Summary
Pure red cell aplasia (PRCA) is a rare disorder characterized by inhibition of erythroid precursors in the bone marrow and normochromic, normocytic anaemia with reticulocytopenia. Among 51 PRCA patients, we identified 12 (24%) patients having monoclonal gammopathy, monoclonal gammopathy of undetermined significance or smouldering multiple myeloma, with presence of monoclonal protein or abnormal serum free light chains and atypical bone marrow features of clonal plasmacytosis, hypercellularity and fibrosis. Thus far, three patients treated with anti-myeloma based therapeutics have responded with reticulocyte recovery and clinical transfusion independence, suggesting plasma cells play a key role in the pathogenesis of this specific monoclonal gammopathy-associated PRCA.
Keywords: MGUS, Pure red cell aplasia, Myeloma, Plasmacytosis, Anaemia
Introduction
The cardinal bone marrow features of patients with pure red cell aplasia (PRCA) include marked erythroid hypoplasia with maturation arrest and sparing of other marrow elements. In several patient reports, the destruction of erythroid precursors has been attributed to immune-mediated mechanisms, either humoral or cellular (Peschle et al. 1975, Dessypris et al. 1982, Casadevall et al. 1996, Prabhakar & Muhlfelder 1997, Fisch et al. 2000). The aetiology of idiopathic PRCA remains elusive and immunosuppressive treatments have become the cornerstone of management. A number of secondary causes have been linked to acquired PRCA, including thymoma, lymphoid malignancies, viral infection, drugs and connective tissue disorders (Dessypris 1991, Mamiya et al. 1997, Young & Brown 2004, Sawada et al. 2007). A few case reports have been described that have associated monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM) with PRCA (Kobayashi et al. 1987, Orchard et al. 1997, So et al. 2013, Lv & Qian 2015). However, no systematic assessment of PRCA associated with plasma cell dyscrasias has been conducted. While anaemia is a common end-organ feature of MM, PRCA with erythroid maturation arrest is not a typical finding in MM-inflicted bone marrow (Kyle et al. 2003). Rather, anaemia associated with MM usually still demonstrates full erythroid maturation and may be the result of iron dysregulation with increased hepcidin levels due to interleukin 6, abnormal erythropoietin levels linked to renal insufficiency or large marrow replacement by clonal plasma cells (Silvestris et al. 2001, Sharma et al. 2008, Maes et al. 2010).
In order to estimate the prevalence of MGUS associated with PRCA, 51 PRCA patients were assessed for bone marrow plasmacytosis and presence of serum and/or urine monoclonal protein (M-protein) or abnormal serum free light chains.
Methods
A series of bone marrow biopsies from 51 PRCA patients referred to the National Institutes of Health (NIH) Clinical Center between 2001 and 2013 were morphologically reviewed by two experienced haematopathologists. Immunohistochemical staining of bone marrow biopsy for CD138 (DAKO, Carpinteria, CA, USA) enabled the enumeration of plasma cells and was performed using an automated stainer. Plasma cell clonality was evaluated by kappa or lambda light chain immunohistochemistry or in situ hybridization. Reticulin stain was performed on bone marrow biopsies to assess the degree of fibrosis. All patients underwent testing for presence of M-protein by serum and/or urine protein electrophoresis (SPEP/UPEP) with immunofixation (IFE). Serum free light chains (FLC) and FLC ratio was determined subsequently on stored frozen sera (−80° C), using the Freelite™ immunoassays from The Binding Site Ltd (Birmingham, UK) according to the manufacturer’s protocol. Patient charts were reviewed for available cytogenetic karyotyping data and clinical data including prior treatment regimens and relevant responses. Patients were tested for JAK2 V617F, CALR Type 1 and Type 2 mutations and BCR-ABL1 mutations using standard clinical laboratory testing. Molecular analysis for additional receptor tyrosine kinase (RTK) mutations was performed using RTK Panel I mutation polymerase chain reaction (PCR) array kit (Qiagen, Frederick, MD,USA) on 9 patients with available stored DNA.
