Abstract
Background: This study examined the influence of step goals with pedometers to improve children's weight loss, physical activity, and psychosocial health during obesity treatment.
Methods: Overweight and obese children ages 8–17 years (n = 105) participated in a 10-week family-based weight management intervention, including physical activity, nutrition, and behavioral modification. A quasi-experimental design was used to group eight cohorts into three conditions: no pedometer (n = 24), pedometer only (n = 25), and pedometer with step goals (i.e., 500 steps/day weekly increase above baseline; n = 56). Height and weight were measured at baseline and week 10 and used to calculate BMI. Analysis of covariance was performed to examine difference by condition for change in weight, BMI, and BMI z-score, controlling for age and baseline value. Differences in steps per day and psychosocial health were compared between the two pedometer conditions.
Results: Participants were 12.4 ± 2.5 years of age, including 70% girls and 64% African Americans. The pedometer with goals condition significantly reduced BMI (p = 0.02) and BMI z-score (p = 0.01) compared with the no-pedometer group. The pedometer with goals condition significantly increased steps per day (+1185 ± 425 steps/day) compared with the pedometer-only condition (−162 ± 620 steps/day; p < 0.05). Both pedometer groups similarly increased in subjective health and quality of life.
Conclusions: Providing children with pedometers and individualized step goals was an effective approach to produce weight loss. Further work is needed to increase the strength of interventions to achieve clinically meaningful weight reduction for children with obesity.
Keywords: : behavior goals, pedometer, physical activity, obesity, self-monitoring
Introduction
Obesity affects 17% of children and adolescents in the United States.1 Insufficient levels of physical activity are related to obesity,2 and youth with obesity spend significantly less time each day in moderate-to-vigorous physical activity compared with their normal-weight peers.3 Physical activity counseling has been identified as a critical component of weight management in children by several organizations, including the American Medical Association,4 the US Preventive Services Task Force,5 and healthcare professionals.6 The identification of tools and strategies to effectively increase physical activity within pediatric weight-loss programs is a research priority.7
Wearable activity trackers like pedometers are inexpensive, objective ways for children to self-monitor physical activity, and are suggested as a way to increase children's awareness and regulation of their physical activity.6 In adults, a recent systematic review indicated that behavioral physical activity interventions that include an activity monitor increased physical activity levels, but there was inconclusive evidence that activity monitors affect weight loss.8 For instance, a 12-week physical activity and dietary counseling intervention observed significantly more weight loss among adults who received a pedometer vs. those who did not,9 whereas a 2-year randomized controlled trial observed no beneficial effect of integrating pedometers into a comprehensive weight management program for adults.10
Similarly, in children and adolescents, the addition of pedometers to interventions has demonstrated moderately increased physical activity, but findings are equivocal for weight loss. The addition of pedometers to physical activity and weight management programs has increased children's physical activity by 1000–3000 steps per day above baseline.11–14 A 12-week study targeting adolescents with type 1 diabetes observed no significant change in physical activity in a group who received a pedometer with motivational text messages vs. a control group.15 Additionally, there are few data available on the influence of pedometers on children's weight loss. A 12-week study, where children were provided with pedometers and a standard daily step goal, indicated no difference in BMI percentile change from a control condition.16
Despite a lack of effectiveness data, family-based weight management programs are using pedometers to help children track their physical activity. A recent survey on pediatric weight management programs from 25 children's hospitals indicated that all programs included physical activity counseling and the majority (82%) used an objective measurement of physical activity, including 22% that used pedometers.6 A missing component of prior pedometer-based weight management trials is integrating behavior change strategies in conjunction with the activity tracker. For instance, the use of pedometer-specific goals may motivate children's physical activity. Self-monitoring and learning to self-regulate behaviors are mechanisms for weight loss,17 and providing physical activity goals that gradually increase in difficulty has been suggested as a way to increase children's motivation for behavior change.6
The primary purpose of the study was to examine the influence of pedometers with step goals to improve children's weight loss during behavioral treatment. Secondary aims were to examine the effects of pedometers with step goals on the children's physical activity and psychosocial health.
