Abstract
Tuberous Sclerosis Complex is one example of a syndromic form of autism spectrum disorder associated with disinhibited activity of mTORCl in neurons (e.g., cerebellar Purkinje cells). mTORCl is a complex protein possessing serine/threonine kinase activity and a key downstream molecule in a signaling cascade beginning at the cell surface with the transduction of neurotransmitters (e.g., glutamate and acetylcholine) and nerve growth factors (e.g., Brain-Derived Neurotrophic Factor). Interestingly, the severity of the intellectual disability in Tuberous Sclerosis Complex may relate more to this metabolic disturbance (i.e., overactivity of mTOR signaling) than the density of cortical tubers. Several recent reports showed that rapamycin, an inhibitor of mTORCl, improved sociability and other symptoms in mouse models of Tuberous Sclerosis Complex and autism spectrum disorder, consistent with mTORCl overactivity playing an important pathogenic role. NMDA receptor activation may also dampen mTORCl activity by at least two possible mechanisms: regulating intraneuronal accumulation of arginine and the phosphorylation status of a specific extracellular signal regulating kinase (i.e., ERK1/2), both of which are “drivers” of mTORCl activity. Conceivably, the prosocial effects of targeting the NMDA receptor with agonists in mouse models of autism spectrum disorders result from their ability to dampen mTORC1 activity in neurons. Strategies for dampening mTORC1 overactivity by NMDA receptor activation may be preferred to its direct inhibition in chronic neurodevelopmental disorders, such as autism spectrum disorders.
Keywords: D-Cycloserine, mTOR, NMDA receptor, Sociability, Tuberous sclerosis
1. Mouse models of Tuberous Sclerosis Complex
Tuberous Sclerosis Complex (TSC), a syndromic form of autism spectrum disorder (ASD) due to the effects of single major genes, has stimulated interest in a metabolic contribution to impaired sociability, intellectual disability and seizures seen in nonsyndromic forms of ASDs (Cambiaghi et al., 2013; Ehninger, 2013; Ehninger and Silva, 2011; Meikle et al., 2007, 2008; Tsai et al., 2012). TSC is an autosomal dominant disorder resulting from mutations in either the TSC1 or TSC2 gene, whose protein products (i.e., hamartin or tuberin, respectively) form a heterodimer. The heterodimeric TSC protein is an important negative regulator of mammalian target of rapamycin complex 1 (mTORC1), which possesses serine/threonine kinase activity and regulates mRNA translation in the periphery of neurons, among other functions (Crino, 2011; Ehninger, 2013; Talos et al., 2012). Ordinarily, the heterodimeric TSC protein inhibits the activity of Rheb (Ras homolog enriched in brain), whose function is to activate mTORCl. Thus, genetic mutations of hamartin or tuberin result in disinhibition of Rheb and (over)activation of mTOR. Disinhibited mTORC1 activity may contribute significantly to the social impairment, cognitive deficits and comorbid seizures of patients with TSC, an autosomal dominant disorder; comorbid presentations of ASDs are seen in about 25–60% of patients with TSC (Ehninger, 2013; Ehninger and Silva, 2011; Sahin, 2012). Thus, targeting the primary metabolic disturbance of TSC (i.e., mTORC1 overactivity) may improve core neuropsychiatric symptom domains even in the presence of cortical tubers in brain; in fact, there are data to suggest that the density of cortical tubers is not solely responsible for the shift to the left in IQ scores observed in these patients (Ehninger and Silva, 2011). Importantly, in addition to TSC, other syndromic forms of ASDs due to major effects of single genes that impact mTOR signaling have been well-described (Brodkin, 2008; Garg et al., 2014; Kim et al., 2015; Peça and Feng, 2012; Sharma et al., 2010; Won et al., 2012). For example, mutations of the gene for the ‘phosphatase and tensin homolog (PTEN)’ are associated with human cancers, extreme macrocephaly and ASDs, whose pathogeneses include disruption of metabolic regulation of mTOR signaling pathways (Buxbaum and Hof, 2012). Given the fact that mTOR signaling pathways affect an array of cellular functions, including cell cycle kinetics, cellular differentiation, cell growth, cell survival, metabolism and protein synthesis, it is not surprising that genetic variations of genes coding for upstream regulators and downstream effectors of mTORCl have been associated with ASDs (Crino, 2011; Hoeffer and Klann, 2010).