Results
Patient Characteristics and Serum Studies
Among 51 PRCA patients, 12 (24%) patients were identified as having monoclonal gammopathy associated PRCA (Table I). These patients had evidence of M-protein on SPEP/UPEP with IFE or abnormal serum FLCs, increased bone marrow plasmacytosis and classical PRCA findings consisting of selective erythroid hypoplasia with maturation arrest, low reticulocyte count, and the majority were transfusion dependent. Nine patients demonstrated M-protein on SPEP and/or IFE. IgG isotype of M-protein was demonstrated in 8 patients and immunofixation was not performed in one patient. Among patients with measurable M-protein, mean M-protein concentration was 11 g/l (range 6–25 g/l). Six of 8 evaluable patients with M-protein (Patient 3 was not included), had a skewed serum FLC ratio. Serum FLC assay was not performed on Patient 3 due to lack of available sample. Three patients did not have a detectable M-protein. Two of these 3 patients (Patients 10 and 11) had a skewed FLC ratio, while 1 patient (Patient 9) had a normal FLC ratio and serum free lambda chain level, but elevated serum free kappa chains with 10% plasma cells on bone marrow biopsy.
Table I.
Monoclonal Gammopathy-Associated PRCA Patient Characteristics and Clinical History
| Patient | Age (years) | Gender | SPEP/SIFE (g/l) | UPEP/UIFE | FLC Ratio (kappa/lambda)** | Hb (g/l) | Absolute Reticulocyte Count (× 109/l) | Transfusion dependent? | Lytic Lesions on Skeletal Survey? | Calcium (mmol/l) | Creatinine (μmol/l) | Prior Lines of Therapy | Response to Daclizumab? | Response to MM treatment? Yes/No (months follow-up) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | F | IgG kappa (13) | * | 31.33 | 107 | 2.6 | Yes | No | 2.4 | 123.8 | 0 | + | Response unknown |
| 2 | 48 | M | IgG kappa (12) | IgG kappa and kappa | 11.87 | 64 | 6.9 | Yes | No | 2.0–2.7 | 63.6–114.9 | 0 | + | Yes (54) |
| 3 | 43 | F | IgG lambda | * | * | 75 | 4.3 | No | * | 2.25 | 61.9 | 0 | + | ++ |
| 4 | 59 | F | IgG lambda (10) | IgG lambda and lambda | 0.06 | 80 | 4.3 | Yes | No | 2.24 | 61.9–97.2 | 2 | No | No (3) |
| 5 | 56 | M | IgG lambda (6) | * | 0.23 | 69 | 7.8 | Yes | * | 2.45 | 97.2–129.9 | 4 | No | Response unknown |
| 6 | 51 | F | (9) | * | 12.04 | 64 | 2.6 | Yes | * | 2.38 | 70.7 | 2 | No | ++ |
| 7 | 39 | F | IgG kappa (25) | IgG kappa | 1.99 | 118 | 4 | Yes | No | 3.04 | 123.8–150.3 | 1 | + | No (2) |
| 8 | 57 | F | IgG lambda (8) | Normal UPEP | 0.92 | 86 | 15.4 | Yes | No | 2.44 | 63.6 | 6 | No | Yes (47) |
| 9 | 23 | F | No M spike | Normal UPEP | 1.57 | 68 | 3.4 | Yes | * | 2.23 | 60.1 | 2 | No | ++ |
| 10 | 30 | F | No M spike | * | 1.94 | 90 | 2.6 | Yes | * | 2.36 | 79.6 | 2 | No | ++ |
| 11 | 44 | F | No M spike | Normal UPEP | 3.07 | 78 | 3.3 | Yes | * | 2.09 | 61.9–141.4 | 1 | + | ++ |
| 12 | 67 | M | IgG lambda (6) | lambda | 0.45 | 80 | 6.4 | Yes | No | 2.19 | 83.98 | 0 | + | Yes (6) |
Test not performed.