Methods
Participants
The participants included 105 children recruited over eight cohorts. Eligibility criteria included children ages 8–17 years old with BMI ≥95th percentile or a BMI ≥85th percentile with comorbidities, such as fatty liver disease, hypertension, heart disease, insulin resistance, orthopedic problems, sleep apnea, or type 2 diabetes. Community physicians referred eligible participants to the Obesity Clinic at the Our Lady of the Lake Children's Hospital. During a clinic visit with a pediatric gastroenterologist or dietitian, patients and their parents underwent an initial evaluation with complete history and physical examination, dietary evaluation, laboratory studies, determination of program readiness, and assurance of family commitment. Once admitted to the intervention, parents and children provided informed consent and assent, respectively (verbal assent if child was between 8 and 11 years of age, written assent if child was 12 years or older). The Institutional Review Boards of the Our Lady of the Lake Children's Hospital and the Pennington Biomedical Research Center approved all study procedures. The trial was registered at Clinicaltrials.gov number NCT02965729.
Behavioral Intervention
The “Our Lifestyles, Our Lives” weight management intervention consisted of a series of 10 weekly 90-minute group sessions focused on physical activity, nutrition, and behavioral modification. For each cohort, eight sessions were taught in the Translational Research Clinic for Children at Pennington Biomedical Research Center, one session was held at a local grocery store, and one session was held at a quick service restaurant. Parents and siblings were encouraged to join the participant during each session. Sessions were taught by a multidisciplinary staff, including a pediatric gastroenterologist, pediatricians, clinical psychologist, dietitian, and kinesiology-trained fitness specialists. Sessions were interactive and included cooking demonstrations, light-to-moderate intensity physical activity that engaged all family members and behavioral counseling sessions in both mixed (parent and child) and parent-only format. The program aligned with the American Medical Association expert committee recommendations for stage three of treatment for childhood obesity consisting of comprehensive multidisciplinary intervention.4 A synopsis of the curriculum is displayed in Table 1.
Table 1.
Standardized Curriculum of the 10-Week Behavioral Intervention for Childhood Obesity
| Session | Location | Physical activity lesson | Nutrition lesson | Behavior modification |
|---|---|---|---|---|
| Session 1 | TReCC | Steps to goal setting | ||
| Session 2 | TReCC | Introduction to barriers | Let's talk sugar | |
| Session 3 | TReCC | I don't know what to do | My plate & lunch in a crunch | Rules for eating |
| Session 4 | TReCC | It's raining, it's too hot | Cutting back the fat | |
| Session 5 | TReCC | I don't have money | Hurray for whole grains | 10 Tips for parents |
| Session 6 | Quick serve restaurant | I have other stuff to do | Eating healthy while on the go | |
| Session 7 | TReCC | I'm too tired, it's boring, it's too hard | Fruits and veggies | ABCs of behavior |
| Session 8 | TReCC | I don't have time, it's raining, it's too hot | Portion control & energy balance | |
| Session 9 | Grocery store | Grocery store scavenger hunt | ||
| Session 10 | TReCC | Challenge your barriers & 1 mile walk/run | Motivation and review | Individual check-in with parents/review |
ABCs, antecedents, behaviors, consequences; TReCC, Translational Research Clinic for Children.
Participants received a 72-page program guide, including weekly goal sheets and handouts with healthy recipes. The weekly goal sheet was completed by the family and included a prescribed nutrition goal, tracking of daily water consumption, minute-goals for physical activity, pedometer target step goals (cohorts 3–6 only), and space to write in self-selected behavioral goals. Families used the goal sheet to track goal achievement throughout each week.
Participants received small incentive items of negligible value (e.g., bouncing ball, jump rope, water bottle, and nail polish) throughout the program to reward and encourage participation, compliance, attendance, and achievement of program goals. Each participant received a $25 gift card at the grocery store session (session 9) and a $25 gift card at the final session (session 10).
Conditions
Eight cohorts of between 8 and 19 participants sequentially attended the program over the course of 2 years. Cohorts were grouped into three conditions: no pedometer (NP; n = 24), pedometer only (P; n = 25), or pedometer with individualized step goals (PG; i.e., 500 steps/day increase each week above baseline, n = 56). Cohorts were scheduled throughout the year to control for seasonal effects between PG vs. NP and P, such that NP occurred in summer and fall, P occurred in winter and spring, and PG occurred in each of the four seasons. Participants in cohorts 1 and 2 did not receive a pedometer. In cohorts 3–8, participants were given a pedometer and instructions at session 1. Participants were asked to wear the pedometer every day for the entirety of the program and return it at session 10. In cohorts 3–6, participants were given a step goal to increase their activity by 500 steps per day each week (above baseline calculated as average daily steps/day during week 1), for a total of 4500 steps per day increase by the end of the program. In cohorts 7 and 8, participants were given the pedometer, but no step goals.
