Abstract
Chronic hepatitis B virus infection is a global health concern as it affects over 240 million people worldwide and an estimated 686,000 people die annually as a result of complications of the disease. With the development of newer antiviral drugs, viral suppression of HBV is achievable, however elimination of HBV from infected individuals (functional cure) remains an issue. Due to persistence of HBV DNA (cccDNA) in infected cells, chronically infected patients who discontinue therapy prior to HBsAg loss or seroconversion are likely to relapse. Several novel therapeutic strategies are being researched and studied in clinical trials. Here we review these novel strategies to achieve sustained cure or elimination of HBV. These strategies include the targeting of the host or viral factors required for viral persistence as well as therapeutic vaccines.
KEYWORDS : cccDNA, eradication, HBV, hepatitis B, Toll-like receptors, TLRs
Background
The long-term management of chronic hepatitis B (CHB) is a significant challenge. Approximately 240 million people have CHB worldwide [1], making it a major public health problem. HBV persistence is the result of a combination of viral factors and the inadequate immune system activation seen in patients with acute HBV, which naturally clears the HBV infection. CHB leads to liver cirrhosis and hepatocellular carcinoma (HCC), and is the major cause of noncirrhotic HCC worldwide [2]. These sequelae result in an estimated 686,000 deaths annually [3]. Therefore, it is important to successfully treat those who are infected in order to decrease the burden on health care systems worldwide. Current therapies suppress viral replication and liver inflammation with the goal of reducing liver-related morbidity and mortality [4]. Viral suppression is easily achieved in most patients using medication currently available; however, they do not lead to sustained remission, requiring indefinite treatment as viral load frequently rebounds once suppressive therapy is stopped [5].
This review will provide an overview of the basis of HBV persistence, current therapy and new therapeutic approaches.
• Basis of HBV persistence
In order to design new treatment strategies to eradicate HBV, it is important to understand the factors that contribute to viral persistence in the liver. Viral factors and host immune factors interplay to create the complicated problem of persistence of HBV. The virus utilizes a replication system that uses covalently closed circular DNA (cccDNA) as a transcriptional template. cccDNA is sequestered in the nucleus of infected cells and avoids detection by the DNA sensing cellular machinery (part of the innate immune response). Furthermore, the virus produces tolerogenic proteins (HBsAg, HBeAg) that lead to T-cell exhaustion [6]. Obtaining a more complete understanding of how these viral and immune factors lead to viral persistence has allowed for novel therapies that might lead to sustained viral response to be designed. The current therapeutics (reverse transcriptase inhibitors) prevent formation of new cccDNA, but have no effect on existing cccDNA [7].
• Viral factors
Error-prone replication
HBV is a small, enveloped virus with a unique genomic organization and replication mechanism. Its genome is only 3200bp long and has multiple overlapping open reading frames (ORFs). These are associated with constraints on variation of other viruses, such as HIV. In HBV, however, in spite of the ORFs replication is error prone because of the lack of proofreading mechanism on the HBV polymerase (error rate of 10- 4 to 10- 5). The high error rates allow for the accumulation of a heterogeneous viral population, or quasispecies [5,8].
Precore/core mutants
The major nucleocapsid protein and RNA dependent DNA polymerase are encoded by the viral core mRNA, which also acts as the pregenomic RNA – the template for reverse transcription [9]. Precore mRNA encodes the precore protein, which is processed in the endoplasmic reticulum to produce HBeAg. Mutations in HBV in the genome, in turn, influences HBeAg production. There are multiple reported precore and core mutants. Two well-studied precore mutations include: stop codon mutation at nt 1896, which results in cessation of HBeAg expression, and a mutation in the basal core promoter (BCP) at nt 1762 and nt 1764, which results in diminished production of HBeAg and an increased immune response as a result. These precore mutants are associated with increased pathogenicity, severe chronic liver disease, and acute liver failure. Double mutations of BCP at nt 1762 and nt 1764 are reported to be associated with severe liver disease, fulminant hepatitis, cirrhosis and HCC [9].
S mutants
The S gene codes for HBsAg. One particular region, the major hydrophilic region (amino acids 120–160), is especially prone to mutation. The most commonly transmitted mutation is G145R, which results in a transcriptionally competent virus even after vaccination [10]. The G145R variant is associated with high HBV DNA levels and viral persistence [11].
HBV genotypes
The ten known HBV genotypes (A–J) and multiple subtypes account for the heterogeneity seen in clinical manifestations and differential treatment response among patients with CHB in different parts of the world. Genotypes are associated with clinical outcomes of treatment, especially interferon treatment [12]. Furthermore, specific genotypes have different risks for disease progression. Having genotype A infection is an independent risk factor for progression to chronic infection after acute hepatitis B infection [13]. Acute infection with genotypes A and D are more likely to progress to chronicity than acute genotype B or C infection. HBV genotypes are also associated with precore and BCP mutations. For example, genotype C has higher frequency of double mutation in BCP A1762T/G1764A, pre-S deletion and is associated with higher viral load than genotype B. Genotypes C and D are also associated with more severe liver disease. Genotypes A and B respond better to interferon-based therapy than genotypes C and D, but there are limited consistent differences for nucleos(t)ide analogs (NAs) [14].
cccDNA
cccDNA is the main barrier to treating CHB due to its sequestration in the nucleus of infected cells. It avoids the cellular machinery that is part of the innate immune response by forming a supercoiled minichromosome, complete with histones and DNA chaperone proteins. These infected hepatocytes have a long half-life, which allows cccDNA to be maintained in the nucleus indefinitely. Thus, cccDNA acts as a reservoir for the reactivation of HBV genome replication after natural or treatment-induced silencing. Additionally, mutations that confer drug resistance may be maintained with the cccDNA found in the nucleus [15–17]. When reactivation of replication occurs, this drug resistance presents a problem for further treatment with antivirals. Thus, cccDNA serves as the latent reservoir of CHB. Currently approved antiviral agents, including lamivudine (3TC), adefovir, telbivudine, entecavir and tenofovir, suppress viral replication, but not reverse transcription, allowing cccDNA persistence. Novel strategies for HBV eradication will need to eliminate or inactivate cccDNA.
• Host factors
Host genetics
Multiple host factors influence HBV persistence and the observed variability in outcomes. An individual’s human leukocyte antigen (HLA) type is one of the important factors that determine variability in response to HBV. HLA DRB locus alleles DRB1*1301/2 are consistently associated with spontaneous resolution upon initial infection with HBV [18]. HLA-DR7 (DRB*07), HLA-DR3 (DRB*0301) and HLA-DPA2 are associated with increased susceptibility to CHB [19–24]. Decreased expression of HLA-DPA1 is associated with HBV persistence [25]. Multiple other alleles are associated with increased susceptibility to chronic HBV infection in addition to failure to respond to HBsAg-based vaccine, including HLA-DRB1*0701 and DRB1*0301 [26,27]. Another study of the genomes of siblings with CHB has discovered that a region of linkage on chromosome 21 with single nucleotide polymorphisms spanning the IFN-α receptor II and IL-10 receptor II was associated with chronicity [28,29]. HLA-DP polymorphism might be a useful therapeutic predictor for PEG-IFN.
Host–viral interactions
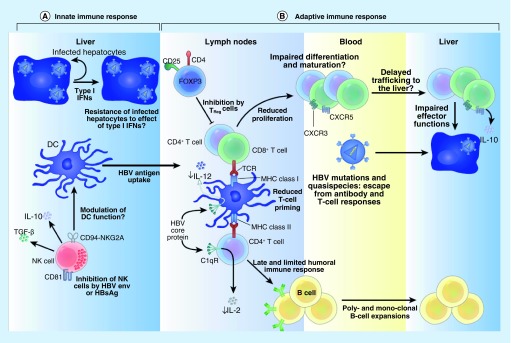
HBV antigens have been shown to hinder signaling of Toll-like receptor (TLR) 2, 3, 7 and 9 molecules, which allows for diminished innate immune recognition of the virus. TLRs are vital in HBV immunity due to their role in generating effective innate adaptive crosstalk. In CHB subjects, innate immune responses are blunted and facilitate persistence, as described in Figure 1. TLRs are down regulated in hepatocytes and intrahepatic type-I interferon antiviral response is virtually absent [30]. This will in turn lead to inadequate priming of adaptive immune responses. Both humoral and cellular immunity to HBV antigens are impaired. Protective antibodies are not formed, since B cells are not optimally activated by CD4+ T cells. Moreover, the T cells from these subjects with CHB have an exhausted phenotype and are less responsive to HBV antigens [30]. Altogether, these lead to establishment of HBV persistence.
