Abstract
Background and Purpose
To assess the rate of neurological complications and mortality after tuberculous meningitis in the United States.
Methods
The authors performed a retrospective cohort study of all patients 18 years or older hospitalized for tuberculous meningitis in California between 2005–2010, New York between 2006–2012, and Florida between 2005–2012. Outcomes of interest were mortality and the following neurological complications: stroke, seizure, hydrocephalus requiring a ventriculoperitoneal shunt, vision impairment, and hearing impairment. Kaplan-Meier survival statistics were used to assess the cumulative rate of neurological complications and death. Cox proportional hazards regression was used to compare rates of complications in patients with and without human immunodeficiency virus (HIV) after adjustment for comorbidities.
Results
806 patients with tuberculous meningitis were identified, among whom the cumulative rate of any complication or death was 55.4% (95% CI, 51.5–59.3%). More than two-thirds of complications occurred during the initial hospitalization for tuberculous meningitis. Individual neurological complications were not uncommon: the cumulative rate of stroke was 16.8% (95% CI, 14.0–20.0%), the rate of seizure was 18.8% (95% CI, 15.4–22.8%), and the rate of ventriculoperitoneal shunting was 8.4% (95% CI, 6.4–10.9%). Vision impairment occurred in 21.6% (95% CI, 18.5–25.1%) of patients and hearing impairment occurred in 6.8% (95% CI, 4.9–9.4%). The mortality rate was 21.5% (95% CI, 18.4–24.9%). Patients with HIV infection were not at increased risk of complications compared to patients without HIV (hazard ratio, 1.2; 95% CI, 0.9–1.6).
Conclusions
Tuberculous meningitis is associated with significant risk of neurological complications and death even in the United States.
Keywords: Tuberculous meningitis, stroke, seizure, ventriculoperitoneal shunt, outcome studies, United States
Introduction
Tuberculosis (TB) is a global disease epidemic, with an estimated 9.6 million new cases occurring in 2014 [1]. Tuberculous meningitis (TBM) occurs in 1–5% of patients with TB and is characterized by a progressive granulomatous inflammation of the basal meninges. TBM is associated with a mortality of 19–28% in HIV-uninfected patients and 40–58% in HIV-infected patients [2–5]. TBM also frequently leads to neurological complications including hydrocephalus [6–11], stroke [9, 10, 12–24], seizure [5, 6, 9, 24], vision loss [5, 25, 26], and hearing loss [27–29]. However, the majority of studies on TBM have been performed in resource-limited settings where there may be suboptimal prevention, diagnosis, and treatment of the disease [2–5]. Furthermore, the average length of follow-up in previous studies was relatively short and therefore may not have accurately captured delayed neurological complications [2–5]. Our aim was to evaluate the mortality and long-term risk of neurological complications after TBM in a high-resource setting such as the United States using a large heterogeneous cohort of patients.
Methods
Design
The authors performed a retrospective cohort study using administrative claims data from all emergency department (ED) visits and hospitalizations at nonfederal acute care hospitals in California from 2005 to 2011, New York from 2006 to 2013, and Florida from 2005 to 2013. Trained analysts used standardized methods to collect data regarding discharges and reported these to state health agencies for regulatory purposes. After quality checking, these data were provided in a de-identified format to the Agency for Healthcare Research and Quality for its Healthcare Cost and Utilization Project [30]. Each patient was assigned an anonymous, unique linkage number that allowed for longitudinal tracking of ED encounters and hospitalizations [30, 31]. The Weill Cornell Medicine institutional review board approved our analysis of these data.
Patient Population
Our cohort consisted of all patients 18 years or older discharged at the time of their first recorded hospitalization for TBM, as defined by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 013.x in any discharge diagnosis position. Non-residents of New York, Florida, and California were excluded to maximize follow-up. In order to ensure at least 1 year of follow-up, patients whose index visit for TBM occurred in 2013 in New York or Florida or 2011 in California were excluded.