Consistent with the pathogenic role of mTORC1 overactivity, several proof of concept/proof of principle studies showed that treatment with rapamycin attenuated severity of behavioral and neuropathological findings in transgenic mouse models of TSC (Meikle et al., 2008; Sahin, 2012; Talos et al., 2012; Tsai et al., 2012). Additionally, there are data from relevant mouse models of TSC to suggest that addressing the metabolic lesion (i.e., disinhibited mTOR signaling activity) may have beneficial effects on behavior in “adolescent” mice (Ehninger, 2013; Talos et al., 2012). Because TSC is a chronic disease, an ideal strategy to dampen mTOR overactivity must be effective, safe and tolerable upon sustained long-term administration.
Increased levels of mTOR activity are found in transgenic mice with absent expression of TSC1 in cerebellar Purkinje cells; these mutant mice show deficits of social interaction, impairments of spatial memory and cerebellar abnormalities (Tsai et al., 2012). Importantly, postnatal chronic treatment with rapamycin (6 mg/kg), an inhibitor of mTORCl, improved sociability, spatial working memory, and increased the number of cerebellar Purkinje cells to levels observed in the wild type controls (Tsai et al., 2012). A recently described transgenic knockout mouse model of TSC (Tsc1null-neuron), lacks TSC1 expression in most neurons starting at embryonic day 13 and exhibits well-characterized TSC mutant phenotypes and deficits similar to those seen in patients with TSC pathology (Meikle et al., 2007, 2008). For instance, these mice develop enlarged and dysplastic neurons, and show reduced myelination and overactivation of phosphorylated-S6, a ribosomal protein located downstream of mTORCl. Importantly, postnatal treatment with rapamycin (6 mg/kg) in this model also dramatically improved the TSC phenotype (Meikle et al., 2008).
2. NMDA receptor regulates normal sociability
The NMDA receptor is an example of a highly regulated glutamategated cationic channel receptor; the receptor is a heteromeric protein complex constructed from four constituent polypeptide subunits (Millan, 2005; Millan et al., 2014). The likelihood that the binding and cooperative interaction of glutamate and the obligatory glycine co-agonist will result in channel opening depends on the unique combination of polypeptide receptor subunits (i.e., combinatorial diversity), the extent to which intracellular domains are phosphorylated, and the concentration of allosteric modulators, such as neurally active steroids and polyamines, among other factors (Deutsch et al., 2011b; Millan, 2005). Transgenic mice that have diminished expression of the obligatory NR1 NMDA receptor subunit or NMDA receptors with approximately five-fold reduced affinity for the obligatory glycine co-agonist display impaired sociability in standard paradigms (Halene et al., 2009; Labrie et al., 2008). Recently, genetically engineered mice, whose expression of the NR1 subunit is conditionally “knocked out” in parvalbumin-containing GABAergic inhibitory interneurons, showed diminished sociability, relative to their wild type littermates, confirming a regulatory role for the NMDA receptor in normal mouse sociability (Billingslea et al., 2014). Also, in the resident-intruder mouse model of social interaction, D-cycloserine (320 mg/kg), a partial glycineB site agonist, increased social exploration and diminished aggression in the resident mouse; importantly, the mice tested in the resident-intruder model had “normal” expression of NMDA receptors (McAllister, 1994). In summary, these data support an important role of the NMDA receptor in regulation of sociability, and suggest that targeting this receptor with agonists may improve impaired sociability in disorders, such as ASDs.