Measurements
Body weight, height, and BMI
Height was measured at session 1 (baseline) and session 10 using a wall-mounted stadiometer. Weight was measured at the beginning of each session using a calibrated scale (excluding off-site sessions 6 and 9).
Pedometer
The pedometer provided to the P and PG conditions was the Omron HJ-324U, Omron Healthcare, Lake Forest, IL (cost between $30–40 each). In cohorts 3–8 (the PG and P conditions), the physical activity data, including daily steps, were downloaded from the pedometer through USB and/or manually during weekly sessions; therefore, physical activity during the session was not captured.
Psychosocial questionnaire
A pre- and posttest psychosocial questionnaire was completed by each participant at the beginning (session 1) and end (session 10) of the program. The questionnaire included the following validated instruments: the KIDSCREEN-10 Index to assess quality of life18; a single Likert scale assessment of subjective health (“In general, how would you say your health is?”)19; and the Physical Activity Enjoyment Scale, a 16-item measure of enjoyment during physical activity.20
Statistical Analysis
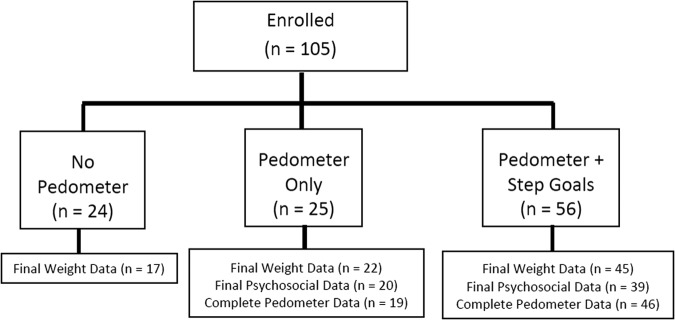
Twenty-one participants did not attend the last two clinic sessions so they did not provide a final weight (7 in NP condition, 3 in P condition, and 11 in PG condition), leaving an analytical sample size of 84. Nearest height and weight within 2 weeks were imputed for those missing week 0 and week 10 values, and baseline height was imputed for 28 participants missing week 10 height. BMI z-score was calculated from the CDC SAS macro program based on the sex, height, and age of the child.21 Of the 81 participants in the P and PG conditions, 16 were missing all step data due to damaged, lost, forgotten, or malfunctioning pedometer. Of the 65 participants with step data, 83% of data were complete. The missing data were due to absences or device malfunction. See Figure 1 for the flow of participants through the intervention.
Figure 1.
Flow diagram of participants through the intervention.
The primary endpoints of the program were change in body weight, BMI, and BMI z-score. Secondary endpoints were change in physical activity (average weekly steps), physical activity enjoyment, subjective health, and health-related quality of life. Difference scores were calculated between initial and final assessment. Analysis of covariance models was calculated to examine change in each primary endpoint, with condition as the independent variable and age and baseline value as covariates. Post hoc Tukey's tests were used to examine differences between conditions. In secondary models, the covariates of sex, race, and attendance were added. Paired samples t-tests were used to examine change in psychosocial variables collapsed across the conditions. Paired samples t-tests were used to examine change in daily steps from baseline for the PG and P conditions. In addition, independent samples t-tests were used to compare daily steps each week between the two conditions.
Results
There were a total of 105 participants. Participants were on average 12 ± 2.5 years of age, and the sample included 70% girls and 64% African Americans, 31% Caucasians, and 5% other races. The majority (65%) were insured by Medicaid, with the remaining on private insurance. There was no significant difference by condition in baseline BMI z-score or BMI percentile or by sex, race, or insurance status. The PG group was older (p = 0.01) and had a significantly higher weight (p = 0.02) and BMI (p = 0.03) compared with the other groups; therefore, age and baseline value were included as covariates in all analyses. There was no difference between those who did and did not provide a final weight for age, sex, race, insurance status, or baseline weight, BMI, BMI z-score, or BMI percentile. See Table 2 for baseline characteristics overall and by condition.