Measurements
Our outcomes of interest were death and the following neurological complications: stroke, seizure, hydrocephalus requiring ventriculoperitoneal shunt (VPS), vision impairment, and hearing impairment. Stroke was a composite of both ischemic and hemorrhagic stroke; ischemic stroke was defined using ICD-9-CM codes 433.x1, 434.x1, or 436 in any diagnosis code position in the absence of a primary discharge code for rehabilitation (V57) or any codes for subarachnoid hemorrhage (430), intracerebral hemorrhage (431) or trauma (800–804 and 850–854). Hemorrhagic stroke was defined by the presence of a discharge code for intracerebral hemorrhage (431) or subarachnoid hemorrhage (430) in the absence of a primary discharge code for rehabilitation (V57) or trauma (800–804 and 850–854). This algorithm has been validated to have a sensitivity of ≥82% and a specificity of ≥93% for both ischemic and hemorrhagic stroke subtypes [32]. Seizure was defined using ICD-9-CM codes 345.x in any discharge diagnosis position; this schema has a positive predictive value ranging from 84–98% in adult patients [33, 34]. Hydrocephalus was considered as only those cases that required a permanent VPS, defined by the presence of an ICD-9-CM procedure code 0.23 and 0.24 in any discharge diagnosis position; these codes have a 95% sensitivity and 100% specificity based on medical record review [35]. Using the methods of prior studies [36], vision impairment was defined as ICD-9-CM codes 360–379 in any discharge diagnosis position and hearing impairment as ICD-9-CM codes 380–390 in any discharge diagnosis position. Finally, subgroup analyses were performed stratified by the presence or absence of HIV, and compared the risks of complications associated with HIV after adjustment for the following covariates: age, sex, race, insurance status, HIV status, and the Elixhauser comorbidity index [37].
Statistical Analyses
Kaplan-Meier survival statistics were used to calculate cumulative rates of individual complications as well as the composite of any complication or death. Patients were censored at the time of death or at the end of the follow-up period. Cox proportional hazards regression was used to compare the likelihood of complications in patients with and without HIV after adjustment for demographics and the Elixhauser comorbidity index [38]. All analyses were performed using Stata/MP, version 13 (StataCorp, College Station, TX). The threshold of statistical significance allowed for an alpha error of 0.05.
Results
806 patients with TBM were identified. Their mean age was 50.7 (±17.1) years, 62.9% were male, and 14.5% had HIV. During a mean follow-up of 2.9 (±2.4) years, the cumulative rate of any complication or death was 55.4% (95% CI, 51.5–59.3%) (Figure 1). Patients with complications were slightly older and had a higher prevalence of medical comorbidities compared to TBM patients without complications (Table 1).
Figure 1. The Cumulative Rate of Complications or Death after Tuberculous Meningitis.
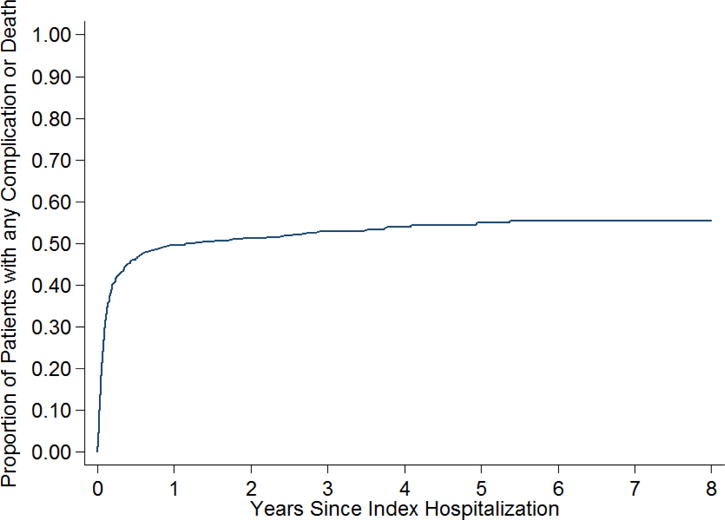
Kaplan-Meier curve showing the cumulative rate of neurological complications or death after tuberculous meningitis.
Table 1.