3. NMDA regulation of mTOR activity
NMDA receptor activation is an important regulator of mTOR signaling activity (Huang et al., 2007) (Fig. 1). Specifically, sustained NMDA receptor activation leads to the rapid internalization of two isoforms of the cationic amino acid transporter, resulting in diminished arginine transport into cortical neurons (Huang et al., 2007). Intraneuronal concentrations of arginine are detected by ‘nutrient sensors’ that influence mTORCl activity. Thus, when intraneuronal concentrations of arginine are low, mTORCl activity is dampened, resulting in diminished rates and amounts of protein synthesis (Huang et al., 2007). Further, NMDA receptor activation regulates the duration of signaling by the phosphorylated form of extracellular signal regulated kinase 1/2 (ERK1/2), an important driver of mTORCl activity. Specifically, NMDA receptor activation leads to the calcium ion-dependent activation of calcineurin, an important phosphatase that cleaves phosphate from and, thereby, activates ‘STriatal Enriched protein tyrosine Phosphatase (STEP)’ (Fitzpatrick and Lombroso, 2011; Paul and Connor, 2010; Paul et al., 2003). STEP is an important phosphatase enriched within anatomic brain areas that serve as important nodes within circuits necessary for sociability and cognition, including frontal cortex and hippocampus (Fitzpatrick and Lombroso, 2011). Sustained NMDA receptor activation leads to dephosphorylation of ERK1/2 by STEP, which may dampen mTOR signaling activity. As noted above, the phosphorylated form of ERK1/2 is an important driver of mTOR signaling. Thus, the data suggest that a potential mechanism contributing to the therapeutic action of NMDA receptor activation is down-regulating mTOR signaling activity; of course, future studies must confirm this hypothesis.
Fig. 1.
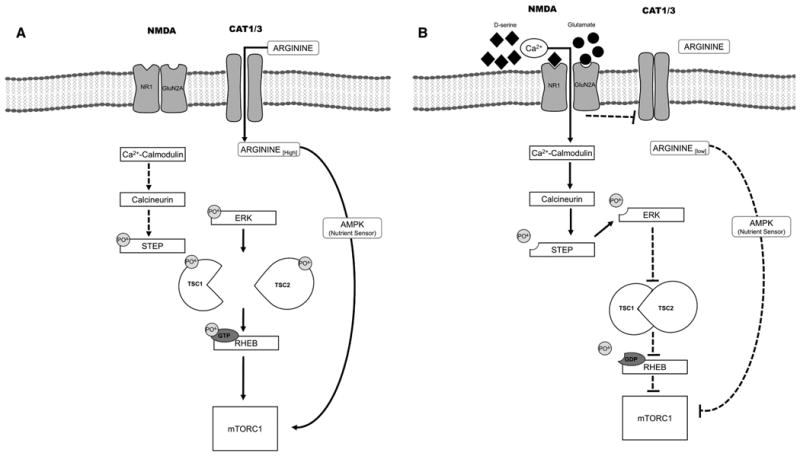
The figure is a cartoon depiction of hypothesized mechanisms by which NMDA receptor activation dampens mTOR signaling and, thereby, improves socialization in several syndromic (e.g., Tuberous Sclerosis Complex) and nonsyndromic forms of autism spectrum disorders. Panel A depicts heightened mTOR signaling activity in several syndromic and nonsyndromic forms of autism spectrum disorders, whereas Panel B depicts two possible consequences of NMDA receptor activation. NMDA receptor activation (Panel B) decreases the activity of specific cationic amino acid transporters (CAT 1/3) responsible for arginine uptake into the neuron. Arginine is detected by nutrient sensors, which contribute to regulation of mTOR signaling activity; lowered intraneuronal concentrations of arginine lead to dampening of mTOR signaling activity. NMDA receptor activation (Panel B) also leads to the Ca2+-dependent activation of calcineurin, a phosphatase that activates STEP, which, in turn, dephosphorylates and inactivates ERK1/2. Phosphorylated ERK1/2 is a major driver of mTOR signaling activity. NMDA receptor activation may be preferred to direct inhibition of mTORC1 in disorders requiring chronic, even lifelong, treatment, such as autism spectrum disorders (see text for additional derails).