Table 2.
Baseline Descriptive Characteristics of the Sample
| No pedometer (n = 24) | Pedometer only (n = 25) | Pedometer + step goals (n = 56) | Overall (n = 105) | |
|---|---|---|---|---|
| Age, years | 11.4 ± 2.5 | 11.7 ± 2.1 | 13.1 ± 2.6 | 12.4 ± 2.5 |
| Girls, % | 75 | 64 | 70 | 70 |
| Race, % | ||||
| African American | 54 | 64 | 68 | 64 |
| White | 38 | 28 | 30 | 31 |
| Other | 8 | 8 | 2 | 5 |
| Weight, kg | 73.0 ± 22.9 | 86.2 ± 24.8 | 90.1 ± 24.0 | 85.3 ± 24.7 |
| BMI, kg/m2 | 32.2 ± 7.3 | 35.2 ± 6.4 | 36.1 ± 5.7 | 35.0 ± 6.4 |
| BMI, z-score | 2.3 ± 0.4 | 2.5 ± 0.3 | 2.4 ± 0.3 | 2.4 ± 0.3 |
| BMI, percentile | 98.4 ± 1.7 | 99.2 ± 0.7 | 99.0 ± 1.3 | 98.9 ± 1.3 |
| Steps/day | — | 4889 ± 1492 | 4376 ± 2188 | 4487 ± 2048 |
| Physical activity enjoymenta | — | 66.1 ± 8.6 | 60.8 ± 12.5 | 62.4 ± 11.7 |
| Subjective healtha | — | 3.1 ± 1.1 | 3.3 ± 1.0 | 3.3 ± 1.1 |
| Quality of lifea | — | 39.0 ± 6.2 | 38.4 ± 7.1 | 38.6 ± 6.8 |
Data are reported as mean ± standard deviation.
Data were not collected for the “No-Pedometer” cohorts and data were missing for 3 Pedometer-Only participants and 17 Pedometer + Step Goals participants.
Four participants (4%) only attended the first session and were lost to follow-up; therefore, they were not included in the analyses. Excluding these dropouts, attendance averaged 74% (i.e., 7.4 of 10 sessions) and did not vary by condition.
Body Mass
Overall, the mean weight change was +0.02 kg, the mean BMI change was −0.20, and the mean BMI z-score change was −0.02. As indicated in Table 3, there was a significant difference by condition in change in weight, BMI, and BMI z-score (p < 0.05). Post hoc Tukey's tests indicated that the PG condition had a significantly greater reduction in weight (p = 0.045), BMI (p = 0.017), and BMI z-score (p = 0.012), compared with the NP condition. When included as covariates, race, sex, and attendance did not attenuate the observed effects.
Table 3.
Absolute Change in Weight-Related Parameters after 10 Weeks
| No pedometer (n = 17) | Pedometer only (n = 22) | Pedometer + step goals (n = 45) | |||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p | Mean ± SD | p | |
| Body weight (kg) | 1.12 ± 1.90 | 0.23 ± 1.87 | 0.522 | −0.49 ± 2.06 | 0.045 |
| BMI (kg/m2) | 0.43 ± 0.82 | −0.24 ± 0.78 | 0.087 | −0.37 ± 0.92 | 0.017 |
| BMI (z-score) | 0.01 ± 0.04 | −0.03 ± 0.04 | 0.098 | −0.03 ± 0.05 | 0.012 |
p-Values are compared with the No-Pedometer group in analysis of covariance models controlling for baseline age and baseline variable (weight, BMI, or BMI z-score).
SD, standard deviation.