Figure 1. . Interactions between hepatitis B virus proteins and the immune system.
(A) Innate immune system: HBV-infected hepatocytes trigger a type 1 interferon response in the liver. This in turn primes DCs to antigen uptake, processing and initiation of an effective adaptive immune response. This interferon response also activates NK cells to lyse target (HBV-infected) cells and promote maturation of DCs. HBsAg can inhibit NK cells thereby downmodulating intrahepatic innate immunity. (B) Adaptive immune system: appropriate priming of T cells results in maturation of an effective HBV response that leads to secretion of protective antibodies and/or cytotoxic T cells in the lymph node. These HBV-specific T and B cells circulate to the liver via blood, targeted by CXCR3 and CXCR5 ligands. Chronic HBV infection is associated with enhanced Treg activity (inhibits T-cell maturation and proliferation), impaired humoral response (low protective antibodies), emergent escape mutants (ineffective cytotoxic T-cell response) and enhanced T-cell exhaustion. Collectively these effects contribute to HBV persistence.
DC: Dendritic cell; HBV: Hepatitis B virus; NK: Natural killer.
• Current therapy for HBV
Increased HBV DNA level is associated with poorer outcomes, including cirrhosis and HCC [31–33]. Therefore, current therapy suppresses HBV DNA levels (by inhibiting viral replication but not affecting cccDNA) with the goal of slowing the progression to liver fibrosis and cirrhosis and preventing HCC, which are the primary complications of the disease. Currently, interferon or pegylated-IFN-α and/or long-term use of NAs are the primary treatment strategies. The newer NAs, tenofovir (TDF) and entecavir, have a higher barrier to resistance and are well tolerated, thus are prescribed as the first line of treatment more often than older NAs, including lamivudine (3TC), adefovir and telbivudine. Additionally, telbivudine has been shown to cause myopathy and neuropathy with long-term use [34].
In CHB patients that are HBeAg positive, PEG-IFN-α with or without 3TC may be used to achieve durable viral suppression, increased rate of HBsAg seroconversion, and improved fibrosis and cirrhosis, as demonstrated by long-term follow-up studies [35–37]. Genotypes A and B demonstrated increased response rates to this treatment compared with genotypes C and D [38–40]. Additionally, favorable outcomes are more likely in CHB patients with lower levels of HBV DNA and higher alanine aminotransferase (ALT) levels [38,40].
While PEG-IFN is effective in some patient populations, its use is limited greatly by intolerance, thus NAs are likely to be used to achieve durable viral suppression.
One problem with the earlier NAs (3TC, adefovir and telbivudine) is the increased likelihood to develop resistance mutations. Tenofovir and entecavir overcome this problem with high barriers to resistance, as well as having increased potency and good tolerability for CHB patients. Patients show durable viral suppression and improvements in both histological and biochemical signs of disease while taking tenofovir or entecavir, according to long-term follow-up studies [41,42]. However, it has been demonstrated that persistence of HBeAg and high ALT remain risk factors for HCC despite patients receiving suppressive therapy [2].
There remain several limitations to current therapy. As stated earlier, despite being effective at suppressing viral replication, for the majority of patients, current therapy will not result in cure as they do not target cccDNA. Furthermore, long-term use of current antiviral agents is hindered by the emergence of drug resistance as changes within the viral polymerase – the target of NAs – generates HBV surface protein variants due to the overlapping nature of the viral genome [5,43,44], which may influence treatment response [45].
• Current end points of HBV treatment
Currently, biochemical, serological, virological and histological endpoints defined below and measured during treatment course are used in HBV treatment [46]. Percentage of participants who normalize elevated levels of ALT after initiation of therapy constitute biochemical endpoint [46]. Similarly, proportion of patients with undetectable HBV DNA after initiation of therapy is used for the virological end point for HBV treatment. Since sustained suppression of HBV DNA levels after cessation of therapy seldom happens, this is not a completely accurate measure of HBV elimination. For patients with CHB who are HBeAg positive, serological response is defined as loss of HBeAg and seroconversion to anti-HBe antibodies [46]. For CHB patients who are HBsAg positive, serological response is defined as the loss of HBsAg and seroconversion to anti-HBs antibodies [46]. Loss of HBsAg is associated with a diminished likelihood of developing complications such as cirrhosis and HCC; therefore it is the most valuable clinical outcome marker for treatment endpoint [47–49]. Finally, the histological treatment endpoint is defined by lowering in necroinflammatory activity by two points or more ≥2 points in hepatic activity index (HAI) or Ishak’s system without worsening fibrosis compared with baseline histology [46]. According to a long-term follow-up study of patients treated with TDF, suppression of HBV over time can reduce fibrosis and cirrhosis [50]. While many patients achieve viral suppression on current therapy, only 10–25% of patients seroconvert [51]. It is because numbers remain so small that novel therapeutics are necessary.
Ultimately, HBV elimination can be defined by complete suppression of HBV DNA levels, the loss of HBsAg and seroconversion to anti-HBs antibodies after stopping antiviral therapy. Loss of HBsAg levels is critical since HBsAg levels are surrogate markers for levels of transcriptionally active cccDNA, meaning that if HBsAg is eliminated, the virus is most likely inactivated [52,53].
• Novel treatment strategies
The purpose of this review is to highlight potential newer treatment for HBV aimed at achieving a functional cure of sustained virologic remission. This is defined as absence of HBV DNA levels and HBsAg with or without HBsAg seroconversion after cessation of therapy.
• Improved nucelos(t)ide analogs
In order to overcome concerns about long-term safety of entecavir and tenofovir, which are the current first-line treatments for CHB, besifovir and tenofovir alafenamide are being studied. Besifovir has been shown to be safe and efficacious over 96 weeks, with potency similar to that of entecavir. The only problem found was the depletion of L-carnitine, which was easily rectified with a carnitine supplement [54]. Tenofovir alafenamide is a phosphonate prodrug of tenofovir that optimizes potency and safety [55]. These will be valuable in conjugation with other treatments in development.
• Targeting the virus
Targeting viral entry
HBV viral entry is mediated through interactions between viral envelope proteins and cellular receptors. This provides a new target for treatment with receptor antagonists that would prevent entry of the virus into the cell. Yan et al. reported that sodium taurocholate cotransporting polypeptide (NTCP) is a functional receptor for HBV in vitro [56]. NTCP is expressed at the basolateral membrane of hepatocytes and responsible for most of the sodium-dependent bile acid uptake. The synthetic lipopeptide, Myrcludex-B, specifically targets the NTCP and has been shown to efficiently block HBV infection in vitro [57,58] and in mouse models by efficaciously blocking entry of the viral particles into the uninfected hepatocytes [59,60]. In the upA/SCID human mouse model, administration of Myrcludex-B results in suppression of not only HBV DNA levels but HBsAg levels as well [60]. These results are promising that Myrcludex-B could be an adjunct to antiviral therapy for CHB.
Targeting cccDNA
Current NA-based therapies can prevent the formation of new cccDNA, but do not affect the existing cccDNA in already infected cells, which has a long half-life (33–50 days) and is dependent on several host factors. The viral cccDNA in the nucleus serves as a store of viral escape variants, which can cause a viral rebound once treatment is stopped. cccDNA also serves as a reservoir of escape mutants that confer drug resistance.
Epigenetic silencing of cccDNA
Recent studies have demonstrated that cccDNA can be epigenetically silenced so that they remain transcriptionally inactive even after cessation of antiviral therapy. Using uPA-SCID mouse model with HBV-infected human hepatocytes, IFN-α was shown to reduce pgRNA and pre-S1 RNA even though cccDNA levels were not affected. Hence, it is possible to suppress HBV replication by targeting the epigenetic control of cccDNA function and transcription [61]. A mapping of post-translational histone modifications in cccDNA from infected liver revealed chromatin targets for epigenetic manipulation as potential therapy [62].
Transcriptional silencing of cccDNA
One of the recently developed therapeutic approaches involves using zinc-finger nucleases (ZFNs) that directly target HBV cccDNA. Zinc finger proteins (ZFP) can be used to block transcription of cccDNA and have two components. One is a restriction endonuclease enzyme that is capable of introducing DNA breaks and the other (zinc finger motifs) provides DNA binding specificity. ZFPs can be used specifically to target the cccDNA of duck HBV infection and inhibit viral transcription and replication in vitro [63]. Expression of ZFPs in LMH cells undergoing the DHBV viral lifecycle resulted in decreased expression of viral RNA and decreased protein expression compared with the empty vector control. No toxicity effects were observed. Additionally, the viral particle production decreased in the presence of the expressed ZFPs [63].