4. Nonsyndromic mouse models of ASDs
Unlike transgenic knockout mouse models, whose impaired sociability may be causally-related to diminished or absent expression of single major genes, the genetically-inbred Balb/c and BTBRT+ Itpr3tf/J (BTBR) mouse models are particularly interesting models of ASD because their impaired sociability may reflect subtle epistatic interactions within a network of related genes, many of which may be normal polymorphisms (Cuscó et al., 2009; Levitt and Campbell, 2009). Syndromic forms of ASD due to the effects of single major genes (e.g. tuberous sclerosis, neurofibromatosis type 1 and fragile X syndrome) occur much less commonly than nonsydromic presentations. The genetic mechanisms contributing to or underlying the impaired sociability of Balb/c and BTBR mice may more closely resemble what occurs in most presentations of ASD (Cuscó et al., 2009; Veenstra-Vanderweele et al., 2004).
The Balb/c mouse has altered endogenous tone of NMDA receptor-mediated neurotransmission as reflected in its hypersensitivity to the behavioral effects of MK-801 (dizocilpine), a noncompetitive NMDA receptor antagonist that binds to a hydrophobic channel domain of the NMDA receptor (Burket et al., 2010a; Deutsch et al., 1997, 1998). Previous data showed that Balb/c mice are more sensitive to MK-801's antagonism of electrically precipitated tonic hindlimb extension, and elicitation of irregular episodes of intense jumping behavior, termed “popping,” and circling behavior than other strains (Burket et al., 2010a; Deutsch et al., 1997, 1998). Balb/c mice also have impaired sociability and serve as an established model of ASD (Brodkin, 2007; Burket et al., 2010b; Deutsch et al., 2011a). For example, Balb/c mice show diminished locomotor activity in the presence of an enclosed and freely-behaving social stimulus mouse, relative to the outbred Swiss Webster comparator strain (Burket et al., 2010b). Also, in a standard social paradigm, Balb/c mice spend less time in the compartment containing an enclosed social stimulus mouse; spend less time exploring the enclosed social stimulus mouse; and make fewer discrete episodes of social approach, mounting and anogenital sniffing of a freely-behaving stimulus mouse, among other examples of impaired social behavior, relative to the Swiss Webster comparator strain (Jacome et al., 2011a, 2011c). Finally, although the Balb/c mouse did not differ from the comparator Swiss Webster strain in terms of time spent exploring an inanimate object and did not spend less time in the open arms of an elevated plus maze, this mouse strain differed from the comparator Swiss Webster strain in terms of decreased time spent exploring an enclosed social stimulus mouse (Jacome et al., 2011b). Thus, the Balb/c mouse displays selective reduction of social exploration, although it is not more anxious than the comparator strain in nonsocial situations, nor does it have a diminished exploratory drive, in general (Jacome et al., 2011b). Because the Balb/c mouse is behaviorally hypersensitive to MK-801, D-cycloserine and D-serine, a partial and full glycineB agonist acting at the NMDA receptor, respectively, were investigated for their ability to improve sociability in this mouse strain. The data showed that these targeted NMDA receptor agonist interventions improved several quantitative measures of sociability in 4- and 8-week-old Balb/c mice (Deutsch et al., 2011a, 2012; Jacome et al., 2011a). Further, the C57B1/6 strain of mouse spent more time exploring D-cycloserine-treated Balb/c mice than they did exploring vehicle-treated Balb/c mice, suggesting that D-cycloserine “normalized” social signals emitted by the Balb/c strain (Benson et al., 2013). Overall, a growing body of evidence implicates the NMDA receptor in regulation of normal mouse sociability; additionally, the NMDA receptor serves as a therapeutic target for the treatment of impaired sociability in several relevant mouse models of ASD.