Physical Activity
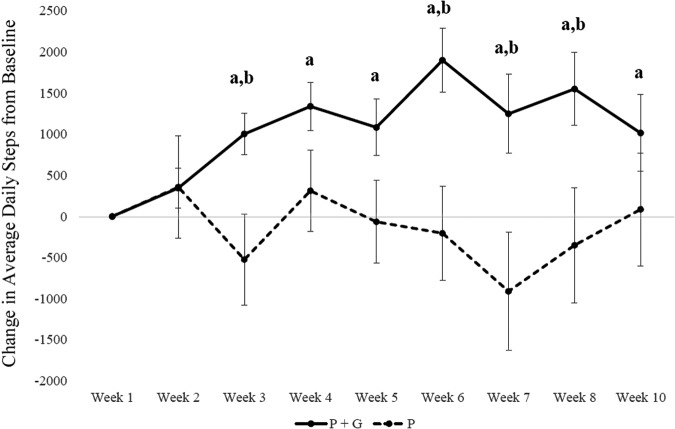
Average change in daily steps compared with baseline was 1185 ± 425 daily steps for the PG condition vs. −162 ± 620 daily steps for the P condition. Compared with baseline daily steps, the PG condition had significantly higher daily steps at every week after the goals were implemented (week 3: p < 0.001, week 4: p < 0.001, week 5: p < 0.001, week 6: p < 0.001, week 7: p = 0.01, week 8: p = 0.001, week 9: p = 0.04). The P condition did not vary in steps from baseline values at any week. Compared with the P condition, the PG condition accumulated significantly more daily steps at week 3 (p = 0.006), week 6 (p = 0.005), week 7 (p = 0.005), and week 8 (p = 0.03), but differences at the other weeks were not significant (Figure 2).
Figure 2.
Change in pedometer-measured steps from baseline. Error bars indicate standard error. aIndicates a significant differences from baseline using paired samples t-test. bIndicates a significant difference between conditions using independent samples t-test.
Psychosocial Health
With the two pedometer groups combined, there was a significant increase in subjective health (p < 0.0001) and health-related quality of life (p < 0.01), but no change in physical activity enjoyment (Table 4). There were no differences between the P and PG conditions for change in physical activity enjoyment, subjective health, or health-related quality of life.
Table 4.
Absolute Changes in Psychosocial Factors after 10 Weeks
| Pedometer only (n = 20) Mean ± SD | Pedometer + step goals (n = 39) Mean ± SD | p | |
|---|---|---|---|
| Physical activity enjoyment | 0.02 ± 8.25 | 2.66 ± 10.90 | 0.175 |
| Subjective health | 0.55 ± 1.34 | 0.66 ± 1.00 | 0.083 |
| Health-related quality of life | 2.2 ± 4.5 | 0.9 ± 5.3 | 0.343 |
p-Values are obtained from analysis of covariance models controlling for baseline age and baseline psychosocial variable. Data were not assessed in the No-Pedometer group.
Discussion
This study investigated the additive benefit of adding pedometers plus step goals to a family-based weight management intervention to increase weight loss, increase physical activity, and improve psychosocial health among children and adolescents. Children showed high adherence to wearing the pedometer, with 83% of the pedometer data being complete, indicating the feasibility of using pedometers in a structured weight management program. Given the recent proliferation of wearable technology,22 wearable devices are expected to be attractive to children and families.
Children who received the pedometers and individualized step goals reduced weight and BMI z-score to a greater extent than children who did not receive a pedometer or step goals. Over the 10-week period, total weight loss in the pedometer and goals group (−0.49 kg) was similar to the amount observed in a meta-analysis (∼0.05 kg/week) of nine pedometer-based walking studies in adult cohorts.23 However, when converted to z-score, the weight loss did not meet criteria for clinically meaningful loss of at least 0.25 z-score reduction.24,25 Therefore, a longer duration behavioral intervention that meets or exceeds current recommendations5 is recommended for clinically meaningful weight reduction.
