Abstract
Higher order structure (HOS) is a crucial determinant for the biological functions and quality attributes of protein therapeutics. Mass spectrometry (MS)-based protein footprinting approaches play an important role in elucidating the relationship between protein biophysical properties and structure. Here, we describe the use of a combined method including hydrogen-deuterium exchange (HDX), fast photochemical oxidation of proteins (FPOP) and site-specific carboxyl group footprinting to investigate the HOS of protein and protein complexes. The work focuses on implementing complementary solution-phase footprinting approaches that differ in time scale, specificity for protein residue side chains vs. backbone as well as selectivity for different residue types to map integratively the epitope of human interleukin-6 receptor (IL-6R) for two adnectins with distinct affinities (Kd, Adnectin1 ∼ 6.2 pM vs. Kd, Adenctin2 ∼ 46 nM), and evaluate the resultant conformation/dynamic change of IL-6R. The suggested epitope, which is conserved for adenctin1 and adenctin2 binding, is a flexible loop that connects two β-strands in the cytokine-binding domain (DII) of IL-6R. We also found that adnectin1, the more strongly binding ligand, induces structural perturbations on two unstructured loops that are distally located beyond the epitope. Those changes are either attenuated or not detected for the case of adnectin2 binding. In addition to providing credibility in epitope determination, utilization of those combined approaches reveals the structural effects that can differentiate protein therapeutics with similar apparent biophysical properties.
Graphical abstract
Introduction
HOS describes the three-dimensional arrangement of a protein structure required for biological function. Monitoring protein HOS is critical for understanding the impact of molecular conformation on biotechnological applications in the protein-discovery pipeline.1-2 Furthermore, maintaining HOS presents one of the key challenges for achieving robust and stable formulations of therapeutic proteins.3 For the design of monoclonal antibodies (mAbs) and other therapeutic protein products, protein HOS is essential because binding of the therapeutic to the target is based on the specific recognition of the epitope on the protein. This is not only related to the primary sequence but also to conformation and post-translational modifications.4
Although atomic-level mapping of a protein or a protein complex can be achieved by high-resolution X-ray crystallography, the resulting static structure may have limited biological relevance and not reveal solution phase dynamics or long-range protein-protein interactions.5-6 The complexity and low-throughput of X-ray crystallography restrict its application in the initial research stages where many potential therapeutic protein candidates may be of interest. Spectroscopy-based approaches including circular dichroism (CD),7 infrared (FTIR)8 and fluorescence spectroscopy9 provide quick, global measurements of protein conformation, but the profile obtained from those methods often contains no local or regional structural information. In contrast, protein footprinting, an evolving bioanalytical tool in structural biology, can reveal coarse-grained structural information relevant to proteins and their complexes. High sensitivity and fast data acquisition recommend MS-based footprinting for characterization of protein structure and macromolecular interactions at regional and even residue-specific levels of detail.10-12
Here, we describe a combination of MS-based protein footprinting methods, including hydrogen-deuterium exchange (HDX), fast photochemical oxidation of proteins (FPOP) and carboxyl group footprinting for mapping the extracellular region of human interleukin-6 receptor α-chain (referred as IL-6R hereafter) interacting with adnectins. Interleukin-6 (IL-6) plays critical roles in the pathogenesis of multiple myeloma, autoimmune diseases, and prostate cancer, appearing in abundant IL-6/IL-6R complexes.13 Inhibition of the IL-6/IL-6R complex is a primary goal to antagonize the action of IL-6 in vivo.14 The interacting partners of IL-6R selected for this study, adnectins, belong to a class of therapeutic proteins designed based upon the 10th human fibronectin type III domain.15 The two adnectins (adnectin1 and adnectin2) bind to IL-6R with picomolar and nanomolar affinity, respectively. X-ray structures of the IL-6R/adnectin complexes are not available, however, further motivating protein footprinting.
Among the methods to footprint IL-6R in this work, HDX is already well-established for protein HOS characterization.16-17 HDX occurs via formation of covalent bonds in a reversible manner.18 Its sensitivity to structural change is high provided there is little back exchange due to the labile nature of the N-D bond. HDX may be insensitive to subtle differences in conformation or dynamics when the exchange at the local region is low or rapid with respect to the HDX timescale.19
As an alternative to HDX, footprinting by incorporating irreversible modifications is emerging because it provides site-specific information by targeting amino acid side chains. Unlike HDX, irreversible labeling can survive extensive sample treatment and digestion. Footprinting by the hydroxyl radical, a common approach, involves irreversible oxidation of surface-accessible amino acid side chains as the primary product formation pathway. The radical probe has high reactivity with many residues, particularly those with sulfur-containing, aromatic, and aliphatic side chains.20 FPOP, the method used to generate hydroxyl radicals in this work, utilizes pulses of 248 nm KrF laser radiation to induce photolysis of hydrogen peroxide.21 By introducing a radical scavenger, usually a free amino acid, the lifetime of labeling with primary hydroxyl radicals can be controlled within microseconds.22 To date, FPOP has been implemented for HOS characterization of several therapeutic targets,23-26 showing its suitability for proteins of interest in drug discovery.
As a complement to free-radical footprinting, a variety of chemical reagents that target amino acid residues in a site-specific manner (e.g., N-ethylmaleimide27 and diethylpyrocarbonate28-29) can also provide information on site-specific solvent accessibility but react with protein substrates more slowly than do free radicals.30 In this work, we performed carboxyl group footprinting with glycine ethyl ester (GEE) to corroborate the findings from HDX and FPOP, taking advantage of the presence of the many Asp/Glu residues in the flexible loops of IL-6R. The chemical modification occurs for solvent-accessible Asp/Glu side chains (and the C-terminus) as a result of activation by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) through formation of an O-acylisourea intermediate, which is subsequently displaced by the amine group of a GEE molecule via nucleophilic attack.31 The labeling product, which is stable and remains intact in post-sample handling and digestion processes, can be quantified to determine the solvent accessibility at various carboxylic acid sites.32-33
In practice, application of protein footprinting to structural characterization projects including epitope mapping requires careful consideration of time and resource allocation because, at this stage, experiments still require considerable instrument and interpretation time. Questions arise whether time is better spent doing many replicates or time points with one approach or instead employing other complementary approaches each in a less rigorous manner. Although an exhaustive evaluation by one approach will often provide an answer, we have chosen an integrative course, and the results presented here demonstrate the value of applying that approach for epitope mapping and HOS characterization.