NMDA receptor activation affects mTOR signaling by its influence on the cascade of intracellular phosphorylations along the Ras and PI3K signal transduction pathways, upstream of mTORC1, which could serve as one of its potential mechanisms of prosocial effects (Crino, 2011; Peça and Feng, 2012; Talos et al., 2012). In addition to the Balb/c mouse, the BTBR mouse is another inbred mouse strain that models impaired sociability and stereotypic behavior displayed by persons with ASDs (McFarlane et al., 2008; Silverman et al., 2010; Wöhr et al., 2011; Yang et al., 2007). Interestingly, the BTBR mouse strain differs from at least 12 other inbred strains with respect to genetic variation of the gene encoding kynurenine 3-hydroxylase, an enzyme regulating the pool of kynurenic acid (McFarlane et al., 2008). Kynurenic acid is involved both as a ligand and part of a metabolic pathway that influences neurotransmission mediated by glutamate. Furthermore, a very recent signaling pathway analysis using quantitative proteomics to explore cortical and hippocampal tissues of aged 15 month-old male BTBR mice, compared to age and sex-matched C57B1/6 mice, showed that the mTOR signaling pathway in cortex was among “the top 10 most significantly-populated neuronally-specific” signaling pathways (Jasien et al., 2014). Also, levels of mature brain-derived neurotrophic factor (BDNF) and total TrkB receptor, a receptor tyrosine kinase on the cell surface that transduces the BDNF signal, were reduced in cortical and hippocampal tissues of aged BTBR mice, compared with C57B16 mice (Jasien et al., 2014). BDNF signal transduction is a known regulator of mTOR signaling pathways; thus, these data suggest a compensatory decreased transduction of BDNF in response to elevated and dysregulated mTOR signaling in BTBR mice. Importantly, the aged BTBR mice continued to show deficits of sociability. Of course, these provocative differences in protein abundance and, possibly, mTOR signaling between BTBR and C57B16 mice could be related primarily to their different genetic backgrounds and reflect nonspecific effects of genetic drift. In summary, there is strong support for therapeutic interrogation of glutamatergic neurotransmitter systems and mTOR signaling pathways in these two mouse models of ASDs. Since NMDA receptor activation regulates sociability and mTOR signaling activity, and the latter is upregulated in several syndromic forms of ASD, we wondered whether D-cycloserine, a pharmacological strategy for producing NMDA receptor activation, would improve sociability in the BTBR mouse model of ASD. D-Cycloserine improved sociability in the BTBR mouse, supporting the hypothesis that its prosocial effects are mediated, at least in part, by dampening effects on mTOR signaling activity in brain (Burket et al., 2013). Specifically, a hypothesized mechanism of D-cycloserine's prosocial effects could include: inhibition of cationic amino acid transporters that are responsible for carrying arginine into the neuron, and Ca2+-calcineurin-dependent activation of STEP. STEP dephosphorylates the active form of ERK1/2, regulating the duration of ERK1/2 signaling, which is a driver of mTOR signaling (Fitzpatrick and Lombroso, 2011; Paul and Connor, 2010; Paul et al., 2003). Moreover, consistent with a hypothesis of possible upregulated mTOR signaling activity in the BTBR mouse strain, rapamycin, an inhibitor of mTORCl, was shown to improve sociability in the BTBR mouse strain (Burket et al., 2014).