The secondary aim was to examine the effect of the pedometer plus goals on youths' physical activity levels and psychosocial health. The pedometer plus goals condition significantly increased daily steps by an average of 1185 steps per day, whereas the condition that received only the pedometer without step goals did not change steps from baseline. When using pedometers to measure physical activity, a total of 9000 steps per day is recommended for children and adolescents to reach 60 minutes of moderate-to-vigorous physical activity.26 At baseline, participants accumulated only one-half of the recommended number of steps per day.26 By the end of the intervention, the children were closer to 65% of recommended daily steps, but did not meet the study-specific physical activity goals of an overall 4500 steps per day increase. The increase of 1185 steps per day is similar to one pediatric trial that observed ∼1000 steps per day increase over 9 months using pedometers and structured outdoor activities,12 but lower than other pediatric trials that achieved between 1500 and 3000 steps per day increase above baseline.11,13,14,27 The latter studies taught additional behavior change strategies such as coping skills training11 and behavior modification skills for parents.27 Therefore, the use of additional behavior change strategies beyond individualized step goals may be needed to increase children's physical activity to recommended levels. Additional recommendations to increase children's physical activity include enhanced physical education, classroom activity breaks, developing behavioral skills to increase children's confidence related to physical activity, and replacing inactivity with activity, such as walking or bicycling to school and engaging in physical activity during screen time.28
Both pedometer groups significantly improved in subjective health and health-related quality of life, without a difference between the two groups. These findings build on a prior study that observed a nonsignificant trend in higher quality of life for children aged 6–12 years who were randomized to a pedometer-based intervention with structured weekend outdoor activities vs. a control group.12 Future studies should examine the specific interventional elements that improve quality of life, such as self-esteem or self-efficacy improvements from using a pedometer to self-regulate physical activity or the other behavior change strategies that were taught in the weight management program. Health-related quality of life is identified as an important outcome of obesity intervention research by the NIH29 and as a key measure of population health in the Healthy People 2020 report.30 Because youth with obesity are at risk for low quality of life related to both physical health and psychosocial health,31 it is important to identify interventions such as the present one that improve health-related quality of life among children and adolescents.32
Strengths of this study include the use of objective measurements of physical activity and weight, as well as a focus on a population at high risk for obesity and associated comorbidities, with the sample being majority African American and insured by Medicaid.
There are several limitations to this study. The quasi-experimental design prevented the use of randomization, and there were baseline differences between conditions that were corrected using covariates in the analyses. Although the program remained consistent in intervention delivery, there may have been variation across seasons and time due to the sequential nature of the conditions. Seasonal effects have been observed for children's physical activity;33 this limitation was addressed by holding the pedometer plus goals condition across one full year (each of four cohorts occurred during each season). Participants were recruited from several pediatric clinics, but streamlined through one pediatric gastroenterology clinic which determined a family's readiness to participate in the program, thereby limiting generalizability. Psychosocial surveys were not added until the third cohort, so there are no data for the no-pedometer group. Future research should involve randomized controlled trials with larger samples to isolate the effects of specific tools and strategies to effectively promote physical activity and weight loss among children and adolescents.
Conclusion and Clinical Implications
Pedometers are low-cost devices that are popular among consumers and can be integrated into clinical settings to help children self-regulate their physical activity.6 The addition of a pedometer coupled with step goals based on baseline values increased both physical activity and weight loss in children participating in a family-based behavioral treatment program for weight management. However, the pedometer alone without step goals was not sufficient to significantly impact weight or physical activity. Identifying adjuncts to in-person treatment of childhood obesity coupled with behavior change strategies remains a priority to achieve clinically meaningful weight loss and behavior change in children and adolescents.
Acknowledgments
This research study was funded by a grant from the American Council on Exercise and the Franciscan Missionaries of Our Lady. A.E.S. and P.T.K. are supported, in part, by the 1 U54 GM104940 grant from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. P.T.K. is supported, in part, by the Marie Edana Corcoran Endowed Chair in Pediatric Obesity and Diabetes and the NORC Center Grant No. P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions.”