Reference range for FLC ratio is 0.26 – 1.65.
Not treated on daclizumab trial.
Myeloma treatment not administered.
Patient 3 had serum IFE positive only with no quantifiable M-protein on SPEP. Serum IFE was not performed for Patient 6 and isotype could not be confirmed, however plasma cell clonality (Table II) was demonstrated by kappa-light chain restriction on histopathology.
PRCA, pure red cell aplasia; SPEP/SIFE, serum protein electrophoresis/serum immunofixation; UPEP/UIFE, urine protein electrophoresis/urine immunofixation; FLC, free light chains; Hb, haemoglobin concentration; MM, multiple myeloma.
Evidence of additional MM related end-organ damage, as established by “CRAB” criteria (Calcium [elevated], Renal failure, Anaemia, Bone lesions), was investigated (Kyle et al. 2003). Among patients with available data for review, six patients had no evidence of lytic lesions or myeloma on skeletal surveys. No patients demonstrated a serum creatinine above 176.8 μmol/l, and 2 of the 12 patients had evidence of hypercalcaemia but the cause was determined to be benign. Consistent with PRCA histories, all patients demonstrated haemoglobin levels less than 100 g/l while 11/12 patients were clinically dependent on transfused red blood cells. Mean reticulocyte count was 5.3 x 109/l (2.6–15.4 x 109/l). Excluding anaemia as a confounding criterion that fulfils MM-related end-organ damage, 10/12 patients were diagnosed with smouldering plasma cell myeloma and 2/12 patients with MGUS. All 12 patients were ruled out for alternative secondary causes of PRCA including viral infections, such as Parvovirus B19, thymomas or other haematological malignancies (B-cell or T-cell lymphoid malignancies and myelodysplastic or myeloproliferative neoplasms) based on clinical chart review, available imaging, molecular and histopathological data.
Histopathological Bone Marrow Features and Mutational Analysis
In addition to the PRCA findings and plasmacytosis, patient bone marrows also demonstrated atypical features including hypercellularity, increased reticulin fibrosis, and eosinophilia (Table II). Based on CD138 immunohistochemistry, 10 of the monoclonal gammopathy-associated PRCA patients had between 10–30% plasma cells on marrow biopsies; two patients demonstrated plasma cells less than 10%. Light chain restriction of plasma cells on bone marrow was demonstrated by immunohistochemistry or in situ hybridization in 7 patients (4 kappa and 3 lambda). Among the remaining 4 patients with indeterminate plasma cell light chain clonality, 2 patients had abnormal serum FLC ratios (Patients 10 and 11), 1 had elevation of serum kappa light chains (kappa 24.1 mg/l; reference range 3.30–19.4 mg/l) with normal serum free lambda and FLC ratio (Patient 9), and 2 patients had positive serum IFE showing IgG lambda (Patients 3 and 12) (Table I). All patients demonstrated mildly-moderately increased reticulin fibrosis (grade 2–3 out of 4), while 11/12 patients had increased overall marrow cellularity (>60%). Megakaryocytes were normal in number and there was no increase in micromegakaryocytes or abnormal hyperchromatic megakaryocytes. Cytogenetics were normal in 10 out of 11 patients where data was available, and one patient had pericentric inversion of chromosome 2, inv (2)(p11.2q13), which was deemed to be a constitutional normal variant finding. One patient was found to have a KIT M541L mutation. All patients were negative for JAK2 V617F and CALR Type 1 and Type 2 mutations; 4 patients were tested for BCR-ABL1 and found to be negative.
Table II.