5. Conclusion
NMDA receptor activation may be an important regulator of mTOR signaling activity in the brain. Possible mechanisms of the dampening of mTOR signaling by NMDA receptor agonists include, internalization of specific cationic amino acid transporters in relevant areas of the brain, leading to a reduction in intraneuronal arginine concentrations and a dampening of mTOR signaling activity. Additionally, NMDA receptor activation and the opening of the associated cation channel lead to a cascade that begins with the activation of calcineurin and, ultimately, the dephosphorylation of ERK1/2, which, in turn, dampens mTOR signaling. Data from syndromic forms of ASD suggest that dampening of mTOR activity can improve core domains of ASD symptomatology, including socialization. Unfortunately, strategies for the direct inhibition of mTORCl may not be applicable for a disorder, such as ASD, whose origin is during fetal brain development and persists throughout the lifespan (e.g., rapamycin is immunosuppressive). Thus, effective pharmacotherapeutic strategies to activate the NMDA receptor may safely dampen mTOR signaling activity in brain on a long-term basis for a chronic neuropsychiatric disorder, such as ASD. Also, because the NMDA receptor is enriched in brain, NMDA receptor activation may avoid systemic effects, while targeting the social and cognitive impairments of TSC and ASDs in general. A recent translational clinical trial of D-cycloserine in adolescents and young adults with ASDs with good expressive language ability and normal to near-normal IQs reported promising results, supporting future exploration of this provocative hypothesis (Urbano et al., 2014, in press); D-cycloserine has been used as a treatment intervention and validated tool to study the activation of the NMDA receptor in a variety of neuropsychiatric disorders, including schizophrenia, obsessive compulsive disorder, and posttraumatic stress disorder (PTSD) (de Kleine et al., 2014; Difede et al., 2014; Goff, 2012; Goff et al., 2008; Scheeringa and Weems, 2014; Wilhelm et al., 2008).
Importantly, there are exciting complementary strategies for activating the NMDA receptor, in addition to administration of direct glycineB site partial and full agonists, including inhibitors of the glycine transporter type 1 (GlyT1) and allosteric modulators of the metabotropic glutamate receptor 5 (mGluR5) (Canitano, 2014; Millan et al., 2014). Stimulation of mGluR5 results in phosphorylation of the NMDA receptor and, thereby, increases its sensitivity to activation by drugs mimicking glutamate and glycine. Conceivably, some of these complementary strategies will enjoy greater precision, acting “when and where” endogenous glycine and D-serine, are released in brain; moreover, these strategies may not be associated with receptor desensitization or potential excitotoxicity.
Acknowledgments
The authors acknowledge the support they received from grant funding from Virginia's Commonwealth Health Research Board. The authors also acknowledge the support they received from the Office of the Dean of Eastern Virginia Medical School.
Abbreviations
- TSC
Tuberous Sclerosis Complex
- ASD
Autism spectrum disorder
- mTORCl
Mammalian target of rapamycin complex 1
- Rheb
Ras homolog enriched in brain
- mTOR
Mammalian target of rapamycin
- PTEN
Phosphatase and tensin homolog
- NMDA
N-methyl-D-aspartate
- ERK 1/2
Extracellular signal regulated kinase 1/2
- STEP
Striatal enriched protein tyrosine phosphatase
- BDNF
Brain-derived neurotrophic factor
- PTSD
Posttraumatic stress disorder
- GlyT1
Glycine transporter type 1
- mGLuR5
Metabotropic glutamate receptor 5
References
- Benson AD, Burket JA, Deutsch SI. Balb/c mice treated with D-cycloserine arouse increased social interest in conspecifics. Brain Res Bull. 2013 Oct;99:95–9. doi: 10.1016/j.brainresbull.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014 Jun;39(7):1603–13. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007 Jan 10;176(1):53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008) Behav Neurosci. 2008 Apr;122(2):483–9. doi: 10.1037/0735-7044.122.2.483. [DOI] [PubMed] [Google Scholar]
- Burket JA, Cannon WR, Jacome LF, Deutsch SI. MK-801, a noncompetitive NMDA receptor antagonist, elicits circling behavior in the genetically inbred Balb/c mouse strain. Brain Res Bull. 2010 Nov;83(6):337–9. doi: 10.1016/j.brainresbull.2010.08.014. 20a. [DOI] [PubMed] [Google Scholar]