While it has been shown that ZFNs can cleave HBV DNA in vitro, there are concerns about off target effects in vivo and the challenge of delivering ZFNs specifically to the patients’ infected liver [64]. To overcome this challenge, a vector platform may be applied for delivery. Adeno-associated virus vectors are being investigated as delivery vehicles for designer nucleases to cells. These vectors were found to be safe in clinical and preclinical applications [65].
Transcription activator-like effector nucleases (TALENs) can cleave sequence specific DNA targets, making them an ideal treatment strategy for silencing of cccDNA [66]. In cells transfected with monomeric linear full-length HBV DNA, expression of TALENs decreased production of HBeAg, HBsAg, HBcAg and pgRNA. Furthermore, it suppressed the cccDNA level and misrepaired cccDNA without any apparent cytotoxic effects. In a mouse model, it was shown that TALENs can be used in combination with IFN-α to specifically target and inactivate the sequestered cccDNA, potentially providing a new treatment option for patients with CHB [66].
Disruption of cccDNA
Two disubstituted sulfonamides, CCC-0975 and CCC-0346 were recently shown to inhibit cccDNA production [67]. Mechanistic studies demonstrated the reduction of both cccDNA and its precursor, deproteinized relaxed circular DNA (DP-rcDNA) without inhibition of HBV DNA replication or reduction of viral polymerase activity. Furthermore, it was shown that the disubstituted sulfonamide compounds did not promote intracellular decay of DP-rcDNA and cccDNA, which suggests that these compounds interfere with the conversion of rcDNA into cccDNA [67].
Recent studies have also shown that IFN-α and lymphotoxin-β receptor activation in primary human hepatocytes and differentiated HepaRG (dHepaRG) cells can induce degradation of cccDNA via the induction of the APOBEC3 family of proteins, which restrict foreign DNAs. HBV core protein facilitates interaction between nuclear cccDNA and APOBEC3A and APOBEC3B cytidine deaminases resulting in cytidine deamination, apurinic/apyrimidinic site formation. This culminates in the degradation of cccDNA and prevents further HBV reactivation [68].
Additionally, clustered regularly interspaced short palindromic repeat (CRISPR)/CAS-9 can be used to target the HBV genome and inhibit HBV infection by cleaving the cccDNA of HBV transfected cells. This method has been tested in a mouse model and provides a promising novel technique for treating humans [69].
Targeting viral assembly/encapsidation
Viral assembly is a critical step in the viral life cycle, thus is an attractive target for therapeutics. The assembled core particle of the virus is composed of a capsid protein, polymerase and pregenomic RNA [7]. Proper assembly is vital to the ability of the virus to persist in humans. Heteroaryldihydropyrimidines are known to inhibit HBV virion encapsidation both in vitro and in vivo, thus preventing assembly [70,71]. Bay 41–4109 is a heteroaryldihydropyrimidine that both inhibits capsid formation and also reduces the half-life of the core protein. Furthermore, these drugs misdirect viral assembly when in excess, which decreases the stability of normal capsids [72–74]. Furthermore, these compounds are active against HBV mutants resistant to NAs, making them an even more attractive therapeutic agent [75].
Phenylpropenamides also inhibit viral encapsidation by inducing changes in tertiary and quaternary structures of HBV capsid proteins. Phenylpropenamides are active against 3TC-resistant strains [75–77]. A phenylpropenamide derivative, AT-130, acts by binding to a promiscuous pocket at the dimer–dimer interface and decreases viral production by initiating virion assembly at the wrong time, resulting in normal capsids that are empty and noninfectious [78,79]. While these are promising therapeutic agents, their clinical efficacy still needs to be studied. It is unclear whether capsid inhibitors have any effect on HBsAg levels, which could determine their utility in achieving sustained viral remission.
NVR 3-778, a core inhibitor, is another newly discovered drug that may be used for the treatment of CHB. In the humanized mouse model, NVR 3-778 demonstrated high antiviral activity. In combination with PEG-IFN-α, antiviral activity was higher than with NVR 3-778 or PEG-IFN-α alone [80]. This may be a beneficial treatment in conjunction with currently approved therapies.
Targeting envelopment
The HBV genome contains four ORFs that code for three envelope proteins, the viral polymerase and capsid-forming core protein. The three envelope proteins: large (LHBs), middle (MHBs) and small hepatitis B surface antigens share the same S-domain and contain N-linked glycosylation at amino acid 146 within that domain. Within the S-domain are the pre-S1 and pre-S2 domains. MHBs contain the pre-S1 domain, while LHBs contain both of the pre-S domains [81]. The interaction between non-glycosylated pre-S sequences with specific regions on the core particle leads to envelopment of viable hepatitis B capsids. There is evidence that the transport of the enveloped virus and subviral empty envelope particles (SVPs) depends on glycosylation and the processing of the MHBs glycoprotein [82,83]. Glucosidase inhibitors suppress viral infectivity and morphogenesis, likely by inhibition of the glycosylation of the envelope protein in endoplasmic reticulum [84–88].
Targeting HBsAg levels
Typically, antiviral response starts with the production of type I interferons (IFN-α and IFN-β), signaled by the innate immune response. In HBV-infected chimpanzees, it has been shown that type I interferons are not produced at all during the early stages of infection. Furthermore, type I interferon response is completely lacking in human patients with HBV as well [89,90]. In the early stages of acute HBV infection, IL-10 is produced rather than type I interferon, accompanied by a temporary attenuation of natural killer (NK) cell and T-cell responses [91].
Patients with HBV experience a suppressed immune response to the virus that is likely caused by direct interference of HBV antigens with host cells. HBV-infected patients have high levels of HBsAg [81,92,93], which are thought to suppress immune activation via direct action on dendritic cells’ production of cytokines. Reduction of HBsAg production can be achieved with several classes of drugs [94]. Specifically, it has been demonstrated that nitazoxanide and its active metabolite, tizoxanide reduce not only extracellular HBsAg, but also HBeAg and intracellular HBcAg in a dose-dependent manner in vitro. In combination with lamivudine or adefovir, nitazoxanide has synergistic activity and inhibits HBV replication [94].
Triazolo-pyrimidine inhibitors of HBsAg secretion were recently identified using high-throughput screening of the HBV-expressing HepG2.2.15 cell line [93]. The parent compound does not inhibit viral replication, but does selectively inhibit HBV envelope secretion. The triazolo-pyrimidine derivatives were active in inhibiting HBsAg secretion of HBV variants that are resistant to current NAs [92,93]. It is still unclear whether these compounds would be able to enhance HBV specific immunity in vivo or impact secretion of important host translated proteins of significance.
HBV produces two other types of particles in addition to infectious virion: SVPs and naked capsid particles. Noninfectious subviral particles share antigenic features of the virus envelope and are thought to act as a decoy for the immune system. Recently, it has been found that SVPs and infectious virions do not share a pathway to production, as it was originally hypothesized [82].
Nucleic acid polymers are amphipathic oligonucleotides shown to have both entry and postentry antiviral activity in the duck hepatitis B virus model and can be engineered to remove the secondary proinflammatory and immunostimulatory effects associated with single-strand nucleic acids [95,96]. In the same in vivo duck model, the NAP REP 2055 resulted in rapid clearance of duck HBsAg with an increase in antiduck HBsAg antibodies with no viral antigens detected in the liver, and only trace amounts of intrahepatic cccDNA post-treatment [97]. Two recent proof-of-concept studies evaluated the safety and antiviral response to REP 2055 (and its calcium chelate formulation, REP 2139-Ca) monotherapy and in combination with immunotherapy in patients with CHB infection. HBsAg and HBV DNA levels were reduced in participants treated with REP 2055, accompanied by detection of HBsAb. However, HBsAb titers were not consistently maintained with REP 2055 monotherapy. The majority of participants experience virologic rebound at follow-up after monotherapy. Immunotherapy with either PEG-IFN or Thymosin-α (compound with immunomodulatory activity) was added on to nine other participants who had HBsAg reductions but persistent low grade viremia after REP 2139-Ca monotherapy. With the addition of immunotherapy, HBV DNA levels declined to below the lower limit of detection in seven of the of the nine participants, and eight participants had HBsAg loss by the end of initial follow-up; however, the majority did eventually experience virologic rebound with reappearance of HBsAg and loss of HBsAB [98,99]. A follow-up trial evaluating the safety and efficacy of triple combination therapy with nucleic acid polymers, PEG-IFN and tenofovir in patients with HBeAg negative CHB is currently underway and we await the results (NCT02565719).