Experimental Section
Recombinant human IL-6R alpha extracellular region (residue 20-358, referred as IL-6R below) was purchased from R&D systems (Minneapolis, MN). Adnectin1 (Kd ∼ 6.2 pM) and adnectin2 (Kd ∼ 46 nM) were expressed and purified at BMS as previously described.34 All surface plasmon resonance (SPR) experiments for binding affinity measurement were performed using a Biacore T100 instrument (GE Healthcare) (details of SPR can be found in SI Materials and Methods). To form the IL-6R/adnectin complex, bound state IL-6R was prepared by mixing 50 μM IL-6R with adnectin1 or adnectin2 at a 1:1 molar ratio and incubated at room temperature for 1 h.
H/D exchange
HDX was performed by following a standardized protocol (see SI Materials and Methods). Briefly, a mapping experiment of IL-6R peptic peptides was performed under non-denaturing condition, and the common peptides identified were further monitored for their deuterium uptake levels with a Synapt G2 High Definition mass spectrometer (Waters). HDX was initiated by mixing the labeling buffer (10 mM phosphate buffer in D2O, pD 6.99) with the protein solution. The labeling reaction was allowed for different periods of times: 20 s, 1 min and 10 min.
FPOP
Prior to injection into the FPOP tubing, the protein sample in PBS was mixed with 20 mM H2O2 and 500 μM histidine. The final concentration of IL-6R for FPOP labeling was 10 μM. No dosimeter or reporter peptide was used (see SI for explanation). To avoid repeated laser exposure, the flow rate was adjusted to give ∼20% irradiation-excluded volume. The laser beam was from a KrF excimer laser (GAM Laser Inc.), providing an excitation wavelength of 248 nM to initiate H2O2 photolysis into hydroxyl radicals. After laser irradiation, the sample solution was collected in a tube containing 10 mM catalase and 20 mM Met to remove leftover H2O2 and prevent post-labeling oxidation artifacts. Control samples of IL-6R with all the reagents added (including H2O2) were handled in the same manner, but not laser-irradiated. Samples of each state were subjected to FPOP in triplicate.
Carboxyl Group Footprinting
For carboxyl group labeling, glycine ethyl ester (GEE), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) stock solutions were prepared freshly in PBS buffer. GEE was added to each pre-equilibrated sample to a concentration of 200 mM, followed by adding EDC to a concentration of 50 mM. The final concentration of IL-6R was 10 μM. In this reaction regime, the by-products of the reaction (e.g., Lys-Asp/Glu cross-links) were essentially eliminated because the excessive amount of GEE dominates the cross-linking reactions.35-36 Time-dependent labeling was carried out at room temperature and quenched at 1 min, 3 min and 10 min by adding an equal volume of 1 M ammonium acetate. Samples were further desalted using a Zeba column (Thermo Scientific, Rockford, IL).
Mass Spectrometry
FPOP or GEE-labeled protein was deglycosylated using PNGas F (New England Biolabs, Ipswich, MA) and digested using Trypsin/Lys-C or chymotrypsin (Promega, Madison, WI). In LC-MS/MS analysis, peptide fragments were separated on a custom-packed C18 column (CSH, 75 μm × 15 cm, 3.5 μm, 130 Å) using a Nano UltiMate 3000 Rapid Separation system (Dionex Co.) and analyzed with a Q Exactive Plus Orbitrap mass spectrometer (Thermo Scientific). The relative FPOP or GEE modification fraction was calculated by dividing the intensity of modified peptide/residue (Iox) by the summed intensity of modified and unmodified peptides (Iu) (i.e., fraction of modified = Iox/(Iox + Iu)). A detailed description of MS methodology and data analysis of FPOP and GEE footprinting is given in SI Materials and Methods.
Results and Discussion
HDX mapping
HDX is a widely used method for exploring protein conformation and monitoring protein-ligand interactions based on mapping the hydrogen bonding of protein backbone amide; a number of examples of using HDX MS for epitope mapping were reported.37-40 We first applied HDX to probe the structure of IL-6R and its complexes with the adnectins and found modest changes in the region 130-141 (Figure 1a). Although there is usually a more significant change in protection (reduction in HDX rates) of the amide backbone with epitope binding37-39, the results clearly suggest an epitope at this site. At this point, we considered repeating the HDX study and extending it over longer times with improved sequence coverage (78% residues mapped by peptic peptides (Figure S1)) by using other acid-insensitive proteolysis enzymes.41 We reasoned, however, that more time points or higher coverage would not alter our conclusion about the 130-141 region or address the distinctive affinities of adnectin 1 and adnectin 2. In fact, HDX kinetic curves often show convergence at longer times owing to fast off rates, suggesting that extending the time for HDX is not productive.24, 37, 39 Furthermore, all other detected regions follow identical HDX kinetics for the apo and holo forms (Figure 1b and Figure S1). We were thus motived to seek orthogonal footprinting methods to provide corroborating evidence for the epitope determination as well as to uncover conformational effects that may impact the binding of the two adnectins.
Figure 1.