- Burket JA, Herndon AL, Deutsch SI. Locomotor activity of the genetically inbred Balb/c mouse strain is suppressed by a socially salient stimulus. Brain Res Bull. 2010 Oct;83(5):255–6. doi: 10.1016/j.brainresbull.2010.07.006. 30b. [DOI] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. D-Cycloserine improves sociability in the BTBRT+ Itpr3tf/J mouse model of autism spectrum disorders with altered Ras/Raf/ ERK1/2 signaling. Brain Res Bull. 2013 Jul;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. Rapamycin improves sociability in the BTBR T(+)Itpr3(tf)/J mouse model of autism spectrum disorders. Brain Res Bull. 2014 Jan;100:70–5. doi: 10.1016/j.brainresbull.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Hof PR. The neuroscience of autism spectrum disorders. Academic Press; 2012. [DOI] [PubMed] [Google Scholar]
- Cambiaghi M, Cursi M, Magri L, Castoldi V, Comi G, Minicucci F, et al. Behavioural and EEG effects of chronic rapamycin treatment in a mouse model of tuberous sclerosis complex. Neuropharmacology. 2013 Apr;67:1–7. doi: 10.1016/j.neuropharm.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2:61. doi: 10.3389/fped.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol Med. 2011 Dec;17(12):734–42. doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Cuscó I, Medrano A, Gener B, Vilardell M, Gallastegui F, Villa O, et al. Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum Mol Genet. 2009 May 15;18(10):1795–804. doi: 10.1093/hmg/ddp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kleine RA, Hendriks G-J, Smits JAJ, Broekman TG, van Minnen A. Prescriptive variables for d-cycloserine augmentation of exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2014 Jan;48(1):40–6. doi: 10.1016/j.jpsychires.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Paul SM, Riggs RL, Mastropaolo J. Inbred mouse strains differ in sensitivity to “popping” behavior elicited by MK-801. Pharmacol Biochem Behav. 1997 Jun;57(1-2):315–7. doi: 10.1016/s0091-3057(96)00347-4. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Mastropaolo J, Powell DG, Rosse RB, Bachus SE. Inbred mouse strains differ in their sensitivity to an antiseizure effect of MK-801. Clin Neuropharmacol. 1998 Aug;21(4):255–7. [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Jacome LF, Cannon WR, Herndon AL. D-Cycloserine improves the impaired sociability of the Balb/c mouse. Brain Res Bull. 2011a Jan 15;84(1):8–11. doi: 10.1016/j.brainresbull.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Urbano MR, Herndon AL, Winebarger EE. Impaired sociability of the Balb/c mouse, an animal model of autism spectrum disorders, is attenuated by NMDA receptor agonist interventions: clinical implications. In: Mohammad-Reza M, editor. Compr Book Autism Spectr Disord. InTech; 2011b. [Internet] [Available from: http://www.intechopen.com/articles/show/title/impaired-sociability-of-the-Balb-c-mouse-an-animal-model-of-autism-spectrum-disorders-is-attenuated-] [Google Scholar]
- Deutsch SI, Pepe GJ, Burket JA, Winebarger EE, Herndon AL, Benson AD. D-cycloserine improves sociability and spontaneous stereotypic behaviors in 4-week old mice. Brain Res. 2012 Feb 23;1439:96–107. doi: 10.1016/j.brainres.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014 Apr;39(5):1052–8. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D. From genes to cognition in tuberous sclerosis: implications for mTOR inhibitor-based treatment approaches. Neuropharmacology. 2013 May;68:97–105. doi: 10.1016/j.neuropharm.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Rapamycin for treating tuberous sclerosis and autism spectrum disorders. Trends Mol Med. 2011 Feb;17(2):78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Lombroso PJ. The role of striatal-enriched protein tyrosine phosphatase (STEP) in cognition. Front Neuroanat. 2011;5:47. doi: 10.3389/fnana.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Plasschaert E, Descheemaeker M-J, Huson S, Borghgraef M, Vogels A, et al. Autism spectrum disorder profile in neurofibromatosis type I. J Autism Dev Disord. 2014 Dec;:5. doi: 10.1007/s10803-014-2321-5. [DOI] [PubMed] [Google Scholar]