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016;315:2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez-Pavon D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: Systematic review. Int J Pediatr Obes 2010;5:3–18 [DOI] [PubMed] [Google Scholar]
- 3.Carson V, Staiano AE, Katzmarzyk PT. Physical activity, screen time, and sitting among U.S. adolescents. Pediatr Exerc Sci 2015;27:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007;120(Suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics 2010;125:361–367 [DOI] [PubMed] [Google Scholar]
- 6.Kist C, Gier A, Tucker J, et al. Physical activity in clinical pediatric weight management programs: Current practices and recommendations. Clin Pediatr 2016;55:1219–1229 [DOI] [PubMed] [Google Scholar]
- 7.Flodmark CE. What's new in childhood obesity and what do we still need to establish? Acta Paediatr 2016;105:1116–1118 [DOI] [PubMed] [Google Scholar]
- 8.de Vries HJ, Kooiman TJ, van Ittersum MW, et al. Do activity monitors increase physical activity in adults with overweight or obesity? A systematic review and meta-analysis. Obesity 2016;24:2078–2091 [DOI] [PubMed] [Google Scholar]
- 9.Harrington DM, Champagne CM, Broyles ST, et al. Steps ahead: A randomized trial to reduce unhealthy weight gain in the lower Mississippi delta. Obesity 2014;22:E21–E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA 2016;316:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry D, Savoye M, Melkus G, et al. An intervention for multiethnic obese parents and overweight children. Appl Nurs Res 2007;20:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein EA, Tan Y-T, Malhotra R, et al. A cluster randomized controlled trial of an incentive-based outdoor physical activity program. J Pediatr 2013;163:167.e1–172.e1 [DOI] [PubMed] [Google Scholar]
- 13.Rodearmel SJ, Wyatt HR, Stroebele N, et al. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: The America on the Move family study. Pediatrics 2007;120:e869–e879 [DOI] [PubMed] [Google Scholar]
- 14.Walders-Abramson N, Wamboldt FS, Curran-Everett D, et al. Encouraging physical activity in pediatric asthma: A case-control study of the wonders of walking (WOW) program. Pediatr Pulmonol 2009;44:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: Randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care 2009;32:813–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney BL, Gritt LR, Havens SJ, et al. Growing healthy families: Family use of pedometers to increase physical activity and slow the rate of obesity. Wis Med J 2005;104:54–60 [PubMed] [Google Scholar]
- 17.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight. JAMA 2007;298:1661–1673 [DOI] [PubMed] [Google Scholar]
- 18.Ravens-Sieberer U, Erhart M, Rajmil L, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: A short measure for children and adolescents' well-being and health-related quality of life. Qual Life Res 2010;19:1487–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias C, Gold PB, Ongur D, et al. Are single-item global ratings useful for assessing health status? J Clin Psychol Med Settings 2015;22:251–264 [DOI] [PubMed] [Google Scholar]
- 20.Moore JB, Yin Z, Hanes J, et al. Measuring enjoyment of physical activity in children: Validation of the Physical Activity Enjoyment Scale. J Appl Sport Psychol 2009;21:S116–S129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). A SAS Program for the 2000 CDC Growth Charts [Computer Program]. Atlanta, GA: CDC, 2016. Available at www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (last accessed July16, 2016) [Google Scholar]
- 22.Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: Promises and barriers. PLoS Med 2016;13:e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson CR, Newton TL, Abraham JJ, et al. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med 2008;6:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford AL, Hunt LP, Cooper A, et al. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child 2010;95:256–261 [DOI] [PubMed] [Google Scholar]
- 25.Reinehr T, de Sousa G, Toschke AM, et al. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr 2006;84:490–496 [DOI] [PubMed] [Google Scholar]
- 26.Adams MA, Johnson WD, Tudor-Locke C. Steps/day translation of the moderate-to-vigorous physical activity guideline for children and adolescents. Int J Behav Nutr Phys Act 2013;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton RL, Jr., Marker AM, Allen HR, et al. Parent-targeted mobile phone intervention to increase physical activity in sedentary children: Randomized pilot trial. JMIR Mhealth Uhealth 2014;2:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Physical Activity Guidelines for Americans midcourse report: Strategies to increase physical activity among youth. Available at https://health.gov/paguidelines/midcourse Last accessed March15, 2017
- 29.National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Strategic Plan for NIH Obesity Research. Available at www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/strategic-plan-NIH-obesity-research.aspx Last accessed December7, 2016
- 30.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at http://healthypeople.gov/2020 Last accessed December7, 2016
- 31.Ul-Haq Z, Mackay DF, Fenwick E, et al. Meta-analysis of the association between body mass index and health-related quality of life among children and adolescents, assessed using the pediatric quality of life inventory index. J Pediatr 2013;162:280.e1–286.e1 [DOI] [PubMed] [Google Scholar]
- 32.Kolodziejczyk JK, Gutzmer K, Wright SM, et al. Influence of specific individual and environmental variables on the relationship between body mass index and health-related quality of life in overweight and obese adolescents. Qual Life Res 2015;24:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staiano AE, Broyles ST, Katzmarzyk PT. School term vs. school holiday: Associations with children's physical activity, screen-time, diet and sleep. Int J Environ Res Public Health 2015;12:8861–8870 [DOI] [PMC free article] [PubMed] [Google Scholar]