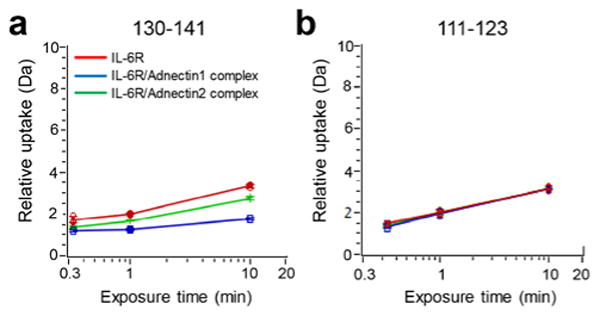
Representative HDX kinetics for IL-6R. (a) Region 130-141 shows reduced rates or extents of exchange in the holo (bound) states. (b) Region 111-123 shows no difference providing an example of a region that is not involved in or affected by adnectin binding.
FPOP mapping
FPOP is capable of reporting on protein transient dynamics, including fast folding42 and alteration in side-chain flexibility43. We also showed that FPOP reveals fast fluctuations occurring remotely upon ligand binding, which is undetectable by slower footprinting methods24. Thus, we applied FPOP as a probe with high sensitivity to monitor changes in structure and dynamics of IL-6R. To obtain structural resolution spanning the IL-6R sequence, we chose two separate proteolysis experiments with Lys-C/trypsin and chymotrypsin. In the LC-MS/MS analysis, peptides and their modifications were identified by relying on their accurate (< 5 ppm) mass and the product-ion spectra (see Figure S2 for an example). In the bottom-up strategy, all tryptic peptides, including those with one missed cleavage site, provide > 90% sequence coverage of IL-6R. By contrast, chymotryptic cleavage is less specific. We found that chymotryptic digestion of IL-6R provides a better overall regional resolution by yielding peptides with shorter average length, the signal intensities for some regions are dispersed among a greater number of overlapping chymotryptic fragments. With 80% sequence coverage from chymotrypsin digestion, we observed loss in the signal intensities for some chymotryptic peptides. This phenomenon is particularly pronounced for modified peptides of low abundance. Nevertheless, performing two sets of digestion experiments afforded a combined coverage of 96% of IL-6R sequence (Figure S3), permitting a detailed investigation into the local structure of IL-6R. In the data analysis, we only selected representative peptides that were relatively short and had desirable signal-to-noise ratios for accurate, label-free quantification (Table S1).
FPOP clearly shows differential modification of peptides from regions in IL-6R (Figure 2a and 2b). Some regions (e.g., 61-65, 119-126, 232-237) are “FPOP-silent”; that is, we could detect no FPOP modification even though we saw signals from the unmodified peptides. We found small modification extents for other regions that are either shielded in the inner core of IL-6R (PDB: 1N26) and not solvent-accessible, or mainly composed of residues less reactive to hydroxyl radicals (e.g. Glu, Ser, Lys and Thr). By contrast, we observed high levels of modifications (modified fraction > 50%) for the C-terminus (288-296 and 301-319), because this region not only contains highly reactive Met292, Trp296 and Met312 residues but also diversified loops and random coils that are inherently flexible and expected to be reactive with short-lived radicals.
Figure 2.
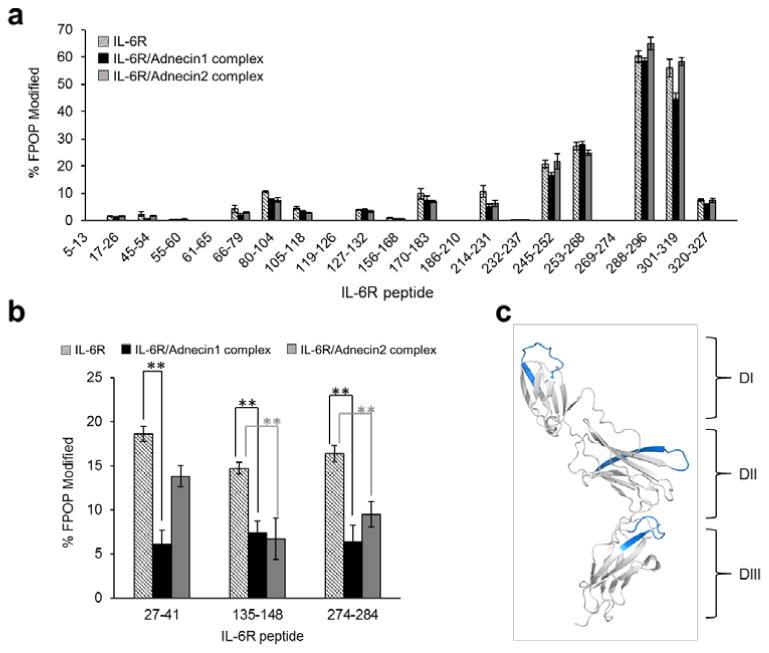
FPOP modification extents of IL-6R peptide regions and the locations of the regions showing altered solvent accessibility. (a) Regions of IL-6R without significant change in their extent of FPOP modification. (b) Regions of IL-6R with significantly decreased (**Δrel. > 40%, p < 0.005) FPOP modification, indicating reduced solvent accessibility upon adnectin1/adnectin2 binding. (c) Regions in (b) mapped onto IL-6R (PDB: 1N26) are colored blue. The three domains of IL-6R are referred as DI, DII and DIII on the structure.
Regions 27-41, 274-284 and 135-148 in adnectin1-bound IL-6R, and regions 274-284, 135-148 in adnectin2-bound IL-6R show remarkably decreased FPOP modification (relative difference > 40%, p < 0.005 in Student's t-test) (Figure 2b), suggesting those regions undergo major conformational changes introducing reduced solvent accessibility upon adnectin binding (Table 1 summarizes the FPOP modifications of those regions exhibiting statistically significant differences in solvent accessibility upon adnectin binding). We also observed minor differences in FPOP modification for region 301-319 in the adnectin1-bound state, which is likely attributed to minor structural or dynamical perturbation on this region upon adenctin1 binding. The 3D structure of IL-6R consists of three domains including the N-terminal Ig-like domain (DI) and two cytokine-binding domains (DII and DIII). All the regions for which solvent accessibility is significantly altered in FPOP adopt a flexible loop structure (Figure 2c). In addition, we found the overlapping tryptic and chymotryptic peptides (e.g., tryptic peptide 133-154 and chymotryptic peptide 135-148) reveal correlated trends of FPOP modification change (Figure S5). This indicates that the observed difference is due to structural changes of IL-6R in the holo states, instead of structure-based proteolytic bias caused by the FPOP modification.