Histopathological Bone Marrow Features of Monoclonal Gammopathy-Associated PRCA
| Patient | Erythroid Precursors in Bone Marrow (%) | Plasma Cells in Bone Marrow (%) | Plasma Cell Expression by Immunohistochemistry | Total Bone Marrow Cellularity (%) | Reticulin Stain | Cytogenetics | BCR/ABL1Mutation Detected? | JAK2 V617F Mutation Detected? | CALR Type 1 and 2 Mutation Detected? | RTK Mutation Detected? | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light Chain Expression Restriction? | CCND1 Expression? | CD56 Expression? | ||||||||||
| 1 | 7 | 30 | Kappa | * | Yes | 60–80 | 2+ | Normal | * | No | No | No |
| 2 | 8 | 20 | Kappa | Yes | No | 70–90 | 2+ | Normal | No | No | No | No |
| 3 | 9 | 15 | No | * | * | 90–100 | 2–3+ | Normal | * | * | * | * |
| 4 | 3 | 20 | Lambda | * | Yes | 80 | 2–3+ | Normal | No | * | * | * |
| 5 | 7 | 15 | Lambda | * | No | 50 | 2–3+ | Normal | * | No | No | No |
| 6 | 0 | 10 | Kappa | * | No | 90–100 | 2–3+ | Normal | * | No | No | No |
| 7 | 0 | 30 | Kappa | * | No | 60 | 2–3+ | Normal | * | No | No | Yes KIT M541L |
| 8 | 4 | 20 | Lambda | No | Yes | 65 | 2–3+ | * | No | No | No | No |
| 9 | 7 | 10 | No | * | No | 95–100 | 2–3+ | Normal | * | No | No | No |
| 10 | 7 | 5 | No | * | No | 70–80 | 2–3+ | Normal | * | No | No | No |
| 11 | 4 | 15–20 | No | * | No | 80–90 | 2+ | Normal | * | No | No | No |
| 12 | 3 | 5–9 | Lambda | * | No | 90–100 | 2–3+ | inv (2)(p11.2q13) | No | * | * | * |
Test not performed.
PRCA, pure red cell aplasia; RTK, receptor tyrosine kinase
Clinical Course of Monoclonal Gammopathy Associated PRCA Patients
All 8 previously treated monoclonal gammopathy-associated PRCA patients were refractory to various forms of immunosuppressive therapy, including ciclosporin, rituximab, and steroids. Six patients in this cohort were treated at the NIH with daclizumab under a protocol for idiopathic PRCA previously described (Sloand et al. 2010). Daclizumab response rates among the monoclonal gammopathy-associated PRCA patients compared to idiopathic PRCA patients were significantly worse 0/6 (0%) vs. 10/23 (43%), p=0.04 (chi-square test). Seven patients were recommended for anti-myeloma treatment. To date, three monoclonal gammopathy-associated PRCA patients have had confirmed responses to anti-myeloma therapy with clinical transfusion independence (Figure 1). Patient 2 was initiated on bortezomib and dexamethasone for 8 cycles with brief erythroid recovery and subsequent relapse with M-protein increase and reticulocyte count decrease. He was then started on lenalidomide therapy and remained on this drug after achieving transfusion independence. Maintenance lenalidomide was discontinued after 4 years, and patient remains disease-free 6 months later without requiring transfusions. Patient 8 had a long-standing history of “idiopathic” PRCA and had received multiple prior lines of therapy. She was initiated on lenalidomide, bortezomib and dexamethasone (RVd) for 4 cycles with immediate resolution of her haemoglobin, reticulocyte counts, and declining M-protein numbers. Subsequently, her therapy was gradually decreased to RVd maintenance regiment followed by lenalidomide maintenance. Therapy was discontinued and the patient remains transfusion independent with low M-protein. Finally, Patient 12 was initiated on lenalidomide and dexamethasone. He was subsequently transitioned to lenalidomide maintenance (lenalidomide 10 mg) after achieving haematological recovery and complete response with respect to his monoclonal gammopathy. He currently remains on low dose lenalidomide with stable blood counts. Post-treatment bone marrows demonstrate reduction of plasma cells and erythroid maturation (Figure 2). Two patients were started on therapy but remain transfusion-dependent (Patients 4 and 7), while two patients were lost to follow-up (Table I).
