Abstract
Objective: Lubricin/superficial zone protein (SZP)/proteoglycan4 (PRG4) plays an important role in boundary lubrication in articular cartilage. Lubricin is secreted by superficial zone chondrocytes and synoviocytes of the synovium. The specific objective of this investigation is to optimize the methods for tissue engineering of articular cartilage surface. The aim of this study is to investigate the effect of cell density on the self-assembly of superficial zone chondrocytes and lubricin secretion as a functional assessment.
Design: Superficial zone chondrocytes were cultivated as a monolayer at low, medium, and high densities. Chondrocytes at the three different densities were treated with transforming growth factor beta (TGF-β)1 twice a week or daily, and the accumulated lubricin in the culture medium was analyzed by immunoblots and quantitated by enzyme-linked immunosorbent assay (ELISA).
Results: Cell numbers in low and medium densities were increased by TGF-β1; whereas cell numbers in high-density cell cultures were decreased by twice-a-week treatment of TGF-β1. On the other hand, the cell numbers were maintained by daily TGF-β treatment. Immunoblots and quantitation of lubricin by ELISA analysis indicated that TGF-β1 stimulated lubricin secretion by superficial zone chondrocytes at all densities with twice-a-week TGF-β treatment. It is noteworthy that the daily treatment of TGF-β1 increased lubricin much higher compared with twice-a-week treatment.
Conclusions: These data demonstrate that daily treatment is optimal for the TGF-β1 response in a higher density of monolayer cultures. These findings have implications for self-assembly of surface zone chondrocytes of articular cartilage for application in tissue engineering of articular cartilage surface.
Keywords: : chondrocytes, TGF-beta, cell density, articular cartilage
Introduction
Osteoarthritis (OA) of the diarthrodial joints is one of the challenging diseases in the aged population. The central characteristic of OA is progressive degeneration of articular cartilage, which leads to permanent functional joint failure and disability.1 A critical event observed in early OA is the degeneration and disruption of the surface of the superficial zone of the articular cartilage.2 As articular cartilage lacks the ability for self-repair, the most common treatment currently for advanced OA is arthroplasty. Therefore, tissue engineers have been seeking novel methods for biological approaches to regenerate the surface of articular cartilage such as autologous chondrocyte implantation (ACI),3 osteochondral grafting,4 and self-assembled tissue engineered constructs.5 One of the main goals of tissue engineering is to create tissue substitutes with native articular cartilage surface function, including low friction coefficient and minimization of wear based on principles of biotribology.
Articular cartilage is an anisotropic tissue consisting of three zones, and the properties are different depending on the depth from the articular surface.6,7 The surface zone (10–20% of the total cartilage thickness) consists of flattened chondrocytes, the matrix has low proteoglycan content, and collagen is parallel to the surface.8 The middle zone (40–60% of the total cartilage thickness) consists of larger, spherical chondrocytes that are surrounded by a randomly oriented type II collagen matrix and has higher proteoglycan.8 The deep zone (30% of the tissue thickness) consists of the chondrocytes that are arranged in nests of columnar cells. In this region, type II collagen is aligned perpendicular to the articular surface; has the highest concentration of proteoglycans and the extracellular matrix is mineralized.8,9
Lubricin, also known as superficial zone protein (SZP) and proteoglycan4 (PRG4), is a large glycoprotein with mucin domains that is synthesized and secreted into synovial fluid by synovial cells and superficial zone chondrocytes.10,11 Lubricin plays an important role in the boundary lubrication of joints by reducing friction and wear at the articular surface.12–14 The Camptodactyly-arthropathy-coxa vara-pericarditis syndrome, which is caused by genetic mutations of lubricin, is characterized by noninflammatory synovial cell hyperplasia, adhesions between tendons and tendon sheaths, and precocious OA.15 Lubricin plays an important role in the lubrication of articular cartilage and protects the cartilage from premature degeneration.4–6
Joint injury and arthritis result in profound changes in the concentration of growth factors and morphogens such as transforming growth factor beta (TGF-β) in synovial fluid.16,17 TGF-β plays a significant role in the anabolism of chondrocytes18; it increases expression and secretion of lubricin in chondrocytes and mesenchymal progenitors in the synovium.19,20 TGF-β has a variety of roles and is dependent on cell types and cell density.21 The seeding cell density used for ACI is usually 1–2 × 106 cells/cm2.22 However, the optimized cell density for this treatment is still conflicting. In this study, we investigated the effect of cell density on the self-assembly and secretion of lubricin from bovine articular cartilage superficial zone chondrocytes in response to TGF-β.