Characteristics of Patients, Stratified by Presence of any Complication, including Death
| Characteristica | Any Complication (N = 414) |
No Complication (N = 392) |
|---|---|---|
| Age, mean (SD), y | 56.2 (15.3) | 46.6 (20.1) |
| Female | 155 (37.4) | 144 (36.7) |
| Raceb | ||
| White | 88 (21.9) | 75 (20.0) |
| Black | 97 (24.1) | 82 (21.8) |
| Hispanic | 111 (27.6) | 96 (25.5) |
| Asian | 75 (18.7) | 81 (21.5) |
| Other | 31 (7.7) | 42 (11.2) |
| Payment source | ||
| Medicare | 115 (27.8) | 92 (23.5) |
| Medicaid | 145 (35.0) | 139 (35.5) |
| Private | 112 (27.1) | 98 (25.0) |
| Self-pay | 19 (4.6) | 32 (8.2) |
| Other | 23 (5.6) | 31 (7.9) |
| State | ||
| California | 209 (52.5) | 189 (48.5) |
| New York | 107 (48.4) | 114 (51.6) |
| Florida | 98 (52.4) | 89 (47.6) |
| HIV | 63 (15.2) | 54 (13.8) |
| Elixhauser comorbiditiesc, mean (SD) | 2.9 (1.9) | 2.2 (1.7) |
Data are presented as number (%) unless otherwise specified.
Self-reported by patients or their surrogates. Numbers do not sum to group totals because of missing race/ethnicity data in 3.5% of patients.
The Elixhauser comorbidities represent a comprehensive set of 28 comorbidity measures for use with large administrative datasets.
Abbreviations: SD, standard deviation
By 8 years, 21.5% (95% CI, 18.4–24.9%) of patients had died. Individual neurological complications were not uncommon. The cumulative rate of stroke was 16.8% (95% CI, 14.0–20.0%), of which the majority were ischemic (12.8% (95% CI, 10.3–15.7%)). The cumulative rate of seizure was 18.8% (95% CI, 15.4–22.8%) and that of VPS surgery was 8.4% (95% CI, 6.4–10.9%). Vision impairment occurred in 21.6% (95% CI, 18.5–25.1%) of patients and hearing impairment occurred in 6.8% (95% CI, 4.9–9.4%) of patients. The majority of complications occurred during the index hospitalization for TBM (Table 2), although when individually assessed, the cumulative rate of seizures, hydrocephalus requiring ventriculoperitoneal shunt, hearing impairment and death more than doubled throughout the follow-up period. Finally, the results of our study were similar when each state was analyzed separately.
Table 2.
Rates of Complications over Time
| Complication | During Initial Hospitalization | 6 Months | 1 Year | 8 Years |
|---|---|---|---|---|
| All Strokea | 11.2% (9.0–13.3%) | 13.4% (11.0–16.2%) | 15.0% (12.5–18.0%) | 16.8% (14.0–20.0%) |
| Ischemic Stroke | 8.4% (6.5–10.4%) | 10.5% (8.4–13.1%) | 11.8% (8.6–14.5%) | 12.8% (10.3–15.7%) |
| Seizure | 7.7% (5.8–9.5%) | 10.2% (8.1–12.8%) | 11.9% (9.6–14.7%) | 18.8% (15.4–22.8%) |
| VPS | 3.4% (2.1–4.6%) | 6.6% (4.9–8.8%) | 7.2% (5.5–9.6%) | 8.4% (6.4–10.9%) |
| Vision Impairment | 13.3 % (10.9–15.6%) | 17.5% (14.8–20.6%) | 18.7% (15.9–21.9%) | 21.6% (18.5–25.1%) |
| Hearing Impairment | 3.0% (1.8–4.2%) | 4.9% (3.5–6.8%) | 5.0% (3.6–7.0%) | 6.8% (4.9–9.4%) |
| Death | 10.5% (8.4–12.7%) | 16.8% (14.2–19.7%) | 18.3% (15.7–21.4%) | 21.5% (18.4–24.9%) |
| Any Complication | 36.8% (33.5–40.2%) | 45.7% (42.2–49.5%) | 48.9% (45.3–52.6%) | 55.4% (51.5–59.3%) |
Includes both ischemic and hemorrhagic stroke subtypes
Abbreviations: VPS, ventriculoperitoneal shunt
The cumulative rate of complications in HIV-infected individuals was 62.7% (95% CI, 51.5–74.0%) as compared to 54.1% (95% CI 50.1–58.3%) in HIV-uninfected patients. After adjusting for demographics and the Elixhauser comorbidity index, however, HIV infection was not associated with a higher risk of neurological complications or death (hazard ratio, 1.2; 95% CI, 0.9–1.6) (Table 3). Only hearing impairment was statistically more common in patients with HIV co-infection.