Table 1.
Summary of the regions showing significant changes in conformation and/or dynamics identified by FPOP.
| Ligand | IL-6R peptide | Sequence | Relative difference (%) | p-value |
|---|---|---|---|---|
| Adnectin1 | 27-41 | TCPGVEPEDNATVHW | 67 | 0.001 |
| 274-284 | RAQEEFGQGEW | 61 | 0.004 | |
| 135-148 | QNSPAEDFQEPCQY | 50 | < 0.001 | |
|
| ||||
| Adnectin2 | 135-148 | QNSPAEDFQEPCQY | 54 | 0.001 |
| 274-284 | RAQEEFGQGEW | 42 | 0.004 | |
A significant advantage of FPOP is that it provides residue-level information (Figure S6). His40 and Trp41 are the residues that are predominantly modified by FPOP in region 27-41 (TCPGVEPEDNATVHW). The two residues are located at the front end of a β-stand connected to the loop. The side chain of His40 is exposed on the protein surface, and the aromatic side chain of Trp41 is largely protected inside the protein core. Note that the amino acid residues from the sequence that compose the loop (30GVEPEDNAT38) on region 27-41 are much less reactive than His and Trp, with the most reactive Val and Pro being ∼ 20× and 6× less reactive compared to Trp and His, respectively.20 Therefore, the hydroxyl radical preferentially modifies His40 and Trp41 rather than the less reactive ones from the loop. The local selectivity is also pronounced for some Met containing regions (e.g., 245-252 and 288-296). Moreover, the product-ion spectrum does not definitively indicate what residue is modified on region 274-284, but it does show that the FPOP modification occurs on either Pro138, Phe142, Pro145 or Tyr148.
Although peptide regions in Table 1 undergo significantly reduced FPOP modification in the holo states, one should be cautious in interpreting the data because the change in FPOP extent may result either from the epitope binding, or from decreased dynamics and flexibility induced remotely from the adnectin binding site. For the residues with high intrinsic reactivity with the hydroxyl radical, a relatively modest change in their solvent accessibility in response to the dynamic motion can result in a dramatic difference in their FPOP modification.44-45 Our hypothesis is that dynamic motions occurring within the sub-second time range will be differentiated by fast labeling but not by slow labeling that presents an averaged view over seconds, and applying orthogonal footprinting can distinguish binding from remote dynamics change.
There are ways to improve the confidence in the FPOP experiment. For example, the footprinting can be done as a function of time to give outcomes similar to those typically obtained for HDX. Assigning differences can be elaborated further with kinetic curves46 (e.g., five time points) rather than single point, but this requires considerable investments in LC/MS/MS analysis time, data processing, and interpretation as well as more sample. At this point, we decided to turn instead to another type of footprinting.
Carboxyl group footprinting
The effectiveness of protein footprinting to map the epitope and conformational changes depends on whether the reagent-active residues are located on those regions.12 Although residue-specific labeling provides less structural information due to limited numbers of target residues on the protein surface, fortuitously, the regions of IL-6R with significant changes in the solvent accessibility mapped by FPOP (27TCPGVEPEDNATVHW41, 274QNSPAEDFQEPCQY284 and 135RAQEEFGQGEW148) are rich in Asp/Glu as potential GEE modification sites, and we expect carboxyl group footprinting, as an orthogonal approach to provide more site-specific insights for those acidic regions.
In the GEE reaction, carboxyl groups of Asp/Glu located on the surface are readily modified, whereas ones surrounded by hydrophobic amino acids or buried in the interior of the structure undergo less or even no modifications (assuming there is no additional steric restrictions prohibiting the access of EDC and the GEE molecules).33, 47 To investigate the kinetics of the labeling, we performed sparse, time-dependent GEE footprinting (1, 3 and 10 min) on the apo and adnectin-bound IL-6R. Given that for 16 Asp/Glu-containing chymotryptic peptides, 17-26, 109-134, 247-254, 255-264 and 297-315 have no detectable modification, we performed quantification for the other 11 peptides.
From the time-dependent GEE footprinting, we found two regions 135-148 on DII and 274-284 on DIII show a clear difference upon binding of either adnectin1 or adnectin2 (Figure 3a and 3b). The two regions are mapped onto the IL-6R structure in Figure 3d. The rates of GEE incorporation for region 135-148 in the two holo states decrease significantly with a relative change of 30% at 10 min of labeling, and the differences are made more apparent by the time-dependent results, indicating prominently decreased solvent accessibility of the region upon adnectin binding. Region 135-148 contains Glu140, Asp141 and Glu144 as possible GEE modification sites, but we found the modification is exclusively on Glu140. The structure of IL-6R indicates that the GEE-modified Glu140 is on the surface of a loop with a flexible carboxyl group amenable to EDC/GEE reaction (Figure 3e). By contrast, Asp141 is involved in the front end of a β-strand with its side-chain hydrogen bonded to Arg132, and Glu144 is also located on the same β-strand with its side chain occluded by surrounding residues Phe142, Gln158 and Leu159. The GEE labeling reaction requires activation of the carboxyl group by EDC, and the sizes of EDC and GEE are larger than the small reagents in FPOP and HDX, suggesting steric requirements for the reaction. We reason that although Asp/Glu as charged residues are often prone to be on the protein surface, their solvent accessibility can be largely diminished or even completely blocked by their microenvironments.