Materials and Methods
Cell isolation and monolayer culture
Stifle (knee) joints from 3-month-old calves were obtained from Research 87 (Boston, MA). The joints were dissected under aseptic conditions, exposing the femoral condyles. Surface zone chondrocytes were obtained as previously described.23 Briefly, the surface zone of the articular cartilage was harvested from the anterior half of the lateral and medial femoral condyles (∼100 μm thick) by using a dermatome. Then, they were digested with 0.2% collagenase P (Roche) in Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco, Carlsbad, CA) containing 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO), 0.1% bovine serum albumin (BSA; Sigma-Aldrich), and antibiotics (Medium-A) with 3% fetal bovine serum (FBS; Gibco) for 2 h at 37°C. Cells released from the tissues were filtered through a cell strainer (70 μm; BD Biosciences, San Jose, CA) and rinsed with DMEM/F-12. Isolated superficial chondrocytes were plated as a monolayer on 24-well culture plates (Corning, NY) at three different densities: 5 × 105 cells/well (low), 1.5 × 106 cells/well (medium), and 5 × 106 cells/well (high). After overnight equilibration in 10% FBS in DMEM/F-12 with 3.15 g/L of d Glucose, the medium was changed to fresh Medium-A with 1% ITS + Premix in the presence or absence of 3 ng/mL TGF-β, type 1 isoform (TGF-β1). Culture media (50% of the volume) were changed twice a week or daily, and the equal volume of fresh medium was replaced. The rationale for changing 50% of the medium is that the conditioned cell culture medium may be enriched in anabolic growth factors that are secreted in response to TGF-β1 treatment. Collected media were stored at −80°C for later analysis of lubricin accumulation.
TGF-β1 preparation
TGF-β1 was reconstituted in a filter-sterilized solution of 0.1% BSA in 5 mM hydrochloric acid at 10 mg/mL. Stock concentration was prepared and stored at −20°C, until it was diluted in culture media to its final concentrations. Chondrocytes were treated with or without TGF-β1 (3 ng/mL). We have previously shown that the effect of TGF-β was the highest at 3 ng/mL.24
Immunoblot analysis
For immunoblot analysis, cell culture media were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat dry milk in TBST (25 mM Tris HCl, 125 mM NaCl, and 0.1% Tween 20) for 1 h and incubated overnight at 4°C with the primary antibody mAb S6.79 at a dilution of 1:5000. The membranes were then incubated for 1 h at 4°C with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies at a dilution of 1:3000 for mice (Bio-Rad, Hercules, CA), followed by a 1-min incubation with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA) for visualization.
Enzyme-linked immunosorbent assay for lubricin
The culture media were harvested after treatment with TGF-β and lubricin accumulation in the media was determined by an enzyme-linked immunosorbent assay (ELISA) with purified, bovine lubricin as a standard.24 Briefly, each well of 96-well MaxiSorp plates (Nalge Nunc, Penfield, NY) was coated with 1 μg/mL peanut lectin (EY Laboratories, San Mateo, CA) in 50 mM sodium carbonate buffer (pH 9.5). The wells were then blocked with 1% BSA in the same buffer for at least 2 h. Aliquots of culture medium were incubated in the wells. Thereafter, the wells were incubated overnight with mAb S6.79 as the primary antibody at 4°C, and they were then incubated at room temperature for 1 h with goat anti-mouse immunoglobulin G conjugated with HRP (1:3000; Bio-Rad) as the secondary antibody. SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) was added, and the results were quantified in a luminometer. The wells were washed with phosphate-buffered saline containing 0.05% Tween 20 after each step. Lubricin concentrations were calculated by using a bovine lubricin standard, which was purified by affinity chromatography on a peanut lectin column. Purity was verified by immunoblot analysis. The concentration of the lubricin standard was quantified by using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific).19,24
Statistical analysis
For the determination of cell numbers and ELISA, six replicate wells of culture were used. Analysis of variance and post hoc test were performed for analyzing cell numbers and ELISA data. Student's paired t-test was performed for the analysis for lubricin secretion per 100,000 cells and total lubricin secretion between the two groups in the same density, on the same day and by the same treatment. p Values <0.05 were considered significant. Data are presented as the mean ± standard deviation.