Table 3.
Rates of Complications, Stratified by HIV Status
| Complication | HIV Positive | HIV Negative | Hazard Ratioa |
|---|---|---|---|
| All Strokeb | 17.7% (11.4–26.9%) | 16.6% (13.7–20.1%) | 1.3 (0.7–2.3) |
| Ischemic Stroke | 16.0% (9.9–25.1%) | 12.2% (9.7–15.4%) | 1.5 (0.8–2.8) |
| Seizure | 21.7% (12.8–35.3%) | 18.2% (14.7–22.3%) | 0.6 (0.3–1.2) |
| VPS | 4.9 % (1.8–13.0%) | 9.0% (6.8–11.8%) | 0.4 (0.1–1.2) |
| Vision Impairment | 24.4% (16.8–34.6%) | 21.1% (17.8–25.0%) | 1.1 (0.7–1.7) |
| Hearing Impairment | 11.2% (5.7–21.3%) | 6.0% (4.1–8.6%) | 2.3 (1.0–5.2) |
| Death | 21.1% (14.1–30.8%) | 21.5% (18.2–25.3%) | 1.4 (0.9–2.5) |
| Any Complication | 62.7% (51.5–74.0%) | 54.1% (50.1–58.3%) | 1.2 (0.9–1.6) |
Adjusted for demographics and the Elixhauser comorbidity index
Includes both ischemic and hemorrhagic stroke subtypes
Abbreviations: VPS, ventriculoperitoneal shunt
Discussion
In a large cohort of patients with TBM in the United States, a neurological complication or death occurred in more than half of all patients. The majority of neurological complications occurred during the index hospitalization for TBM, suggesting that the highest at–risk period occurs soon after diagnosis. Concomitant HIV infection was not associated with a higher risk of mortality or neurological complications from TBM.
Comparison with other studies
Our results should be considered in light of several important prior international studies on TBM. Previously reported rates of individual neurological complications vary significantly, potentially in part due to small study populations and differences in patterns of care that may not apply universally. Hearing loss, for example, has previously been reported in 11–54% of patients with TBM [27–29]; however, streptomycin was part of the standard TBM treatment regimen in these studies, and as a known ototoxic agent [27], may partly explain the higher rates of hearing loss. Furthermore, complication rates differ depending on access to medical resources including antimicrobial therapy and advanced diagnostic techniques. The rate of stroke after TBM varies between 15 and 57% [9, 10, 12–24], with lower numbers reported in studies where stroke was identified clinically or by CT [15, 17] and higher numbers in studies in which serial MRIs [10, 14, 16, 21, 22] or autopsies were performed [12, 13]. The previously documented high rates of neurological complications may also reflect the fact that the majority of prior studies were performed in endemic areas, which are typically resource-limited countries with major gaps in healthcare infrastructure; however, in this study more than half of TBM patients died or had a neurological complication even in the United States, a country with general availability of critical care units, specialized healthcare providers including neurosurgeons, advanced neuroimaging, modern laboratory techniques to identify TBM, access to antimicrobial therapy, and rehabilitation centers. The high rate of complications seen in TBM is likely due to several factors including the already advanced severity of disease at presentation in the majority of patients, lack of accurate diagnostic measures to identify TBM, increased prevalence of drug resistant TBM, poor central nervous system (CNS) penetration of antimicrobials, and lack of knowledge regarding the appropriate timing and dose of corticosteroids [1, 2, 39, 40]. Furthermore, as the majority of complications occurred during the index hospitalization for TBM, further investigation into the pathophysiology of neurological injury due to TBM may lead to improved methods of both risk stratification and prevention.
Impact of HIV on risk of death or neurological complications after TBM
Contrary to the majority of prior literature performed in endemic regions, we found that patients with HIV infection were not at increased risk for death or neurological complications. Prior studies, however, recruited patients who were severely immunocompromised and who had poor access to antiretroviral therapy [2, 5]. In the study by Thwaites et al., the median CD4 count was 66 cells per cubic millimeter and none of the patients were receiving antiretroviral therapy [2]. Similarly, in the study by Heemskerk et al., the median CD4 count was 38 cells per cubic millimeter and antiretroviral therapy was not initiated until eight weeks after antituberculosis therapy was started [5]. Our results are, however, consistent with prior research from high-resource countries showing no difference in mortality between patients with TBM and HIV infection compared to HIV-uninfected patients [6, 41].