Targeting viral mRNA
Antisense oligonucleotides, ribozymes and RNAi can be used to directly target viral mRNA. According to in vitro data, the following can be used to decrease HBV transcript levels: antisense oligonucleotides [100], hairpin ribozymes [101], or hammerhead ribozymes using a lentiviral vector for delivery [102].
RNAi uses small interfering RNA molecules to induce gene silencing post-transcriptionally, which effectively knocks down genes of interest. In mammalian cells, it can be used to specifically target the degradation of mRNA [103]. HBV is particularly vulnerable to RNAi because of the use of multiple ORFs in the HBV genome [104]. Multiple studies have shown that in cell cultures and mice, viral mRNA and HBV replication are inhibited through the use of RNAi [105–108]. Furthermore, a recent study has shown that RNAi can be applied in cell culture and in immunocompetent and immunodeficient mice transfected with an HBV plasmid to inhibit the production of HBV replicative intermediates [106]. RNAi inhibited all steps of replication both in culture and in mice. In mice RNAi reduced HBsAg in serum and HBV RNA in the liver. Futhermore, HBV genomic DNA was undetectable in the liver, and fewer cells showed any presence of HBcAg [106].
ARC-520 has recently been shown to significantly decrease HBsAg in CHB patients in a single-dose Phase IIa clinical trial [109]. This drug utilizes a dynamic polyconjugate method of delivery to the infected hepatocytes. In dynamic polyconjugates the RNAi trigger is conjugated to cholesterol and is coinjected with a hepatocyte-targeted membrane-active peptide, which limits off-target effects of the drug. Based on the results of this Phase II trial, ARC-520 may be a useful agent for treating CHB.
• Targeting the host
HBV clearance requires robust immune responses from both the adaptive and innate immune systems. The innate immune response results in the production of type I interferon, which leads to suppression of viral replication, mediation of NK cell-mediated killing of viral infected cells and supports the efficient maturation and site recruitment of adaptive immunity through production of proinflammatory cytokines and chemokines [30,110]. Interferon modulates both innate and adaptive immune cells [91,111]. Plasmacytoid dendritic cells (pDC) are the primary producers of type I interferon. pDC respond to viruses and other pathogens via recognition of pathogen-associated molecular patterns by two intracellular TLRs: TLR7 and TLR9 [91,112]. In addition to the increased production of interferon upon activation of pDC, other cytokines are also released, including TNF-α, IL-6 and cell surface costimulatory molecules. pDC also activate NK cells and T lymphocytes, allowing further priming and regulation of antiviral immunity [111,113,114]. Priming of the adaptive immune system causes the maturation of B- and T-cell clones, which recognize specific infectious agents, leading to infection control and memory responses for future infections with the recognizable pathogen [30].
As described above, in patients with CHB, HBV is associated with diminished adaptive and innate immune responses. Therefore, augmenting the innate immune response may aid in reconstitution of HBV-specific immunity by enhancing intrahepatic innate immunity and priming effective adaptive anti-HBV immunity. TLR 2, 3, 4, 7 and 9 play important roles in controlling infections and eliminating virally infected cells. Studies have shown that HBV interferes with their signaling, and that expression is decreased in CHB [115–118].
TLR agonists
Studies in which HBV transgenic mice were injected with ligands specific for TLR 2–9 have shown that the livers of these mice produce IFN-α, -β and -γ to inhibit HBV replication [119]. This suggests that the activation of the innate immune response in the liver can suppress HBV replication [119]. Inhibition of HBV replication is accomplished post-transcriptionally by suppressing the assembly or stability of HBV RNA-containing capsids [119,120]. Thus, TLR activation directly inhibits HBV replication [121,122]. However, HBV somehow evades detection by TLRs as a strategy to escape innate immune response [121,122]. Expression of TLRs in hepatocytes is downregulated in the presence of multiple HBV viral products [116,123–127].
Although HBV circumvents endogenous type I interferon pathways, the use of a TLR7 agonist could plausibly induce the IFN-α response. When combined with current NA therapy for viral suppression, the use of TLR agonists may allow for the development of protective immunity via induction of exogenous interferon. Various studies have shown that NA therapy results in long term suppression of HBV and partial reconstitution of adaptive immunity [128]. Thus, an adjuvant therapy using TLR agonist may be able to accelerate the process of immune reconstitution and HBV clearance.
TLR agonists are available as oral compounds and could potentially be combined with other NAs as a single pill, making treatment regimens simple for patients to follow. Finally, similar to injected interferon, TLR agonists induce interferon production, triggering the production of cytokines to facilitate intracellular communication and cellular trafficking [128]. While injected interferon causes many adverse events, selective TLR agonists would act specifically in the liver, limiting the systemic innate immune activation by bypassing TLR8 stimulation.
GS-9620 is one of the TLR7 agonists currently being studied for CHB treatment. Oral TLR7 has demonstrated significant reductions in both HBV DNA and HBsAg levels in HBV-infected chimpanzees, although the scope of the study is limited because of a short dosing duration and only three animals were studied [129]. In humans, it has been shown to be safe and tolerable in healthy volunteers [130] as well as in virally suppressed and treatment naive patients with CHB [131]. No reduction in HBsAg or HBV DNA, however, were observed, likely because of the short dosing period [131].
Cytokine therapy
Several cytokines have been studied extensively in relation to immunomodulation of chronic viral infections. The most promising of the compounds that have been studied are IL-7 and IL-21 [132–134].
IL-7 is essential for primary T-cell development and likely plays a role in B-cell development as well [135,136]. It also plays a role in the development of some dendritic cell (DC) subsets. Regulation of peripheral CD4+ T-cell homeostasis is accomplished via IL-7-mediated signaling [137]. In patients with CHB, IL-7 therapy may enhance, rejuvenate and restore immune response to HBV. In addition to its immune restorative effects, IL-7 has vaccine adjuvant effects, as well as beneficial effects in adoptive cell therapy.
IL-21 mediates the induction of maintenance of effector CD8+ T cells. In mice, decreased IL-21 or IL-21R causes a progressive decline in the number of virus-specific effector CD8+ T cells. This correlates with poor viral control [138–140]. In mice induced with CHB, IL-21 is important for promoting infection-controlling immune responses [141]. Patients with CHB who were treated with antivirals and show complete viral suppression also demonstrated significantly higher levels of serum IL-21 than those who did not achieve viral suppression [142]. Furthermore, increased levels of serum IL-21 is associated with better viral control due to HBeAg seroconversion. An option for IL-21 therapy is recombinant IL-21 (rIL-21), which is an immune modulator undergoing Phase I and II trials in cancer patients. In CHB patients receiving NA-based therapy, rIL-21 is a promising new approach to therapy [143].
• Therapeutic vaccines
Development of an efficacious therapeutic vaccine for HBV is a promising avenue for the eradication of the disease. Control of CHB may be accomplished through inducing helper and cytotoxic T-cell response, counteracting immune tolerance and the activating the humoral immune response. Several promising options currently being explored are: vaccines based on recombinant proteins, HBV-envelope subviral particles, naked DNA combined with viral vectors and vaccines, dendritic cell-based vaccines (tarmogens) and those based on T-cell peptide epitopes derived from HBV proteins [144–146]. However, T cells of patients with CHB are associated with an exhausted immunophenotype, with reduced response to HBV proteins. This may contribute to why HBsAg-based vaccines have unsuccessful so far [147].
Therapeutic vaccination based on recombinant HBV proteins or HBV-envelope subviral particles
Immunogenic complexes
Immunogenic complex vaccines, using antigen–antibody complexes, target dendritic cells in order to efficiently prime HBV-specific CD8+ cytotoxic T-cells responses [148]. In a double-blind placebo-controlled Phase IIb trial using HBsAg and anti-HBs immunoglobulin complexes there was initial evidence suggesting clinical and virological efficacy [149]. However the Phase II trial failed to show therapeutic efficacy of the immune-based complex vaccine [150].
HBsAg & HBcAg combination
HBsAg and HBcAg are used in the nasal vaccine candidate. This vaccine complex has been shown to have a good immunogenicity and safety profile in preclinical studies. In a Phase I clinical trial, recombinant HBcAg acted as a potent Th1 adjuvant to HBsAg and also as a strong immunogen [151,152]. A pilot study of nasal and subcutaneous HBsAg/HBcAg combination-based vaccination monotherapy reported good tolerability and safety profile among 18 patients with CHB. HBV DNA levels were suppressed in seven participants during the vaccination period. HBV DNA remained undetectable for all seven participants, and a further two participants at 48-week follow-up. This included two HBeAg positive participants, both of whom seroconverted to HBeAg negativity and HBeAb positivity [153]. Furthermore, production of proinflammatory cytokines (IL-1β, TNF-α and IL-12) by dendritic cells from vaccinated participants were higher than unvaccinated controls. This pilot is followed by a Phase III trial and results are awaited (ClinicalTrials.gov identifier: NCT01374308).