Figure 3.
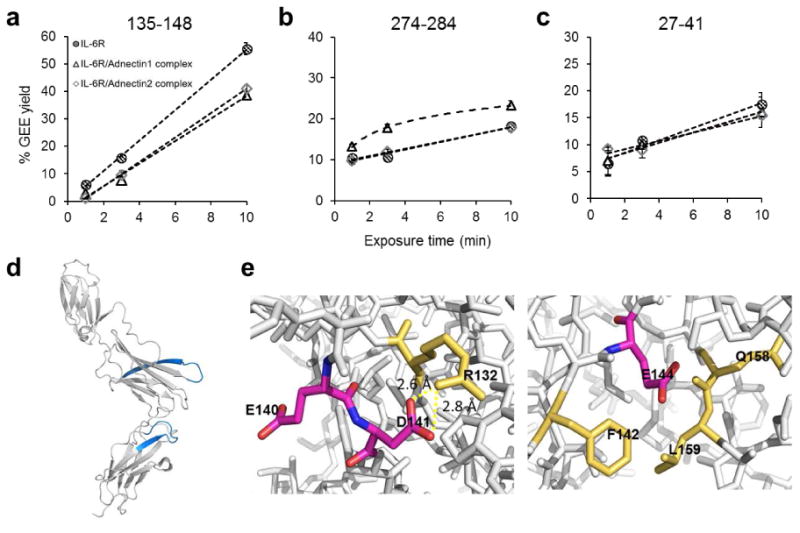
GEE labeling kinetics for selected IL-6R peptides in the ligand-free (gridded circle), adnetin1-bound (triangle) and adnetin2-bound state (diamond) state. (a) Region 135-148 shows decreased GEE incorporation upon adnectin1/adnectin2 binding, whereas (b) region 274-284 shows increased GEE modification upon adnectin1 binding. (c) A representative peptide region without differentiable GEE modification extent between apo and holo as the control. Dashed trend curves in (a), (b) and (c) are generated by linear or 2nd-degree polynomial fitting. (d) Region 135-148 and 274-284 mapped onto IL-6R. (e) Microenvironments of Glu140, Asp141 and Glu144.
We found region 247-284 to be slightly deprotected in the adnectin1-bound state (Figure 3b) as reported for the acidic residues. Due to a steric effect similar to that described above, Glu283, which is located on the unshielded surface loop, is readily modified by GEE. Glu277 and Glu278, however, which are located on or close to the end of a β-strand, remain unmodified owing to the protection from the surrounding loops and hydrogen bonds. The increased solvent accessibility can thus be attributed to the side chain of Glu283. Interestingly, residue-level analysis of FPOP modifications on the same region shows that the Phe279/Trp284 side-chain solvent accessibilities are reduced (Figure S6), and the analysis and interpretation for the motion of the region will be discussed later in section 3.4.2.
By contrast, a majority of peptides, as represented by 27-41, show nearly identical labeling kinetics for bound and unbound states, supporting their role as controls and suggesting their local conformations remain unchanged upon adnectin binding (Figure 3c and Figure S7). The labeling extents of those regions at 10 min, at which time the difference in modification is expected to be greatest, are not differentiable (Figure S8). Unlike FPOP labeling, which is often performed over a single exposure time, kinetic curves of GEE labeling provide statistical weight by tracking the labeling over a time course. We also found the GEE modification extent generally increases with the reaction time, but the labeling of some peptide regions can occur very rapidly. For example, regions 1-16, 288-296 and 316-326 show bursts in their GEE incorporation at the first 1 min of the reaction (Figure S7), indicative of their flexible loop or coil secondary structures.
Structural features from complementary footprinting
HDX rates of exchange of labile amide hydrogens are characteristic of local backbone conformations and related to its hydrogen-bonding pattern and solvent accessibility, which are affected by protein binding. In highly dynamic, unstructured regions, the exchange reaction proceeds on the millisecond to second timescale, whereas amides that are hydrogen bonded will exchange more slowly (minutes to days).48 With comparable reaction rates to HDX, GEE targets solvent-accessible carboxyl group side chains. Labeling by FPOP, however, is considerably faster than HDX and GEE because the lifetime is microseconds for the primary hydroxyl radical and milliseconds for all radicals.22, 49 Hydroxyl radicals react with a variety of amino acid residues, and the labeling extent of a particular residue site is a function of the inherent reactivity and solvent accessibility of the amino acid side chain. Clearly the approaches are complementary in location of footprinting (protein backbone vs. side chain), residue specificity, and rate of reaction. The more rapid approaches offer an opportunity to understand protein dynamics and minor structural fluctuations.43
The epitope and critical contacting residues
In FPOP and GEE footprinting, region 135-148 becomes significantly protected upon binding of adnectin1 or adnectin2, whereas HDX focuses on region 130-141 (region 142-147 does not change--Figure S1). Taken together, the integrated outcome strongly suggests the short segment 135QNSPAED141 to contain the critical binding epitope. The suggested epitope is conserved for adnectin1 and adenctin2 binding. Mapping the proposed epitope onto the IL-6R structure highlights a loop on the DII domain of IL-6R (Figure 4).
Figure 4.