Limitations
Our study has several important limitations. First, this was a retrospective study that relied on administrative data to identify complications of TBM. Although well-validated ICD-9-CM codes were used to identify neurological complications, the authors could not assess certain clinical and microbiological data such as clinical severity of TBM at presentation, presence of multidrug resistance bacteria, and type or efficacy of treatment regimen, all of which may have affected rates of complications. Similarly, there was a lack of granularity regarding clinical characteristics of outcomes and thus, it is uncertain whether complications were directly related to TBM or a consequence of concomitant medical conditions. Second, the authors lacked data on the degree of immunosuppression in patients with HIV such as CD4 count and viral load. Third, patients with TBM who presented with a complication outside of New York, Florida and California were not captured as outcomes. The authors attempted to minimize this risk by only including residents of the three states studied. Lastly, the authors could not account for out-of-hospital death, which may have led to an underestimation in the mortality rate.
Conclusions
TBM is the most devastating manifestation of TB. Neurological complications after TBM were common even in a high-resource country such as the United States. Furthermore, although incident neurological complications most often occurred during the initial hospitalization for TBM, a substantial proportion occurred after hospital discharge, emphasizing the need for close follow-up of these patients.
Highlights.
Tuberculous meningitis is associated with a significant risk of neurological complications and death in the United States.
A neurological complications or death occurred in over half of all patients with tuberculous meningitis.
The majority of neurological complications or death occurred within the hospitalization for tuberculous meningitis.
Concomitant HIV infection does not appear to be associated with a higher risk of mortality or neurological complications in patients with tuberculous meningitis.
Acknowledgments
The authors are grateful to Monica Chen for her copyediting and clerical support.
Funding/role of funding source
This work was supported by National Institute of Neurological Disorders grants K23NS082367 and R01NS097443-01 (Kamel), the Michael Goldberg Stroke Research Fund (Thakur), and NIH grant 1R01HD074944-01 (Thakur). The funding sources played no role in study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None.
References
- 1.World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: WHO Press; 2015. [Google Scholar]
- 2.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 3.Torok ME, Yen NTB, Chau TTH, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis. 2011;52:1374–1383. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woldeamanuel YW, Girma B. A 43-year systematic review and meta-analysis: case-fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol. 2014;261:851–865. doi: 10.1007/s00415-013-7060-6. [DOI] [PubMed] [Google Scholar]
- 5.Heemskerk AD, Bang ND, Mai NTH, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374:124–134. doi: 10.1056/NEJMoa1507062. [DOI] [PubMed] [Google Scholar]
- 6.Berenguer J, Moreno S, Laguna F. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668–672. doi: 10.1056/NEJM199203053261004. [DOI] [PubMed] [Google Scholar]
- 7.Chan KH, Cheung RT, Fong CY, Tsang KL, Mak W, Ho SL. Clinical relevance of hydrocephalus as a presenting feature of tuberculous meningitis. QJM. 2003;96:643–648. doi: 10.1093/qjmed/hcg108. [DOI] [PubMed] [Google Scholar]
- 8.Srikantha U, Morab JV, Sastry S, et al. Outcome of ventriculoperitoneal shunt placement in Grade IV tubercular meningitis with hydrocephalus: a retrospective analysis in 95 patients. Clinical article J Neurosurg Pediatr. 2009;4:176–183. doi: 10.3171/2009.3.PEDS08308. [DOI] [PubMed] [Google Scholar]