Whole recombinant yeast-based therapeutic vaccine
Tarmogens (targeted molecular immunogen), a yeast-based immunotherapy platform, are currently under development. Tarmogen incorporates multiple viral antigens, expressing HBV (X), surface (S) and core Ags (X-S-Core). Tarmogens can be engineered to express HBV antigens by genetically modifying whole, heat-killed yeast. Saccharomyces cerevisiae (yeast) facilitates preferential uptake and processing by dendritic cells in order to effectively prime adaptive immune responses to inserted HBV antigens. This platform favors induction of cellular effector responses (T cells) over humoral immunity (B cells) that arises from the use of subunit vaccines.
In mice studies, it has been shown that GS-4774, a tarmogen currently undergoing clinical trials in humans, protects mice from tumors specifically engineered to express HBV antigens [154]. Additionally, human studies using peripheral blood mononuclear cells collected from healthy donors and patients with CHB to show that tarmogens can elicit HBV-specific T-cell responses ex vivo [154]. GS-4774 has been shown to be safe and tolerable in healthy subjects [154]. The safety and efficacy of GS-4774 was recently evaluated in a Phase II study. In this study, 178 patients with CHB who were already suppressed on antiviral therapy were randomized to continue oral antiviral alone or receive GS-4774 2, 10, or 40 yeast units subcutaneously every 4 weeks until week 20 in addition to oral antiviral therapy. The vaccine was safe and well tolerated; however, no clinical benefit was observed, with no significant reduction in HBsAg levels [155].
Adeno-virus based therapeutic vaccination
TG1050 is a therapeutic vaccination based on a recombinant nonreplicative human adenovirus serotype 5, expressing multiple specific HBV antigens (core, polymerase and envelope) from genotype D. It has been designed to prime de novo and/or stimulate functional T cells expected to control the HBV replication and to clear HBV. In naive mice, TG1050 induced high levels of T cells targeting core, polymerase and HBsAg domains. High frequencies of cytolytic HBV-specific T cells were detected in spleens and livers of immunized mice. Further, induced HBV-specific T cells were detected in the blood of injected mice up to 10 months postimmunization [156].
Therapeutic vaccination based on DNA & T-cell peptide epitope
Based on the results of a woodchuck hepatitis virus (WHV) model study, a T-cell vaccine may be a viable option for treatment of HBV in humans. WHV-transgenic mice were vaccinated with a DNA prime-adenovirus boost vaccine in order to determine whether the vaccinated mice would control the WHV infection and induce WHcAg-specific T-cell response. In 70% of immunized mice, a strong WHcAg-specific CD8+ T-cell response that resulted in reduction of viral load beneath the limit of detection was demonstrated [157]. Combining the vaccine with entecavir resulted in WHsAg- and WHcAg-specific CD4+ and CD8+ T-cell response. These animals demonstrated prolonged response with two out of four chronic carriers developing anti-WHs antibodies in the process [157]. These results show that a combination of antivirals and a vaccine may be a useful approach to a functional cure for HBV.
DNA vaccine
DNA vaccines may be used to induce a T-cell response from CHB patients. The DNA vaccine has been shown to induce both CD8+ and CD4+ cells, with the helper cells producing IFN-γ [158]. This particular vaccine activates HBV- and NK-cell specific T-cell responses [159].
In a proof of concept study, 12 CHB carriers treated with 3TC were vaccinated with a combination of HBV genes and genetically engineered IL-12. Type 1 T-cell responses correlated with viral suppression, and showed a 50% virological response rate. Moreover, T-cell responses were well-tolerated and maintained for at least 40 weeks after cessation of treatment [160].
T-cell peptide epitope vaccine
In a recent Phase I trial the safety of a multiepitope-based (HBV derived) vaccine presented with HLA molecules was tested in healthy volunteers. This vaccine was found to be safe and tolerable in healthy volunteers and future studies are awaited [161].
Therapies involving T-cell response in HBV
T cells control HBV through direct cytolytic action; in addition, TNF-α and IFN-γ released by T cells was recently shown to eliminate HBV replication and reduce cccDNA in infected hepatocytes. This required activation of nuclear APOBEC3 deaminase by these cytokines [162]. Hepatocytes from acute but not CHB patients or healthy controls expressed APOBEC3A/3B. Strategies to restore T-cell response in chronic HBV – such as use of checkpoint inhibitors or adoptive T-cell therapy – might achieve sustained viral control.
PD-1 inhibition
Most T cells specific to HBV are found to be exhausted and expressing PD-1 receptors. These T cells are energized when they interact with PD-1L1/2 on antigen presenting cells in vivo. Ability to reverse this effect by interfering with PD-1/PD-L1/2 interaction presents an opportunity to rejuvenate T-cell responses and aid in HBV clearance as shown in a recent study using the woodchuck model [163]. Studies using PBMCs from CHB patients when stimulated with HBV genome overlapping peptides in the presence of PD-1/L1/2 blockade results in increase in IFN-γ secreting HBV-specific CD8+ T cells [164]. It is unclear if such approaches can be reproduced in vivo. Recently, both anti-PD-1 and PD-L1 monoclonal antibodies have been studied in patients with cancer. Whether such strategy can be tried for CHB patients is not clear.
Chimeric antigenic receptor
The above strategies of stimulating or rejuvenating HBV-specific T cells in vivo would only work in the presence of T cells specific to HBV. In some cases, T-cell deletion may occur, depleting the frequency of HBV specific T cells in vivo. In such cases, passive transfer of engineered chimeric T cells (CARs), may be an effective strategy. At least one patient with CHB and HCC has received CARs and much more are awaited [165].
• Combination approach
Using an antiviral therapy prior to treatment of CHB with a vaccine may lower the HBV DNA enough that there is significantly improved efficacy of the therapeutic vaccination. Multiple studies demonstrate correlations between low HBV DNA in serum at the start of vaccine therapy and increased treatment efficacy [166,167]. Therefore, development of a more effective therapeutic vaccine may hinge on using NAs to lower the HBV DNA levels.
Future perspective
The ultimate goal for HBV treatment is to be able to completely eliminate the virus from the body. By using combinations of the therapies mentioned above, achieving a cure may soon be possible. For example, TALENs, which disrupts cccDNA, has an increased antiviral effect when used in combination with IFN-α, thus may allow the immune system to eliminate the virus when used in combination. Additionally, through the use of an entry inhibitor, such as Myrcludex-B, and a potent antiviral, the body may be able to eventually eradicate the virus due to the lack of production of new cccDNA. Combination therapy using both viral and host targeting approaches offer the best hope for accomplishing a functional cure for CHB, which may be a reality in the near future.
EXECUTIVE SUMMARY.
Chronic hepatitis B (CHB) affects a large number of people worldwide and remains a leading cause of hepatocellular carcinoma, liver failure and mortality.
Current antiviral therapy is effective in suppressing hepatitis B virus (HBV) DNA levels and normalizing toll-like receptor levels, but cessation of therapy is associated with rebound of HBV DNA levels.
Viral and host factors play a major role in establishment of persistence of chronic disease.
Presence of covalently closed circular DNA and secretion of HBsAg and HBeAg subviral particles remain the two major viral causes for HBV persistence.
Blunting of intrahepatic innate immunity and presence of exhausted HBV-specific T cells remain major host immune factors of HBV persistence.
Absence of HBV DNA levels and HBsAg after cessation of therapy is the goal for sustained viral remission for CHB patients.
Targeting various aspects of HBV lifecycle offers unique perspective for combination therapy that may be able to achieve sustained viral response.
Blocking HBV entry, nucleocapsid formation, secretion of subunits and destabilizing or deactivating covalently closed circular DNA are some of the novel strategies to achieve sustained remission.