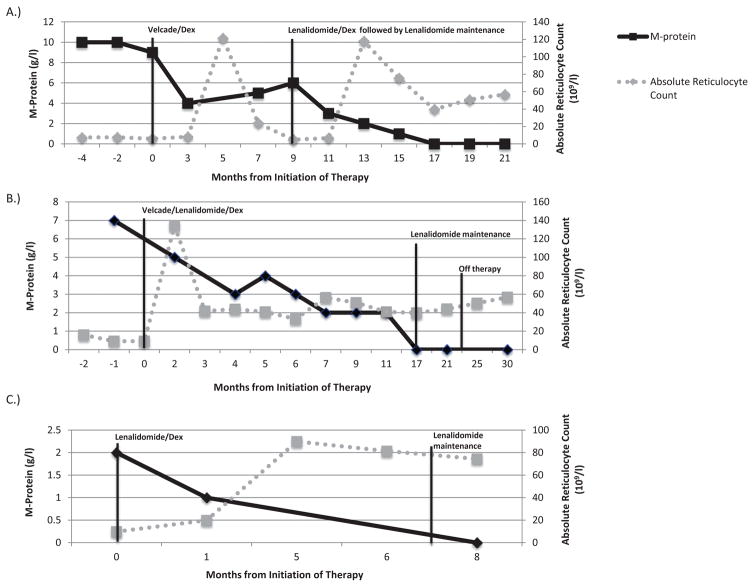
Figure 1. Patient responses with absolute reticulocyte counts and M-protein.
A.) A 51-year-old male diagnosed with pure red cell aplasia with plasma cell dyscrasia (PRCA-PC) (Patient 2, Table I) received bortezomib (1.3 mg/m2 IV days 1, 4, 8 and 11 every 3 weeks) and dexamethasone (40 mg days 1-4) with erythroid recovery and M-protein decline. After relapse, the patient received lenalidomide (25 mg on days 1–21 of a 28-day cycle) and dexamethasone (40 mg weekly) followed by lenalidomide maintenance alone (15 mg daily) for 4 years. Maintenance lenalidomide was discontinued and patient remained transfusion independent 6 months later. B.) A 47-year-old female diagnosed with PRCA-PC (Patient 8, Table I) achieved haematological recovery after receiving lenalidomide, bortezomib and dexamethasone (RVd) for four cycles, followed by maintenance RVd for 12 cycles, and maintenance lenalidomide. Maintenance lenalidomide was discontinued and patient remains transfusion independent. C.) A 67-year-old male with PRCA-PC (Patient 12, Table I) was started on lenalidomide and dexamethasone with normalization of peripheral blood counts and achievement of complete response. Patient switched to lenalidomide maintenance and currently remains on therapy.
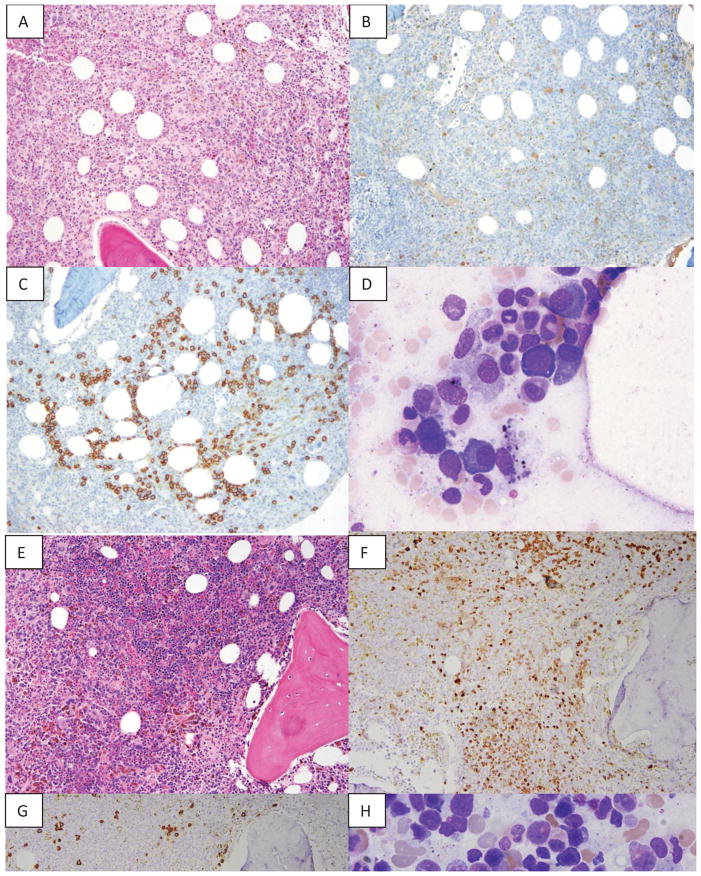
Figure 2. Morphological features of bone marrow biopsy pretreatment and after treatment with anti-myeloma therapy.