Results
Cell morphology and cell numbers
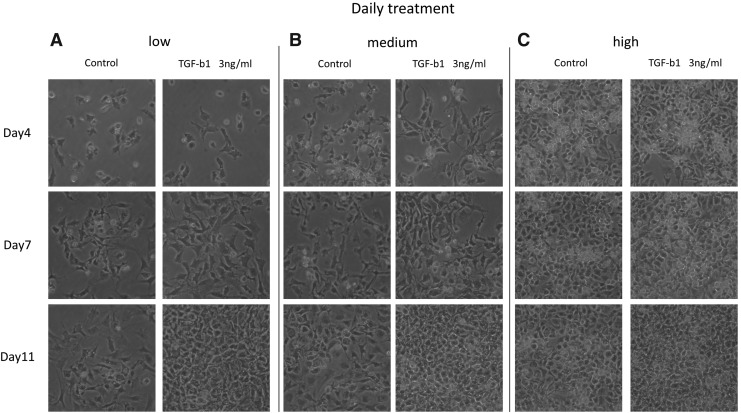
The cell numbers on days 4, 7, and 11 increased steadily after TGF-β1 treatment in low- and medium-density cultures. However, in high-density cultures, the cell number declined in cell cultures treated with TGF-β1 twice a week (Fig. 1A–C). This decline in cell numbers is possibly due to apoptosis in cell cultures treated twice per week. It is noteworthy that daily treatment with TGF-β1 in the high-density group maintained the chondrocyte morphology compared with twice-a-week treatment (Fig. 2C).
FIG. 1.
Morphology of superficial zone chondrocytes after twice-a-week treatment of TGF-β1. Representative images of morphology in low density (A), medium density (B), and high density (C) under 100 × magnification are shown, respectively. TGF-β, transforming growth factor beta.
FIG. 2.
Morphology of superficial zone chondrocytes after daily treatment of TGF-β1. Representative images of morphology in low density (A), medium density (B), and high density (C) under 100 × magnification are shown, respectively.
The cell numbers were counted by using a hemacytometer on day 11. In low- and medium-density cultures, cell numbers were increased by TGF-β1 and there was no difference between twice-a-week and daily TGF-β1 treatment. There was a pronounced decline in cell numbers in high-density cultures treated twice a week with TGF-β1; it is noteworthy that the daily treatment of TGF-β1 prevented the decline in cell number (Fig. 3).
FIG. 3.
Comparison of cell numbers after twice-a-week and daily treatment of TGF-β1. Cell numbers in the same density were compared. Asterisks indicate significant differences between the samples in the same density (*p < 0.01, **p < 0.05).
Immunoblot analysis and quantification of lubricin by ELISA
The secretion of lubricin in low, medium, and high densities was increased on days 7 and 11 by both twice-a-week and daily treatment of TGF-β1 (Fig. 4).
FIG. 4.
Immunoblot analysis for representative samples. Signals of SZP and BSA in cultured medium on days 4, 7, and 11 from low density (A), medium density (B), and high density (C) after twice-a-week and daily treatment of TGF-β1 are shown. SZP, superficial zone protein; BSA, bovine serum albumin.
The quantitative determination of lubricin on days 4, 7, and 11 by twice-a-week TGF-β1 treatment in low, medium, and high densities was stimulatory (Fig. 5A). The degree of stimulation by daily TGF-β1 treatment was pronounced on days 4, 7, and 11, especially in medium- and high-density cell cultures (Fig. 5).
FIG. 5.
Enzyme-linked immonosorbent assays for total SZP accumulation in the culture medium after twice-a-week treatment (A) and daily treatment of TGF-β1 (B). The amount of total SZP accumulation was normalized by the duration. Asterisks indicate the significant differences between the samples in the same density by the same treatment (*p < 0.01, **p < 0.05). Diamonds indicate the significant differences between twice-a-week and daily treated samples in the same density on the same day (♦< 0.01).