Toll-like receptor agonism, therapeutic vaccinations, PD-1 blockade and CARs are novel strategies to target host immunity and aid in HBV clearance.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoulim F. Hepatitis B virus resistance to antiviral drugs: where are we going? Liver Int. 2011;31(S1):111–116. doi: 10.1111/j.1478-3231.2010.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devi U, Locarnini S. Hepatitis B antivirals and resistance. Curr. Opin. Virol. 2013;3(5):495–500. doi: 10.1016/j.coviro.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J. Virol. 2005;79(15):9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor R, Kottilil S. Strategies to eliminate HBV infection. Future Virol. 2014;9(6):565–585. doi: 10.2217/fvl.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak MA, Bonhoeffer S, Hill AM, et al. Viral dynamics in hepatitis B virus infection. Proc. Natl Acad. Sci. USA. 1996;93(9):4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt CM, Mcgill JM, Allen MI, et al. Clinical relevance of hepatitis B viral mutations. Hepatology. 2000;31(5):1037–1044. doi: 10.1053/he.2000.6709. [DOI] [PubMed] [Google Scholar]
- 10.Torresi J, Earnest-Silveira L, Deliyannis G, et al. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293(2):305–313. doi: 10.1006/viro.2001.1246. [DOI] [PubMed] [Google Scholar]
- 11.Zuckerman JN, Zuckerman AJ. Mutations of the surface protein of hepatitis B virus. Antiviral Res. 2003;60(2):75–78. doi: 10.1016/j.antiviral.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Fung SK, Lok AS. Hepatitis B virus genotypes: do they play a role in the outcome of HBV infection? Hepatology. 2004;40(4):790–792. doi: 10.1002/hep.1840400407. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Yotsuyanagi H, Yatsuhashi H, et al. Risk factors for long-term persistence of serum hepatitis B surface antigen following acute hepatitis B virus infection in Japanese adults. Hepatology. 2014;59(1):89–97. doi: 10.1002/hep.26635. [DOI] [PubMed] [Google Scholar]
- 14.Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. J. Gastroenterol. Hepatol. 2011;26(Suppl. 1):123–130. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- 15.Sung JJ, Wong ML, Bowden S, et al. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology. 2005;128(7):1890–1897. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126(7):1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J. Hepatol. 2005;42(3):302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Thursz MR, Kwiatkowski D, Allsopp CE, et al. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N. Engl. J. Med. 1995;332(16):1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 19.An P, Winkler C, Guan L, et al. A common HLA-DPA1 variant is a major determinant of hepatitis B virus clearance in Han Chinese. J. Infect. Dis. 2011;203(7):943–947. doi: 10.1093/infdis/jiq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu L, Zhai X, Liu J, et al. Genetic variants in human leukocyte antigen/DP-DQ influence both hepatitis B virus clearance and hepatocellular carcinoma development. Hepatology. 2012;55(5):1426–1431. doi: 10.1002/hep.24799. [DOI] [PubMed] [Google Scholar]
- 21.Mbarek H, Ochi H, Urabe Y, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum. Mol. Genet. 2011;20(19):3884–3892. doi: 10.1093/hmg/ddr301. [DOI] [PubMed] [Google Scholar]
- 22.Nishida N, Sawai H, Matsuura K, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS ONE. 2012;7(6):e39175. doi: 10.1371/journal.pone.0039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Wu XP, Zhang W, et al. Evaluation of genetic susceptibility loci for chronic hepatitis B in Chinese: two independent case-control studies. PLoS ONE. 2011;6(3):e17608. doi: 10.1371/journal.pone.0017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang DK, Ma XP, Yu H, et al. Genetic variants in five novel loci including CFB and CD40 predispose to chronic hepatitis B. Hepatology. 2015;62(1):118–128. doi: 10.1002/hep.27794. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Yin J, Zhang Y, et al. HLA-DP polymorphisms affect the outcomes of chronic hepatitis B virus infections, possibly through interacting with viral mutations. J. Virol. 2013;87(22):12176–12186. doi: 10.1128/JVI.02073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almarri A, Batchelor JR. HLA and hepatitis B infection. Lancet. 1994;344(8931):1194–1195. doi: 10.1016/s0140-6736(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang YG, Wang YM, Liu TH, et al. Association between HLA class II gene and susceptibility or resistance to chronic hepatitis B. World J. Gastroenterol. 2003;9(10):2221–2225. doi: 10.3748/wjg.v9.i10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frodsham AJ, Zhang L, Dumpis U, et al. Class II cytokine receptor gene cluster is a major locus for hepatitis B persistence. Proc. Natl Acad. Sci. USA. 2006;103(24):9148–9153. doi: 10.1073/pnas.0602800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thursz M, Yee L, Khakoo S. Understanding the host genetics of chronic hepatitis B and C. Semin. Liver Dis. 2011;31(2):115–127. doi: 10.1055/s-0031-1276642. [DOI] [PubMed] [Google Scholar]
- 30.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Postgrad. Med. J. 2013;89(1051):294–304. doi: 10.1136/postgradmedj-2011-301073rep. [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 32.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Yang HI, Liu J, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58(2):546–554. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 34.Fleischer RD, Lok ASF. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. J. Hepatol. 2009;51(4):787–791. doi: 10.1016/j.jhep.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135(2):459–467. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Van Zonneveld M, Honkoop P, Hansen BE, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology. 2004;39(3):804–810. doi: 10.1002/hep.20128. [DOI] [PubMed] [Google Scholar]; • One of the earliest studies to report long-term (median 8.8 years) follow-up of patients with chronic hepatitis B treated with interferon, reporting improvement in survival and development of hepatocellular carcinoma as well are reporting rates of HBeAg and sAg loss.
- 37.Wong VWS, Wong GLH, Yan KKL, et al. Durability of peginterferon alfa-2b treatment at 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(6):1945–1953. doi: 10.1002/hep.23568. [DOI] [PubMed] [Google Scholar]
- 38.Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137(6):2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 39.Janssen HL, Van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365(9454):123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 40.Liaw YF, Jia JD, Chan H, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54(5):1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 41.Chang TT, Gish RG, De Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 42.Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 43.Clements CJ, Coghlan B, Creati M, et al. Global control of hepatitis B virus: does treatment-induced antigenic change affect immunization? Bull. World Health Organ. 2010;88(1):66–73. doi: 10.2471/BLT.08.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locarnini SA, Yuen L. Molecular genesis of drug-resistant and vaccine-escape HBV mutants. Antivir. Ther. 2010;15(3 Pt B):451–461. doi: 10.3851/IMP1499. [DOI] [PubMed] [Google Scholar]
- 45.Velay A, Jeulin H, Eschlimann M, et al. Characterization of hepatitis B virus surface antigen variability and impact on HBs antigen clearance under nucleos(t)ide analogue therapy. J. Viral Hepat. 2016;23(5):387–398. doi: 10.1111/jvh.12498. [DOI] [PubMed] [Google Scholar]
- 46.European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J. Hepatol. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Fattovich G, Olivari N, Pasino M, et al. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57(1):84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 48.Moucari R, Marcellin P. HBsAg seroclearance: prognostic value for the response to treatment and the long-term outcome. Gastroenterol. Clin. Biol. 2010;34(Suppl. 2):S119–S125. doi: 10.1016/S0399-8320(10)70031-2. [DOI] [PubMed] [Google Scholar]
- 49.Ruan P, Xu SY, Zhou BP, et al. Hepatitis B surface antigen seroclearance in patients with chronic hepatitis B infection: a clinical study. J. Int. Med. Res. 2013;41(5):1732–1739. doi: 10.1177/0300060513487643. [DOI] [PubMed] [Google Scholar]
- 50.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 51.Scaglione SJ, Lok ASF. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142(6):1360–1368. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 52.Chan HL, Wong VW, Tse AM, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin. Gastroenterol. Hepatol. 2007;5(12):1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 54.Yuen M-F, Ahn SH, Lee KS, et al. Two-year treatment outcome of chronic hepatitis B infection treated with besifovir vs. entecavir: results from a multicentre study. J. Hepatol. 2015;62(3):526–532. doi: 10.1016/j.jhep.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal K, Fung SK, Nguyen TT, et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J. Hepatol. 2015;62(3):533–540. doi: 10.1016/j.jhep.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 56.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3485615/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 2005;79(3):1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze A, Schieck A, Ni Y, et al. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 2010;84(4):1989–2000. doi: 10.1128/JVI.01902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petersen J, Dandri M, Mier W, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat. Biotechnol. 2008;26(3):335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 60.Volz T, Allweiss L, Ben Mbarek M, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J. Hepatol. 2013;58(5):861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Belloni L, Allweiss L, Guerrieri F, et al. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Invest. 2012;122(2):529–537. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tropberger P, Mercier A, Robinson M, et al. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc. Natl Acad. Sci. USA. 2015;112(42):E5715–E5724. doi: 10.1073/pnas.1518090112. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Presents the first genome-wide mapping of post-translational modifications of covalently closed circular DNA in hepatitis B virus (HBV) that could provide a promising target for treatment.