A. Pre-treatment bone marrow core biopsy showing hypercellularity and absence of erythroid islands (Haematoxylin and eosin [H&E], 10x magnification) B. Virtually absent red cell precursors (Haemoglobin stain, 10x magnification) C. Increased plasma cells forming clusters (CD138 stain, 10x magnification) D. Bone marrow aspirate showing only rare pronormoblast and increased plasma cells before treatment (50x magnification) E. Post-treatment bone marrow core biopsy showing large progressively maturing erythroid islands (H&E, 10x magnification) F. Full erythroid maturation is present (Haemoglobin stain, 10x magnification) G.. Few scattered plasma cells (CD138 stain 10x magnification) H. Bone marrow aspirate showing progressive erythroid maturation and no increase in plasma cells after treatment (50x magnification)
Discussion
We report on a series of monoclonal gammopathy-associated PRCA patients, comprising up to 24% PRCA cases previously labelled “idiopathic”, who had mild-moderate plasmacytosis accompanied by a serum M-protein or skewed serum FLC ratio and atypical bone marrow findings. The distinguishing feature of monoclonal gammopathy-associated PRCA from anaemia of typical MM is the presence of marked erythroid hypoplasia with maturation arrest at the pronormoblast stage. MM patients usually have no selective decrease in erythroid elements and show the full spectrum of maturation. In addition, monoclonal gammopathy-associated PRCA patients did not have very high plasma cell burden and lacked other “CRAB” criteria of end organ damage.Plasma cellularity reached between 10 and 30% in 10 of the 12 patients, suggesting that the mechanism for anaemia was not due to just “overcrowding” of normal haematopoiesis.
Patients demonstrated other unusual bone marrow findings – particularly hypercellularity and reticulin fibrosis, so molecular testing for myeloproliferative neoplasms was performed. JAK2 V617F and CALR Type 1 and Type 2 mutations were negative on all patients. All of the four patients tested for BCR-ABL1 were found to be negative. RTK mutational testing showed that all but one patient was negative; one patient was identified as having KIT M541L mutation. This patient did not seem to respond to bortezomib or lenalidomide therapy despite significant burden of monoclonal plasma cells (30%), possibly suggesting an alternative pathogenetic mechanism. Alternatively, the observed KIT M541L mutation may represent a polymorphic variant, since reports in the literature demonstrate that allele frequencies for the polymorphic variant, 541Leu are 8.1% in healthy Caucasian controls (Kruger et al 2006). The KIT (CD117) sequence variation M541L, but not N564K, is frequent in the general population, and is not associated with CML in Caucasians (Kruger et al. 2006). All patients lacked other clinical features of myeloproliferative neoplasm disorders, such as splenomegaly or other peripheral blood count abnormalities. Cytogenetics were normal in all but one patient, neither demonstrating typical MM or alternative haematological diagnoses.
