D, et al. Next-generation sequencing of 28 ALS-related genes in a Japanese ALS cohort. Neurobiology of aging. 2016;39:219 e1-8. doi: 10.1016/j.neurobiolaging.2015.11.030 . [DOI] [PubMed] [Google Scholar]
- 10.Tokito MK, Howland DS, Lee VM, Holzbaur EL. Functionally distinct isoforms of dynactin are expressed in human neurons. Molecular biology of the cell. 1996;7(8):1167–80. ; PubMed Central PMCID: PMC275970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockmann M, Meyer-Ohlendorf M, Achberger K, Putz S, Demestre M, Yin H, et al. The dynactin p150 subunit: cell biology studies of sequence changes found in ALS/MND and Parkinsonian syndromes. Journal of neural transmission. 2013;120(5):785–98. doi: 10.1007/s00702-012-0910-z . [DOI] [PubMed] [Google Scholar]
- 12.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, et al. Mutant dynactin in motor neuron disease. Nature genetics. 2003;33(4):455–6. doi: 10.1038/ng1123 . [DOI] [PubMed] [Google Scholar]
- 13.Holleran EA, Karki S, Holzbaur EL. The role of the dynactin complex in intracellular motility. International review of cytology. 1998;182:69–109. . [DOI] [PubMed] [Google Scholar]
- 14.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, et al. DCTN1 mutations in Perry syndrome. Nature genetics. 2009;41(2):163–5. doi: 10.1038/ng.293 ; PubMed Central PMCID: PMC2813485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munch C, Rosenbohm A, Sperfeld AD, Uttner I, Reske S, Krause BJ, et al. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Annals of neurology. 2005;58(5):777–80. doi: 10.1002/ana.20631 . [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Seki N, Ishiura H, Mitsui J, Matsukawa T, Kishino A, et al. Development of a high-throughput microarray-based resequencing system for neurological disorders and its application to molecular genetics of amyotrophic lateral sclerosis. Archives of neurology. 2008;65(10):1326–32. doi: 10.1001/archneur.65.10.1326 . [DOI] [PubMed] [Google Scholar]
- 17.Liu ZJ, Li HF, Tan GH, Tao QQ, Ni W, Cheng XW, et al. Identify mutation in amyotrophic lateral sclerosis cases using HaloPlex target enrichment system. Neurobiology of aging. 2014;35(12):2881 e11-5. doi: 10.1016/j.neurobiolaging.2014.07.003 . [DOI] [PubMed] [Google Scholar]
- 18.Steele JC, Guella I, Szu-Tu C, Lin MK, Thompson C, Evans DM, et al. Defining neurodegeneration on Guam by targeted genomic sequencing. Annals of neurology. 2015;77(3):458–68. doi: 10.1002/ana.24346 . [DOI] [PubMed] [Google Scholar]
- 19.He J, Tang L, Benyamin B, Shah S, Hemani G, Liu R, et al. C9orf72 hexanucleotide repeat expansions in Chinese sporadic amyotrophic lateral sclerosis. Neurobiology of aging. 2015;36(9):2660 e1-8. doi: 10.1016/j.neurobiolaging.2015.06.002 . [DOI] [PubMed] [Google Scholar]
- 20.Li C, Ji Y, Tang L, Zhang N, He J, Ye S, et al. Optineurin mutations in patients with sporadic amyotrophic lateral sclerosis in China. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2015;16(7–8):485–9. doi: 10.3109/21678421.2015.1089909 . [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Tang L, Zhang N, Pan L, Hadano S, Fan DS. Six SQSTM1 mutations in a Chinese amyotrophic lateral sclerosis cohort. Amyotroph Lat Scl Fr. 2015;16(5–6):378–84. doi: 10.3109/21678421.2015.1009466 [DOI] [PubMed] [Google Scholar]
- 22.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. 2000;1(5):293–9. . [DOI] [PubMed] [Google Scholar]
- 23.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. doi: 10.1038/gim.2015.30 ; PubMed Central PMCID: PMCPMC4544753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. The Journal of cell biology. 1994;126(2):403–12. ; PubMed Central PMCID: PMC2200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikenaka K, Katsuno M, Kawai K, Ishigaki S, Tanaka F, Sobue G. Disruption of axonal transport in motor neuron diseases. International journal of molecular sciences. 2012;13(1):1225–38. doi: 10.3390/ijms13011225 ; PubMed Central PMCID: PMC3269748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS biology. 2013;11(7):e1001611 doi: 10.1371/journal.pbio.1001611 ; PubMed Central PMCID: PMC3712912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.