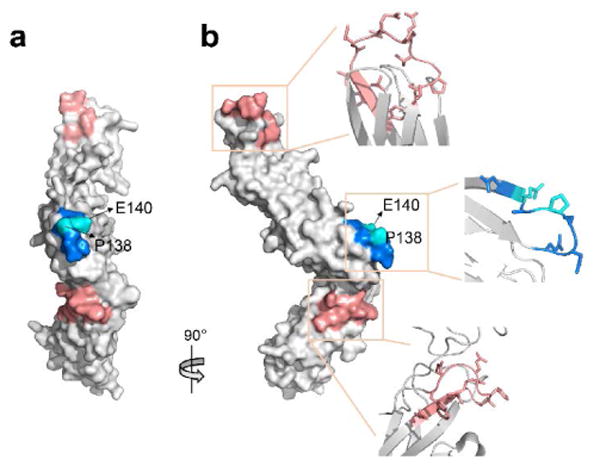
Structural change of IL-6R (PDB: 1N26) upon adnectin binding in the surface representation. IL-6R consists of three domains (DI, DII and DIII, located from the top to the bottom on the structure). (a) Front view and (b) side view of the regions that undergo conformational change in structure or dynamics upon adnectin binding. Close-up views of the region in (b) shows the secondary structure of the region and the side chain orientation. Region 135-141 as the proposed epitope for adentin1/adnectin2 binding are colored in blue with Pro138 and Glu140 highlighted in cyan. Region 27-41 and 274-284 (located on DI and DIII respectively) whose conformation is affected by adnectin binding are colored in coral.
A closer examination of the residue-specific data may reveal the contacting residues. Generally, FPOP modification on a residue is assigned when a +15.9949 Da shift is observed in the product-ion series of the modified peptide. For peptide 135-148, each of FPOP modified residues Pro138/Phe142/Pro145/Tyr148 produces multiple +16 Da products as structural isomers (e.g., by o-/m-/p- oxidation of Phe). Those isomers of peptide 135-148 cannot be distinguished by mass and give very slightly different retention times. The complicated chromatogram for oxidized peptide 135-148 makes FPOP quantification of a specific residue difficult. As for the suggested epitope 135-141, however, we identified Pro138 to be the modified residue responsible for the changes in FPOP. Carboxyl group footprinting also slows for the proposed epitope, but the information is restricted to Glu140 as it is the only residue modified in region 135-148. Although there are Asp141 and Glu144 as potential GEE modification sites in this region, no modification is detected for the two residues owing to shielding of their microenvironments as discussed above.
Summarizing the information from the complementary methods, we posit that 135QNSPAED141 represents the region containing the epitope of IL-6R, with Pro138 and Glu140 being possible binding residues or closely adjoining the critical binding residues (Figure 4 shows Pro138 and Glu140 in the surface presentation with side chains extruding). At this time, we cannot rule out the residues on region 135-141 that are not mapped by the hydroxyl radical or GEE probe (e.g., Gln 135, Asn 136, Ser137 and Ala139), but we can potentially identify some key interacting residues as well as narrow down the epitope to a short segment by FPOP and GEE-reactive residues that are involved in binding. Note that HDX fails to report the backbone solvent accessibility of Pro138 owing to its lack of an amide hydrogen atom, which may account for why the putative epitope region 130-141 only shows a modest protection in HDX, despite the two adnectins both being high-affinity ligands against IL-6R.
Conformational dynamics and side-chain motion of IL-6R loop regions
Protein structure fluctuations will be affected by protein binding.50 If the sampling time of a footprinting reaction is long with respect to the protein dynamics, differences will be averaged and no effect seen. FPOP is capable of reporting on regions showing fast dynamics because its sampling time is short with respect to local motions.19, 42, 51 In the adnectin1-bound IL-6R, the decreased solvent accessibility reported by FPOP for region 27-41 is not observed by HDX or carboxyl group footprinting, indicative of fast changes in the conformational dynamics of region 27-42 upon adenctin1 binding. Region 27-41 is located on the DI domain of IL-6R as a surface-exposed loop (Figure 4b) where changes in dynamics are likely to happen.
For amino-acid residues with high intrinsic reactivities with the hydroxyl radical, drastic changes in their modification occur in response to modest change in the solvent accessibility,45 whereas differences in modification extent of less reactive residues (e.g., Leu, Pro and Val) is expected to be observed for large conformational changes. His40 and Trp41 are the only two residues modified by FPOP in region 27-42, and they do exhibit dramatic differences in FPOP modification with a relatively decrease of 67% in the adenctin1-bound state. The observed protection on the two residues with high susceptibility to the hydroxyl radical suggests relatively modest changes in the structure or dynamics upon adnectin-1 binding. Considering the binding strength of adnectin1 (Kd ∼ 6.2 pM) is greater than that of adnectin2 (Kd ∼ 46 nM), we posit that the binding of adnectin1 stabilizes IL-6R more by reducing the local flexibility of region 27-41.
Interestingly, in contrast to the decreased FPOP modification of residue Phe279/Trp284 in region 274-284 in adnectin1 bound IL-6R, the GEE labeling of Glu283 increases slightly for the adnectin1-bound state, but not for adnectin2, whereas no difference in HDX occurs for this region. This suggests that region 274-284 undergoes minor structural perturbation in dynamic and/or sidechain reorientation upon adnectin binding, which may not cause changes in the amide hydrogen-binding pattern. Considering the hydrophobicity of Phe/Trp and the polarity of Glu, it is possible that the binding causes side-chain motions involving inward rotation of Phe279/Trp284 with its solvent accessibility decreasing, whereas the side chain of Glu283 rotates outward and becomes relatively solvent exposed. These solution-state motions would not be reflected in a single static 3D-structure. Furthermore, region 274-284 spans a flexible loop, where side-chain rotation is more facile than for a more rigid α-helix or β-sheet. This may have implication in the low-affinity binding of IL-6 to IL-6R that precedes the binding to a signal-transducing molecule gp130 to form high-affinity functional complex.52-53 An investigation into the structure of the IL-6R/IL6/gp130 complex (PDB: 1P9M) further reveals that the loop region represented by peptide 274-284 serves as one of the interfaces with IL-6 in this biologically-relevant complex (Figure S9). The structural changes adopted by this region may be inferred by the therapeutic efficacy of the adnectins to block IL-6-mediated signal transduction through inhibiting the binding of IL-6 to IL-6R.