We have demonstrated that monoclonal gammopathy-associated PRCA may be a paraprotein-related phenomenon that has not been well described so far in the literature as a cause of PRCA. There are numerous other examples in the plasma cell literature demonstrating modest plasma cell burden and M-protein levels as a cause of paraprotein-related phenomena; monoclonal gammopathy-associated PRCA may be the newest example (Wood et al. 2009, Voisin et al. 2011, Kuwabara et al. 2012, Kwok et al. 2012). Three patients achieved transfusion independence and restoration of erythroid maturation with anti-myeloma based therapies, including bortezomib and lenalidomide. We speculate that a functional relationship between altered plasma cell and erythroid precursor exists in monoclonal gammopathy-associated PRCA, because there seems to be a causal relationship between M-protein response and haematological response including reversal of PRCA bone marrow findings after treatment. We acknowledge that both bortezomib and lenalidomide may not be myeloma-specific. Further investigation is warranted into possible pathogenetic mechanisms, including erythroid inhibition by the IgG monoclonal protein or direct effects from the neoplastic microenvironment.
Acknowledgments
This manuscript is prepared in memory of E.S. (1953–2010). K.L. and A.P analysed clinical data on PRCA patients. O.S., P.N and I.M. analysed histopathology data on PRCA patients at NIH. A.Z., R.C. and O.L. analysed data and performed SPEP and serum FLC assays. M.K, C.M., R.C. and S.S. treated PRCA-PC patients. E.S. and N.Y. analysed clinical data and led clinical trial for PRCA patients. N.K., E.S., O.L. and I.M. conceived design, analysed data and drafted the manuscript. All authors read the drafted manuscript, gave intellectual input and approved the final version (except for E.S.).
Footnotes
Disclosures
The authors declare no conflict of interests or financial interests.
References
- Casadevall N, Dupuy E, Molho-Sabatier P, Tobelem G, Varet B, Mayeux P. Autoantibodies against erythropoietin in a patient with pure red-cell aplasia. N Engl J Med. 1996;334(10):630–633. doi: 10.1056/NEJM199603073341004. [DOI] [PubMed] [Google Scholar]
- Dessypris EN. The biology of pure red cell aplasia. Semin Hematol. 1991;28(4):275–284. [PubMed] [Google Scholar]
- Dessypris EN, Krantz SB, Roloff JS. Mode of action of the IgG inhibitor of erythropoiesis in transient erythroblastopenia of childhood. Blood. 1982;59:114–123. [PubMed] [Google Scholar]
- Fisch P, Handgretinger R, Schaefer HE. Pure red cell aplasia. Br J Haematol. 2000;111(4):1010–1022. doi: 10.1046/j.1365-2141.2000.02429.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hanada T, Sato Y, Shibuya A, Ninomiya H, Nagasawa T, Abe T. A case of pure red cell aplasia with monoclonal gammopathy: immune-mediated inhibition of erythropoiesis. Rinsho Ketsueki. 1987;28(11):2029–2033. [PubMed] [Google Scholar]
- Kruger S, Emig M, Lohse P, Ehninger G, Hochhaus A, Schackert HK. The c-kit (CD117) sequence variation M541L, but not N564K, is frequent in the general population, and is not associated with CML in Caucasians. Leukemia. 2006;20(2):354–355. doi: 10.1038/sj.leu.2404038. discussion 356–357. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Dispenzieri A, Arimura K, Misawa S, Nakaseko C. Treatment for POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes) syndrome. Cochrane Database Syst Rev. 2012;6:CD006828. doi: 10.1002/14651858.CD006828.