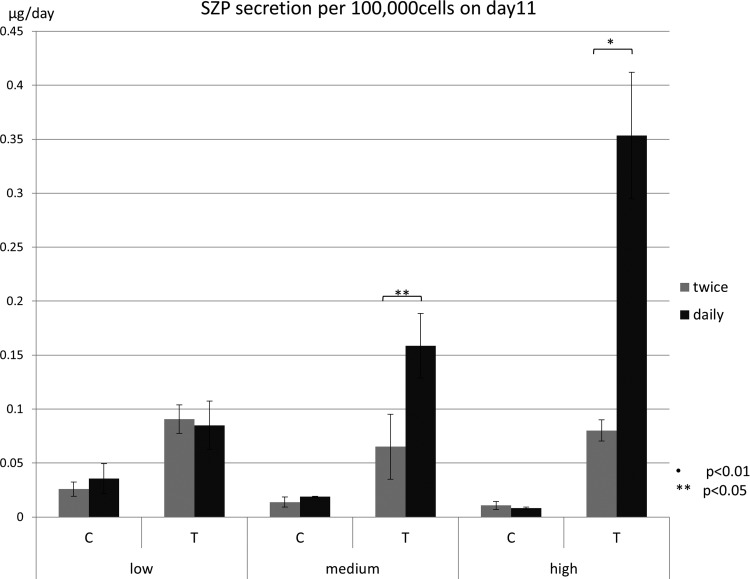
The degree of stimulation of the secretion of lubricin by superficial zone chondrocytes was much higher when the results were normalized based on the cell number (Fig. 6). It is noteworthy that in medium and high densities, daily treatment with TGF-β1 significantly elevated lubricin compared with twice-a-week treatment. Therefore, it is clear that daily treatment with TGF-β1 of superficial zone chondrocytes for 11 days is optimal for self-assembly and lubricin secretion normalized to 100,000 cells.
FIG. 6.
Total SZP accumulation on day 11 was normalized and expressed per 100,000 cells. Samples in the same density by the same treatment were compared. Asterisks indicate the significant difference between twice-a-week and daily treatment (*p < 0.01, **p < 0.05).
Discussion
The articular cartilage surface is critical for the functional integrity of the diarthrodial joints. The damage to the surface of the articular cartilage progressively leads to the degradation of cartilage tissue in OA. The resurfacing of articular cartilage by tissue engineering of the superficial zone chondrocytes and quantitation of lubricin will be immense utility. Therefore, in this investigation, we sought to develop methods to optimize the self-assembly of superficial zone chondrocytes in the surface of articular cartilage. We reasoned that cell density may be critical for optimal self-assembly as determined by the secretion of lubricin, the critical lubricant in the joints. To investigate this approach, we used low, medium, and high cell densities and compared two regimens of TGF-β1 treatment: twice-a-week and daily treatment.
As gleaned from the results of this investigation, at high cell density and twice a week, treatment by TGF-β1 exhibited a significant decline in cell number, possibly due to nutrient limitation and resultant apoptosis. On the other hand, the decrease in cell numbers in the high-density culture was not observed when TGF-β1 was replenished daily. This key finding demonstrates that culturing superficial zone chondrocytes at high density and treating them daily with TGF-β1 promote optimal self-assembly and functional optimization of lubricin secretion.
Cell density in vivo in the articular cartilage varies with depth. Cell density is relatively high in the superficial zone, and the density in this zone of adult bovine articular cartilage is 150 million cells/cm3.25,26 Further, it was demonstrated by Schmidt et al. that lubricin and SZP secretion per cell in explant culture and monolayers were similar.27 Oakes et al. compared high-density (250,000 cells/cm2) and low-density (40,000 cells/cm2) cultures, and they reported that high-density cultures increased cell proliferation and maintained chondrocyte morhphology.28 However, Abbott and Holtzer reported that the synthesis rate of the extracellular matrix was decreased when confluence was approached.29 In the present experiments, we utilized monolayer cell culture at densities of 50,000, 150,000, and 500,000 cells per well, which is equivalent to cell densities of 25,000, 75,000, and 250,000/cm2. The high-density cell culture with daily treatment by TGF-β1 yielded an optimal response compared with twice-a-week treatment.
The optimization of lubricin/SZP secretion is critical for tissue engineering of the surface of the articular cartilage. TGF-β is a useful morphogen and growth factor for articular cartilage tissue engineering by enhancing cartilage phenotype and stimulating lubricin accumulation.30,31 The TGF-β1 dose must be optimized, as higher doses may stimulate OA development.32
In conclusion, we have optimized methods by investigation of cell density and daily TGF-β1 treatment protocols for the self-assembly of superficial zone chondrocytes at high density and lubricin secretion.