Conclusion
Solution-state protein-protein interactions and related conformational changes can be interrogated with high spatial resolution by using orthogonal footprinting and structural mapping. We proposed the epitope of IL-6R is in the region 135-141 and concluded that adnectin binding affects fast dynamics and side-chain reorientation of some of IL-6R's flexible loops. Those “hidden” motions in structure and/or dynamics are invisible to a relatively slow footprinting method like HDX. The results support a more comprehensive understanding of IL-6R HOS and highlight the sensitivity of FPOP towards fast structural changes owing to the short half-life of hydroxyl radicals and higher coverage compared to HDX and site-specific carboxyl group footprinting. Their combined use not only serves to categorize and interpret changes in footprinting as due to protection from binding or to remote structural changes occurring with binding, but also adds confidence to assign the epitope where any stand-alone method has uncertainty. This integrated approach shows great utility for charactering protein and protein complex, which can be applied efficiently to assist understanding and optimizing the design of protein therapeutics.
Supplementary Material
Acknowledgments
We thank research supported by grants NIH NIGMS (8P41 GM103422) (for MLG), and Dr. Sharon Cload and Dr. Bruce Car from Bristol-Myers Squibb for their support of this project.
Associated contents: Supporting Information: Experimental procedures for SPR affinity measurement, H/D exchange, mass spectrometry, quantification of the FPOP/GEE modification level; H/D exchange coverage and complete kinetic curves; example of identification of FPOP modification in LC-MS/MS; IL-6R sequence coverage; investigation of effects of minor modification and digestion on FPOP quantification; FPOP modification for IL-6R residues; representative GEE labeling kinetics for control peptides; 3D-structure of the IL-6/IL-6R/gp130 hetero-trimers.
References
- 1.Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianférani S. Anal Chem. 2013;85(2):715–736. doi: 10.1021/ac3032355. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Warrack BM, Goodenough AK, Wei H, Wang-Iverson DB, Tymiak AA. Drug Discov Today. 2011;16(1-2):58–64. doi: 10.1016/j.drudis.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Weiss WFt, Gabrielson JP, Al-Azzam W, Chen G, Davis DL, Das TK, Hayes DB, Houde D, Singh SK. J Pharm Sci. 2016;105(12):3465–3470. doi: 10.1016/j.xphs.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Beck A, Wurch T, Bailly C, Corvaia N. Nat Rev Immunol. 2010;10(5):345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 5.Acharya KR, Lloyd MD. Trends Pharmacol Sci. 2005;26(1):10–14. doi: 10.1016/j.tips.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Davis AM, Teague SJ, Kleywegt GJ. Angew Chem Int Ed. 2003;42(24):2718–2736. doi: 10.1002/anie.200200539. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield NJ. Nature protocols. 2006;1(6):2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong J, Yu S. Acta Biochimica et Biophysica Sinica. 2007;39(8):549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 9.Tcherkasskaya O, Bychkova VE, Uversky VN, Gronenborn AM. J Biol Chem. 2000;275(46):36285–36294. doi: 10.1074/jbc.M003008200. [DOI] [PubMed] [Google Scholar]
- 10.Huang RYC, Chen G. Anal Bioanal Chem. 2014;406(26):6541–6558. doi: 10.1007/s00216-014-7924-3. [DOI] [PubMed] [Google Scholar]
- 11.Jones LM, Sperry JB, Carroll JA, Gross ML. Anal Chem. 2011;83(20):7657–7661. doi: 10.1021/ac2007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wecksler AT, Kalo MS, Deperalta G. J Am Soc Mass Spectrom. 2015;26(12):2077–2080. doi: 10.1007/s13361-015-1273-0. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. Clinical Science. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard JP, Mani JC, Liautard J, Klein B, Brochier J. European Cytokine Network. 1999;10(1):43–48. [PubMed] [Google Scholar]
- 15.Lipovsek D. Protein Eng Des Sel. 2011;24(1-2):3–9. doi: 10.1093/protein/gzq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins DJ, Espada A, Novick SJ, Molina-Martin M, Stites RE, Espinosa JF, Broughton H, Goswami D, Pascal BD, Dodge JA, Chalmers MJ, Griffin PR. Anal Chem. 2016;88(12):6607–6614. doi: 10.1021/acs.analchem.6b01650. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Zhang A, Xiao G. Anal Chem. 2012;84(11):4942–4949. doi: 10.1021/ac300535r. [DOI] [PubMed] [Google Scholar]
- 18.Engen JR, Wales TE. Annual Review of Analytical Chemistry. 2015;8(1):127–148. doi: 10.1146/annurev-anchem-062011-143113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gau B, Garai K, Frieden C, Gross ML. Biochemistry. 2011;50(38):8117–8126. doi: 10.1021/bi200911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu G, Chance MR. Chem Rev. 2007;107(8):3514–3543. doi: 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- 21.Hambly DM, Gross ML. J Am Soc Mass Spectrom. 2005;16(12):2057–63. doi: 10.1016/j.jasms.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Gau BC, Sharp JS, Rempel DL, Gross ML. Anal Chem. 2009;81(16):6563–6571. doi: 10.1021/ac901054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Wecksler AT, Molina P, Deperalta G, Gross ML. J Am Soc Mass Spectrom. 2017:1–9. doi: 10.1007/s13361-017-1601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Wei H, Krystek SR, Bond D, Brender TM, Cohen D, Feiner J, Hamacher N, Harshman J, Huang RYC, Julien SH, Lin Z, Moore K, Mueller L, Noriega C, Sejwal P, Sheppard P, Stevens B, Chen G, Tymiak AA, Gross ML, Schneeweis LA. Anal Chem. 2017;89(4):2250–2258. doi: 10.1021/acs.analchem.6b03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y, Chen G, Wei H, Huang RYC, Mo J, Rempel DL, Tymiak AA, Gross ML. J Am Soc Mass Spectrom. 