- 63.Zimmerman KA, Fischer KP, Joyce MA, et al. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J. Virol. 2008;82(16):8013–8021. doi: 10.1128/JVI.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cradick TJ, Keck K, Bradshaw S, et al. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol. Ther. 2010;18(5):947–954. doi: 10.1038/mt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Händel EM, Gellhaus K, Khan K, et al. Versatile and efficient genome editing in human cells by combining zinc-finger nucleases with adeno-associated viral vectors. Hum. Gene. Ther. 2012;23(3):321–329. doi: 10.1089/hum.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol. Ther. 2014;22(2):303–311. doi: 10.1038/mt.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows that we can target HBV DNA with specificity using transcription activator-like effector nucleases, whose effects are increased with use of IFN-α.
- 67.Cai D, Mills C, Yu W, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob. Agents Chemother. 2012;56(8):4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the different ways HBV DNA can be specifically targeted without hepatotoxicity, especially focusing on the cytidine deamination that results in covalently closed circular DNA degradation.
- 69.Dong C, Qu L, Wang H, et al. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110–117. doi: 10.1016/j.antiviral.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Deres K, Schröder CH, Paessens A, et al. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299(5608):893–896. doi: 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- 71.Stray SJ, Bourne CR, Punna S, et al. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc. Natl Acad. Sci. USA. 2005;102(23):8138–8143. doi: 10.1073/pnas.0409732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stray SJ, Zlotnick A. BAY 41–4109 has multiple effects on hepatitis B virus capsid assembly. J. Mol. Recognit. 2006;19(6):542–548. doi: 10.1002/jmr.801. [DOI] [PubMed] [Google Scholar]
- 73.Wu GY, Zheng XJ, Yin CC, et al. Inhibition of hepatitis B virus replication by Bay 41–4109 and its association with nucleocapsid disassembly. J. Chemother. 2008;20(4):458–467. doi: 10.1179/joc.2008.20.4.458. [DOI] [PubMed] [Google Scholar]
- 74.Zhu X, Zhao G, Zhou X, et al. 2,4-Diaryl-4,6,7,8-tetrahydroquinazolin-5(1H)-one derivatives as anti-HBV agents targeting at capsid assembly. Bioorg. Med. Chem. Lett. 2010;20(1):299–301. doi: 10.1016/j.bmcl.2009.10.119. [DOI] [PubMed] [Google Scholar]
- 75.Billioud G, Pichoud C, Puerstinger G, et al. The main hepatitis B virus (HBV) mutants resistant to nucleoside analogs are susceptible in vitro to non-nucleoside inhibitors of HBV replication. Antiviral Res. 2011;92(2):271–276. doi: 10.1016/j.antiviral.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Delaney WE, Edwards R, Colledge D, et al. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro . Antimicrob. Agents. Chemother. 2002;46(9):3057–3060. doi: 10.1128/AAC.46.9.3057-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feld JJ, Colledge D, Sozzi V, et al. The phenylpropenamide derivative AT-130 blocks HBV replication at the level of viral RNA packaging. Antiviral Res. 2007;76(2):168–177. doi: 10.1016/j.antiviral.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Katen SP, Chirapu SR, Finn MG, et al. Trapping of hepatitis B virus capsid assembly intermediates by phenylpropenamide assembly accelerators. ACS Chem. Biol. 2010;5(12):1125–1136. doi: 10.1021/cb100275b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katen SP, Tan Z, Chirapu SR, et al. Assembly-directed antivirals differentially bind quasiequivalent pockets to modify hepatitis B virus capsid tertiary and quaternary structure. Structure. 2013;21(8):1406–1416. doi: 10.1016/j.str.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klumpp K, Shimada T, Allweiss L, et al. O115: high antiviral activity of the HBV core inhibitor NVR 3-778 in the humanized uPA/SCID mouse model. J. Hepatol. 2015;62:S250. [Google Scholar]
- 81.Heermann KH, Goldmann U, Schwartz W, et al. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 1984;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prange R. Host factors involved in hepatitis B virus maturation, assembly, and egress. Med. Microbiol. Immunol. 2012;201(4):449–461. doi: 10.1007/s00430-012-0267-9. [DOI] [PubMed] [Google Scholar]
- 83.Watanabe T, Sorensen EM, Naito A, et al. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc. Natl Acad. Sci. 2007;104(24):10205–10210. doi: 10.1073/pnas.0704000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durantel D, Alotte C, Zoulim F. Glucosidase inhibitors as antiviral agents for hepatitis B and C. Curr. Opin. Investig. Drugs. 2007;8(2):125–129. [PubMed] [Google Scholar]
- 85.Lazar C, Durantel D, Macovei A, et al. Treatment of hepatitis B virus-infected cells with alpha-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res. 2007;76(1):30–37. doi: 10.1016/j.antiviral.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Mehta A, Carrouée S, Conyers B, et al. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology. 2001;33(6):1488–1495. doi: 10.1053/jhep.2001.25103. [DOI] [PubMed] [Google Scholar]
- 87.Mehta A, Zitzmann N, Rudd PM, et al. Alpha-glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 1998;430(1–2):17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- 88.Simsek E, Lu X, Ouzounov S, et al. alpha-Glucosidase inhibitors have a prolonged antiviral effect against hepatitis B virus through the sustained inhibition of the large and middle envelope glycoproteins. Antivir. Chem. Chemother. 2006;17(5):259–267. doi: 10.1177/095632020601700503. [DOI] [PubMed] [Google Scholar]
- 89.Ganem D, Prince AM. Hepatitis B virus infection – natural history and clinical consequences. N. Engl. J. Med. 2004;350(11):1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 90.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 91.Dunn C, Peppa D, Khanna P, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137(4):1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 92.Dougherty AM, Guo H, Westby G, et al. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob. Agents Chemother. 2007;51(12):4427–4437. doi: 10.1128/AAC.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu W, Goddard C, Clearfield E, et al. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J. Med. Chem. 2011;54(16):5660–5670. doi: 10.1021/jm200696v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korba BE, Montero AB, Farrar K, et al. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77(1):56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo . Antimicrob. Agents Chemother. 2013;57(11):5299–5306. doi: 10.1128/AAC.01005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro . Antimicrob. Agents Chemother. 2013;57(11):5291–5298. doi: 10.1128/AAC.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noordeen F, Scougall CA, Grosse A, et al. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS ONE. 2015;10(11):e0140909. doi: 10.1371/journal.pone.0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Mahtab M, Bazinet M, Vaillant A. Effects of nucleic acid polymer therapy alone or in combination with immunotherapy on the establishment of SVR in patients with chronic HBV infection. J. Clin. Virol. 2015;69:228. [Google Scholar]
- 99.Al-Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS ONE. 2016;11(6):e0156667. doi: 10.1371/journal.pone.0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zu Putlitz J, Wieland S, Blum HE, et al. Antisense RNA complementary to hepatitis B virus specifically inhibits viral replication. Gastroenterology. 1998;115(3):702–713. doi: 10.1016/s0016-5085(98)70150-7. [DOI] [PubMed] [Google Scholar]
- 101.Welch PJ, Tritz R, Yei S, et al. Intracellular application of hairpin ribozyme genes against hepatitis B virus. Gene Ther. 1997;4(7):736–743. doi: 10.1038/sj.gt.3300441. [DOI] [PubMed] [Google Scholar]
- 102.Nash KL, Alexander GJ, Lever AM. Inhibition of hepatitis B virus by lentiviral vector delivered antisense RNA and hammerhead ribozymes. J. Viral Hepat. 2005;(4):346–356. doi: 10.1111/j.1365-2893.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 103.Hannon GJ. RNA interference. Nature. 2002;418(6894):244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 104.Chen Y, Cheng G, Mahato RI. RNAi for treating hepatitis B viral infection. Pharm. Res. 2008;25(1):72–86. doi: 10.1007/s11095-007-9504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klein C, Bock CT, Wedemeyer H, et al. Inhibition of hepatitis B virus replication in vivo by nucleoside analogs and siRNA. Gastroenterology. 2003;125(1):9–18. doi: 10.1016/s0016-5085(03)00720-0. [DOI] [PubMed] [Google Scholar]; •• One of the first studies to demonstrate specific anti-HBV responses induced using siRNA, which showed decreased HBsAg levels although HBV DNA could still be detected in the serum.