- Goff DC. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012 Sep;38(5):936–41. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, et al. Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr Res. 2008 Dec;106(2–3):320–7. doi: 10.1016/j.schres.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009 Oct;8(7):661–75. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010 Feb;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, et al. The canonic amino acid transporters CAT1 and CATC mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci. 2007 Jan 17;27(3):449–58. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Cannon WR, Deutsch SI. D-serine improves dimensions of the sociability deficit of the genetically-inbred Balb/c mouse strain. Brain Res Bull. 2011a Jan 15;84(1):12–6. doi: 10.1016/j.brainresbull.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. D-Cycloserine enhances social exploration in the Balb/c mouse. Brain Res Bull. 2011b May 30;85:3–4. 141–4. doi: 10.1016/j.brainresbull.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Res. 2011c;4(6):393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- Jasien JM, Daimon CM, Wang R, Shapiro BK, Martin B, Maudsley S. The effects of aging on the BTBR mouse model of autism spectrum disorder. Front Aging Neurosci. 2014;6:225. doi: 10.3389/fnagi.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Wang Y, Kim S-J, Bornhorst M, Jecrois ES, Anthony TE, et al. Transient inhibition of the ERK pathway prevents cerebellar developmental defects and improves long-term motor functions in murine models of neurofibromatosis type 1. eLife. 2015:4. doi: 10.7554/eLife.05151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2008 Oct;200(2):217–30. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009 Apr;119(4):747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister KH. D-cycloserine enhances social behaviour in individually-housed mice in the resident-intruder test. Psychopharmacology (Berl) 1994 Nov;116(3):317–25. doi: 10.1007/BF02245335. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T + tf/J mice. Genes Brain Behav. 2008 Mar;7(2):152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007 May 23;27(21):5546–58. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008 May 21;28(21):5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005 Apr;179(1):30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2014 May;24(5):645–92. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B–NMDA receptor-mediated increases in intracellular Ca2 + concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010 Aug;114(4):1107–18. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003 Jan;6(1):34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Peça J, Feng G. Cellular and synaptic network defects in autism. Curr Opin Neurobiol. 2012;22(5):866–72. doi: 10.1016/j.conb.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol. 2012 Oct;22(5):895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa MS, Weems CF. Randomized placebo-controlled D-cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. J Child Adolesc Psychopharmacol. 2014 Mar;24(2):69–77. doi: 10.1089/cap.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010 Jan 13;30(2):694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010 Mar;35(4):976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, et al. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7(5):e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tscl mutant mice. Nature. 2012 Aug 30;488(7413):647–51. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano M, Okwara L, Manser P, Hartmann K, Herndon A, Deutsch SI. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clin Neuropharmacol. 2014 Jun;37(3):69–72. doi: 10.1097/WNF.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano MR, Okwara L, Manser P, Hartmann K, Deutsch SI. A trial of D-cycloserine to treat the social deficit in older adolescents and young adults with autism spectrum disorders. J Neuropsychiatry Clin Neurosci. 2015 Apr; doi: 10.1176/appi.neuropsych.13070155. in press. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008 Mar;165(3):335–41. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBRT + tf/J mouse model of autism. Genes Brain Behav. 2011 Feb;10(1):35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee H-R, Gee HY, Mah W, Kim J-I, Lee J, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012 Jun 14;486(7402):261–5. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2007 Dec;25(8):515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