2014;25(12):2084–2092. doi: 10.1007/s13361-014-0993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li KS, Rempel DL, Gross ML. J Am Chem Soc. 2016;138(37):12090–12098. doi: 10.1021/jacs.6b07543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan L, Ronald Kaback H. Nat Protocols. 2007;2(8):2012–2017. doi: 10.1038/nprot.2007.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borotto NB, Zhou Y, Hollingsworth SR, Hale JE, Graban EM, Vaughan RC, Vachet RW. Anal Chem. 2015;87(20):10627–10634. doi: 10.1021/acs.analchem.5b03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Vachet RW. J Am Soc Mass Spectrom. 2012;23(4):708–17. doi: 10.1007/s13361-011-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendoza VL, Vachet RW. Mass Spectrom Rev. 2009;28(5):785–815. doi: 10.1002/mas.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Wen J, Huang RYC, Blankenship RE, Gross ML. Int J Mass spectrom. 2012;312:78–86. doi: 10.1016/j.ijms.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur P, Tomechko SE, Kiselar J, Shi W, Deperalta G, Wecksler AT, Gokulrangan G, Ling V, Chance MR. mAbs. 2015;7(3):540–552. doi: 10.1080/19420862.2015.1023683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Zhang H, King JD, Wolf NR, Prado M, Gross ML, Blankenship RE. Biochim Biophys Acta. 2014;1837(12):1955–1963. doi: 10.1016/j.bbabio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Ramamurthy V, Krystek Jr, Stanley R, Bush A, Wei A, Emanuel Stuart L, Das Gupta R, Janjua A, Cheng L, Murdock M, Abramczyk B, Cohen D, Lin Z, Morin P, Davis Jonathan H, Dabritz M, McLaughlin Douglas C, Russo Katie A, Chao G, Wright Martin C, Jenny Victoria A, Engle Linda J, Furfine E, Sheriff S. Structure. 2012;20(2):259–269. doi: 10.1016/j.str.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Liu H, Blankenship RE, Gross ML. J Am Soc Mass Spectrom. 2016;27(1):178–181. doi: 10.1007/s13361-015-1260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collier TS, Diraviyam K, Monsey J, Shen W, Sept D, Bose R. J Biol Chem. 2013;288(35):25254–25264. doi: 10.1074/jbc.M113.474882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen E, Salinas ND, Huang Y, Ntumngia F, Plasencia MD, Gross ML, Adams JH, Tolia NH. Proceedings of the National Academy of Sciences. 2016;113(22):6277–6282. doi: 10.1073/pnas.1600488113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei H, Mo J, Tao L, Russell RJ, Tymiak AA, Chen G, Iacob RE, Engen JR. Drug Discov Today. 2014;19(1):95–102. doi: 10.1016/j.drudis.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malito E, Faleri A, Lo Surdo P, Veggi D, Maruggi G, Grassi E, Cartocci E, Bertoldi I, Genovese A, Santini L, Romagnoli G, Borgogni E, Brier S, Lo Passo C, Domina M, Castellino F, Felici F, van der Veen S, Johnson S, Lea SM, Tang CM, Pizza M, Savino S, Norais N, Rappuoli R, Bottomley MJ, Masignani V. Proc Natl Acad Sci U S A. 2013;110(9):3304–9. doi: 10.1073/pnas.1222845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Willison LN, Tripathi P, Sathe SK, Roux KH, Emmett MR, Blakney GT, Zhang HM, Marshall AG. Anal Chem. 2011;83(18):7129–36. doi: 10.1021/ac201501z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HM, Kazazic S, Schaub TM, Tipton JD, Emmett MR, Marshall AG. Anal Chem. 2008;80(23):9034–9041. doi: 10.1021/ac801417d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Rempel DL, Gau BC, Gross ML. J Am Chem Soc. 2012;134(45):18724–31. doi: 10.1021/ja307606f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart KM, Ho CMW, Dutta S, Gross ML, Bowman GR. Nature Communications. 2016;7:12965. doi: 10.1038/ncomms12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Moniz H, Wang S, Ramiah A, Zhang F, Moremen KW, Linhardt RJ, Sharp JS. The Journal of Biological Chemistry. 2015;290(17):10729–10740. doi: 10.1074/jbc.M115.648410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charvátová O, Foley BL, Bern MW, Sharp JS, Orlando R, Woods RJ. J Am Soc Mass Spectrom. 2008;19(11):1692–1705. doi: 10.1016/j.jasms.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu B, Mackness BC, Rempel DL, Zhang H, Cui W, Matthews CR, Zitzewitz JA, Gross ML. Journal of the American Society for Mass Spectrometry. 2017;28(2):389–392. doi: 10.1007/s13361-016-1552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen J, Zhang H, Gross ML, Blankenship RE. Proceedings of the National Academy of Sciences. 2009;106(15):6134–6139. doi: 10.1073/pnas.0901691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Englander SW. J Am Soc Mass Spectrom. 2006;17(11):1481–1489. doi: 10.1016/j.jasms.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vahidi S, Konermann L. J Am Soc Mass Spectrom. 2016;27(7):1156–1164. doi: 10.1007/s13361-016-1389-x. [DOI] [PubMed] [Google Scholar]
- 50.Keskin O. BMC Struct Biol. 2007;7(1):31. doi: 10.1186/1472-6807-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hart KM, Ho CM, Dutta S, Gross ML, Bowman GR. Nat Commun. 2016;7:12965. doi: 10.1038/ncomms12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulanger MJ, Chow Dc, Brevnova EE, Garcia KC. Science. 2003;300(5628):2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 53.Ward LD, Howlett GJ, Discolo G, Yasukawa K, Hammacher A, Moritz RL, Simpson RJ. J Biol Chem. 1994;269(37):23286–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