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok M, Korde N, Landgren O. Bortezomib to treat the TEMPI syndrome. N Engl J Med. 2012;366(19):1843–1845. doi: 10.1056/NEJMc1202649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle R, Child JA, Anderson K, Barlogie B, Bataille R, Bensinger W, Blade J, Boccadoro M, Dalton RJ, Dimopoulos M, Djulbegovic B, Drayson M, Durie B, Facon T, Fonseca R, Gahrton G, Greipp PR, Harousseau JL, Harrington D, Hussein M, Douglas J, Ludwig H, Morgan G, Oken M, Powles R, Richardson P, Roodman D, San Miguel J, Shimizu K, Shustik C, Sirohi B, Sonneveld P, Tricot G, Turesson I, Van Ness B, Vesole D, Weber D, Westin J, Wheatley K. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- Lv Y, Qian W. Treatment of pure red cell aplasia associated with multiple myeloma with biclonal gammopathy using cyclosporine A: a case report. Int J Clin Exp Med. 2015;8(1):1498–1500. [PMC free article] [PubMed] [Google Scholar]
- Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys L, Rivera S, Vanderkerken K, Lichtenstein A, Ganz T. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood. 2010;116:3635–44. doi: 10.1182/blood-2010-03-274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya S, Itoh T, Miura AB. Acquired pure red cell aplasia in Japan. Eur J Haematol. 1997;59(4):199–205. doi: 10.1111/j.1600-0609.1997.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Orchard J, Myint H, Hamblin TJ. A patient with myeloma who still has pure red cell aplasia despite the most intensive immune modulation. Leuk Res. 1997;21(4):353–354. doi: 10.1016/s0145-2126(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Peschle C, Marmont AM, Marone G, Genovese A, Sasso GF, Condorelli M. Pure red cell aplasia: studies on an IgG serum inhibitor neutralizing erythropoietin. Br J Haematol. 1975;30(4):411–417. doi: 10.1111/j.1365-2141.1975.tb01855.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar SS, Muhlfelder T. Antibodies to recombinant human erythropoietin causing pure red cell aplasia. Clin Nephrol. 1997;47(5):331–335. [PubMed] [Google Scholar]
- Sawada K, Hirokawa M, Fujishima N, Teramura M, Bessho M, Dan K, Tsurumi H, Nakao S, Urabe A, Omine M, Ozawa K. Long-term outcome of patients with acquired primary idiopathic pure red cell aplasia receiving cyclosporine A. A nationwide cohort study in Japan for the PRCA Collaborative Study Group. Haematologica. 2007;92(8):1021–1028. doi: 10.3324/haematol.11192. [DOI] [PubMed] [Google Scholar]
- Sharma S, Nemeth E, Chen YH, Goodnough J, Huston A, Roodman GD, Ganz T, Lichtenstein A. Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res. 2008;14(11):3262–3267. doi: 10.1158/1078-0432.CCR-07-4153. [DOI] [PubMed] [Google Scholar]
- Silvestris F, Tucci M, Cafforio P, Dammacco F. Fas-L up-regulation by highly malignant myeloma plasma cells: role in the pathogenesis of anemia and disease progression. Blood. 2001;97(5):1155–1164. doi: 10.1182/blood.v97.5.1155. [DOI] [PubMed] [Google Scholar]
- Sloand EM, Olnes MJ, Weinstein B, Wu C, Maciejewski J, Scheinberg P, Young NS. Long-term follow-up of patients with moderate aplastic anemia and pure red cell aplasia treated with daclizumab. Haematologica. 2010;95(3):382–387. doi: 10.3324/haematol.2009.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CC, Choi WW, Kwong YL. Pure red cell aplasia associated with CD20+ myeloma: complete remission with rituximab. Ann Hematol. 2013;92(10):1425–1426. doi: 10.1007/s00277-013-1721-5. [DOI] [PubMed] [Google Scholar]
- Voisin S, Hamidou M, Lefrancois A, Sigaud M, Mahe B, Trossaert M. Acquired von Willebrand syndrome associated with monoclonal gammopathy: a single-center study of 36 patients. Medicine (Baltimore) 2011;90(6):404–411. doi: 10.1097/MD.0b013e3182397166. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Wagner MV, Abbott JJ, Gibson LE. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145(3):279–284. doi: 10.1001/archdermatol.2008.583. [DOI] [PubMed] [Google Scholar]
- Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]