Acknowledgments
The authors thank Dr. Thomas M. Schmid of Rush University for his gift of the antibody S6.79. This investigation was supported by the Lawrence J. Ellison Endowed Chair in Musculoskeletal Molecular Biology at the University of California, Davis, and in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, AR 061496. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Loeser R.F., Goldring S.R., Scanzello C.R., and Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64, 1697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilak F., Ratcliffe A., Lane N., Rosenwasser M.P., and Mow V.C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res 12, 474, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Clair B.L., Johnson A.R., and Howard T. Cartilage repair: current and emerging options in treatment. Foot Ankle Spec 2, 179, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Borazjani B.H., Chen A.C., Bae W.C., Patil S., Sah R.L., Firestein G.S., and Bugbee W.D. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am 88, 1934, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Hu J.C., and Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12, 969, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Klein T.J., Malda J., Sah R.L., and Hutmacher D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev 15, 143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schinagl R.M., Gurskis D., Chen A.C., and Sah R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res 15, 499, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Mow V.C., Ratcliffe A., and Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13, 67, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., Lopez-Puertas J.M., and Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev 16, 617, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Schumacher B.L., Block J.A., Schmid T.M., Aydelotte M.B., and Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 311, 144, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Schumacher B.L., Hughes C.E., Kuettner K.E., Caterson B., and Aydelotte M.B. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res 17, 110, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Swann D.A., Slayter H.S., and Silver F.H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem 256, 5921, 1981 [PubMed] [Google Scholar]
- 13.Jay G.D., Harris D.A., and Cha C.J. Boundary lubrication by lubricin is mediated by O-linked beta(1–3)Gal-GalNAc oligosaccharides. Glycoconj J 18, 807, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Rhee D.K., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V., Jay G.D., Stewart M., Wang H., Warman M.L., and Carpten J.D. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115, 622, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcelino J., Carpten J.D., Suwairi W.M., Gutierrez O.M., Schwartz S., Robbins C., Sood R., Makalowska I., Baxevanis A., Johnstone B., Laxer R.M., Zemel L., Kim C.A., Herd J.K., Ihle J., Williams C., Johnson M., Raman V., Alonso L.G., Brunoni D., Gerstein A., Papadopoulos N., Bahabri S.A., Trent J.M., and Warman M.L. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23, 319, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Fahlgren A., Andersson B., and Messner K. TGF-beta1 as a prognostic factor in the process of early osteoarthrosis in the rabbit knee. Osteoarthritis Cartilage 9, 195, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Marks P.H., and Donaldson M.L. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy 21, 1342, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Grimaud E., Heymann D., and Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev 13, 241, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Lee S.Y., Niikura T., and Reddi A.H. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A 14, 1799, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lee S.Y., Nakagawa T., and Reddi A.H. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: analysis of superficial zone protein/lubricin expression. Tissue Eng Part A 16, 317, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Nallet-Staub F., Yin X., Gilbert C., Marsaud V., Ben Mimoun S., Javelaud D., Leof E.B., and Mauviel A. Cell density sensing alters TGF-beta signaling in a cell-type-specific manner, independent from Hippo pathway activation. Dev Cell 32, 640, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foldager C.B., Gomoll A.H., Lind M., and Spector M. Cell seeding densities in autologous chondrocyte implantation techniques for cartilage repair. Cartilage 3, 108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalafi A., Schmid T.M., Neu C., and Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res 25, 293, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Niikura T., and Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum 56, 2312, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Schumacher B.L., Su J.L., Lindley K.M., Kuettner K.E., and Cole A.A. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec 266, 241, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Jadin K.D., Wong B.L., Bae W.C., Li K.W., Williamson A.K., Schumacher B.L., Price J.H., and Sah R.L. Depth-varying density and organization of chondrocytes in immature and mature bovine articular cartilage assessed by 3D imaging and analysis. J Histochem Cytochem 53, 1109, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt T.A., Schumacher B.L., Klein T.J., Voegtline M.S., and Sah R.L. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum 50, 2849, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Oakes B.W., Handley C.J., Lisner F., and Lowther D.A. An ultrastructural and biochemical study of high density primary cultures of embryonic chick chondrocytes. J Embryol Exp Morphol 38, 239, 1977 [PubMed] [Google Scholar]
- 29.Abbott J., and Holtzer H. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol 28, 473, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galera P., Vivien D., Pronost S., Bonaventure J., Redini F., Loyau G., and Pujol J.P. Transforming growth factor-beta 1 (TGF-beta 1) up-regulation of collagen type II in primary cultures of rabbit articular chondrocytes (RAC) involves increased mRNA levels without affecting mRNA stability and procollagen processing. J Cell Physiol 153, 596, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Awad H.A., Wickham M.Q., Leddy H.A., Gimble J.M., and Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25, 3211, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita A., Saito T., Tomita H., Makita Y., Yoshida K., Ghadami M., Yamada K., Kondo S., Ikegawa S., Nishimura G., Fukushima Y., Nakagomi T., Saito H., Sugimoto T., Kamegaya M., Hisa K., Murray J.C., Taniguchi N., Niikawa N., and Yoshiura K. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat Genet 26, 19, 2000 [DOI] [PubMed] [Google Scholar]