- 106.Mccaffrey AP, Nakai H, Pandey K, et al. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 2003;21(6):639–644. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- 107.Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 108.Shlomai A, Shaul Y. Inhibition of hepatitis B virus expression and replication by RNA interference. Hepatology. 2003;37(4):764–770. doi: 10.1053/jhep.2003.50146. [DOI] [PubMed] [Google Scholar]
- 109.Gish RG, Yuen M-F, Chan HLY, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res. 2015;121:97–108. doi: 10.1016/j.antiviral.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 110.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 111.Woltman AM, Op Den Brouw ML, Biesta PJ, et al. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS ONE. 2011;6(1):e15324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA. 2004;101(17):6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 114.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 115.An BY, Xie Q, Lin LY, et al. [Expression of Toll-like receptor 3 on peripheral blood dendritic cells in HBeAg positive patients with chronic hepatitis B] Zhonghua Gan Zang Bing Za Zhi. 2007;15(10):729–733. [PubMed] [Google Scholar]
- 116.Chen Z, Cheng Y, Xu Y, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin. Immunol. 2008;128(3):400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Hirsch I, Caux C, Hasan U, et al. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31(10):391–397. doi: 10.1016/j.it.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Momeni M, Zainodini N, Bidaki R, et al. Decreased expression of toll like receptor signaling molecules in chronic HBV infected patients. Hum. Immunol. 2014;75(1):15–19. doi: 10.1016/j.humimm.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 119.Isogawa M, Robek MD, Furuichi Y, et al. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo . J. Virol. 2005;79(11):7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 2000;74(9):4165–4173. doi: 10.1128/jvi.74.9.4165-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130(6):1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 122.Zhang X, Kraft A, Broering R, et al. Preclinical development of TLR ligands as drugs for the treatment of chronic viral infections. Expert Opin. Drug Discov. 2012;7(7):597–611. doi: 10.1517/17460441.2012.689281. [DOI] [PubMed] [Google Scholar]
- 123.Lang T, Lo C, Skinner N, et al. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signalling pathway. J. Hepatol. 2011;55(4):762–769. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 124.Visvanathan K, Skinner NA, Thompson AJ, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45(1):102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 125.Wang H, Ryu WS. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 2010;6(7):e1000986. doi: 10.1371/journal.ppat.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu J, Meng Z, Jiang M, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49(4):1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 127.Yu S, Chen J, Wu M, et al. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J. Gen. Virol. 2010;91(Pt 8):2080–2090. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 128.Funk E, Kottilil S, Gilliam B, et al. Tickling the TLR7 to cure viral hepatitis. J. Transl. Med. 2014;12:129. doi: 10.1186/1479-5876-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508–1517. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Chimpanzee model demonstrating HBV suppression with TLR-7 and providing preliminary data for subsequent human studies.
- 130.Lopatin U, Wolfgang G, Tumas D, et al. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral Toll-like receptor 7 agonist. Antivir. Ther. 2013;18(3):409–418. doi: 10.3851/IMP2548. [DOI] [PubMed] [Google Scholar]
- 131.Gane EJ, Lim YS, Gordon SC, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J. Hepatol. 2015;63(2):320–328. doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]; • Phase Ib study demonstrating safety of TLR-7 agonist.
- 132.Nanjappa SG, Kim EH, Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117(19):5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pellegrini M, Calzascia T, Toe JG, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144(4):601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 134.Toe JG, Pellegrini M, Mak TW. Promoting immunity during chronic infection – the therapeutic potential of common gamma-chain cytokines. Mol. Immunol. 2013;56(1–2):38–47. doi: 10.1016/j.molimm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 135.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T-cell maintenance. J. Immunol. 2005;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 136.Kikuchi K, Lai AY, Hsu CL, et al. IL-7 receptor signaling is necessary for stage transition in adult B-cell development through up-regulation of EBF. J. Exp. Med. 2005;201(8):1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fröhlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 140.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Publicover J, Goodsell A, Nishimura S, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J. Clin. Invest. 2011;121(3):1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma SW, Huang X, Li YY, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J. Hepatol. 2012;56(4):775–781. doi: 10.1016/j.jhep.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 143.Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert Opin. Biol. Ther. 2010;10(5):807–817. doi: 10.1517/14712598.2010.480971. [DOI] [PubMed] [Google Scholar]
- 144.Inchauspé G, Michel ML. Vaccines and immunotherapies against hepatitis B and hepatitis C viruses. J. Viral Hepat. 2007;14(Suppl. 1):97–103. doi: 10.1111/j.1365-2893.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 145.Kutscher S, Bauer T, Dembek C, et al. Design of therapeutic vaccines: hepatitis B as an example. Microb. Biotechnol. 2012;5(2):270–282. doi: 10.1111/j.1751-7915.2011.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol. Biol. (Paris) 2010;58(4):288–295. doi: 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 147.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev. Gastroenterol. Hepatol. 2009;3(5):561–569. doi: 10.1586/egh.09.48. [DOI] [PubMed] [Google Scholar]
- 148.Yao X, Zheng B, Zhou J, et al. Therapeutic effect of hepatitis B surface antigen–antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine. 2007;25(10):1771–1779. doi: 10.1016/j.vaccine.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 149.Xu DZ, Zhao K, Guo LM, et al. A randomized controlled Phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE. 2008;3(7):e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu DZ, Wang XY, Shen XL, et al. Results of a phase III clinical trial with an HBsAg–HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J. Hepatol. 2013;59(3):450–456. doi: 10.1016/j.jhep.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 151.Böcher WO, Dekel B, Schwerin W, et al. Induction of strong hepatitis B virus (HBV) specific T helper cell and cytotoxic T lymphocyte responses by therapeutic vaccination in the trimera mouse model of chronic HBV infection. Eur. J. Immunol. 2001;31(7):2071–2079. doi: 10.1002/1521-4141(200107)31:7<2071::aid-immu2071>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 152.Lobaina Y, García D, Abreu N, Muzio V, Aguilar JC. Mucosal immunogenicity of the hepatitis B core antigen. Biochem. Biophys. Res. Commun. 2003;300(3):745–750. doi: 10.1016/s0006-291x(02)02897-8. [DOI] [PubMed] [Google Scholar]
- 153.Al-Mahtab M, Akbar SMF, Aguilar JC, et al. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatology International. 2013;7(4):981–989. doi: 10.1007/s12072-013-9486-4. [DOI] [PubMed] [Google Scholar]
- 154.King TH, Kemmler CB, Guo Z, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and core specific T cells in mice and activates human t cells recognizing epitopes linked to viral clearance. PLoS ONE. 2014;9(7):e101904. doi: 10.1371/journal.pone.0101904. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of the first studies in which an immunotherapeutic platform known as tarmogens were shown to be effective in HBV-specific T-cell responses.
- 155.Lok AS, Pan CQ, Han SB, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016 doi: 10.1016/j.jhep.2016.05.016. Epub aheadof print. [DOI] [PubMed] [Google Scholar]
- 156.Martin P, Dubois C, Jacquier E, et al. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015;64(12):1961–1971. doi: 10.1136/gutjnl-2014-308041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kosinska AD, Zhang E, Johrden L, et al. Combination of DNA prime-adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013;9(6):e1003391. doi: 10.1371/journal.ppat.1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005;55:25–40. doi: 10.1016/S0065-2660(05)55002-8. [DOI] [PubMed] [Google Scholar]
- 159.Scott-Algara D, Mancini-Bourgine M, Fontaine H, et al. Changes to the natural killer cell repertoire after therapeutic hepatitis B DNA vaccination. PLoS ONE. 2010;5(1):e8761. doi: 10.1371/journal.pone.0008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang SH, Lee CG, Park SH, et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13(14):1110–1117. doi: 10.1038/sj.gt.3302751. [DOI] [PubMed] [Google Scholar]
- 161.Depla E, Van Der Aa A, Livingston BD, et al. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J. Virol. 2008;82(1):435–450. doi: 10.1128/JVI.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xia Y, Stadler D, Lucifora J, et al. Interferon-gamma and tumor necrosis factor-alpha produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2015;150(1):194–205. doi: 10.1053/j.gastro.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 163.Liu J, Zhang E, Ma Z, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10(1):e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sherman AC, Trehanpati N, Daucher M, et al. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1/programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res. Hum. Retroviruses. 2013;29(4):665–672. doi: 10.1089/aid.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bertoletti A, Brunetto M, Maini MK, et al. T cell receptor-therapy in HBV-related hepatocellularcarcinoma. OncoImmunology. 2015;4(6):e1008354. doi: 10.1080/2162402X.2015.1008354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pol S, Michel ML. Therapeutic vaccination in chronic hepatitis B virus carriers. Expert Rev. Vaccines. 2006;5(5):707–716. doi: 10.1586/14760584.5.5.707. [DOI] [PubMed] [Google Scholar]
- 167.Senturk H, Tabak F, Ozaras R, et al. Efficacy of pre-S-containing HBV vaccine combined with lamivudine in the treatment of chronic HBV infection. Dig. Dis. Sci. 2009;54(9):2026–2030. doi: 10.1007/s10620-008-0586-2. [DOI] [PubMed] [Google Scholar]