- 9.Anderson NE, Somaratne J, Mason DF, Holland D, Thomas MG. Neurological and systemic complications of tuberculous meningitis and its treatment at Auckland City Hospital, New Zealand. J Clin Neurosci. 2010;17:1114–1118. doi: 10.1016/j.jocn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Javaud N, Certal Rda S, Stirnemann J, et al. Tuberculous cerebral vasculitis: retrospective study of 10 cases. Eur J Intern Med. 2011;22:e99–e104. doi: 10.1016/j.ejim.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Luma HN, Tchaleu BCN, Ngahane BHM, et al. Tuberculous meningitis: presentation, diagnosis and outcome in HIV-infected patients at the Douala General Hospital, Cameroon: a cross sectional study. AIDS Res Ther. 2013;10:16. doi: 10.1186/1742-6405-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dastur DK, Lalitha VS, Udani PM, Parekh U. The brain and meninges in tuberculous meningitis-gross pathology in 100 cases and pathogenesis. Neurol India. 1970;18:86–100. [PubMed] [Google Scholar]
- 13.Poltera AA, Templeton AC. Intracranial tuberculosis in Uganda: a post-mortem survey. Afr J Med Sci. 1973;4:343–349. [PubMed] [Google Scholar]
- 14.Offenbacher H, Fazekas F, Schmidt R, Kleinert R. MRI in tuberculous meningoencephalitis: report of four cases and review of the neuroimaging literature. J Neurol. 1991;238:340–344. doi: 10.1007/BF00315335. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh FY, Chia LG, Shen WC. Locations of cerebral infarctions in tuberculous meningitis. Neuroradiology. 1992;34:197–199. doi: 10.1007/BF00596334. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Gupta S, Singh D, Sharma B, Kohli A, Gujral RB. MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;36:87–92. doi: 10.1007/BF00588066. [DOI] [PubMed] [Google Scholar]
- 17.Ozates M, Kemaloglu S, Gurkan F, Ozkan U, Hosoglu S, Simsek MM. CT of the brain in tuberculous meningitis: a review of 289 patients. Acta Radiol. 2000;41:13–17. doi: 10.1034/j.1600-0455.2000.041001013.x. [DOI] [PubMed] [Google Scholar]
- 18.Lan SH, Chang WN, Lu CH, Lui CC, Chang HW. Cerebral infarction in chronic meningitis: a comparison of tuberculous meningitis and cryptococcal meningitis. QJM. 2001;94:247–253. doi: 10.1093/qjmed/94.5.247. [DOI] [PubMed] [Google Scholar]
- 19.Chan KH, Cheung R, Lee R, Mak W, Ho SL. Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis. 2005;19:391–395. doi: 10.1159/000085568. [DOI] [PubMed] [Google Scholar]
- 20.Koh SB, Kim BJ, Park MH, Yu SW, Park KW, Lee DH. Clinical and laboratory characteristics of cerebral infarction in tuberculous meningitis: a comparative study. J Clin Neurosci. 2007;14:1073–1077. doi: 10.1016/j.jocn.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Shukla R, Abbas A, Kumar P, Gupta RK, Jha S, Prasad KN. Evaluation of cerebral infarction in tuberculous meningitis by diffusion weighted imaging. J Infect. 2008;57:298–306. doi: 10.1016/j.jinf.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Anuradha HK, Garg RK, Agarwal A, et al. Predictors of stroke in patients of tuberculous meningitis and its effect on the outcome. QJM. 2010;103:671–678. doi: 10.1093/qjmed/hcq103. [DOI] [PubMed] [Google Scholar]
- 23.Sheu JJ, Hsu CY, Yuan RY, Yang CC. Clinical characteristics and treatment delay of cerebral infarction in tuberculous meningitis. Intern Med J. 2012;42:294–300. doi: 10.1111/j.1445-5994.2010.02256.x. [DOI] [PubMed] [Google Scholar]
- 24.Pasticci MB, Paciaroni M, Floridi P, Malincarne L, Scavizzi M, Baldelli F. Stroke in patients with tuberculous meningitis in a low TB endemic country: an increasing medical emergency? New Microbiol. 2013;36:193–198. [PubMed] [Google Scholar]
- 25.Lorber J. Long-term follow-up of 100 children who recovered from tuberculous meningitis. Pediatrics. 1961;28:778–791. [PubMed] [Google Scholar]
- 26.Sinha MK, Garg RK, Anuradha HK, et al. Vision impairment in tuberculous meningitis: predictors and prognosis. J Neurol Sci. 2010;290:27–32. doi: 10.1016/j.jns.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Walker AS. Hearing of patients after tuberculous meningitis. Proc R Soc Med. 1952;45:781–782. [PubMed] [Google Scholar]
- 28.Higton DI, James DW. Sequelae of tuberculous meningitis. Br Med J. 1964;2:584–585. doi: 10.1136/bmj.2.5409.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah I, Meshram L. High dose versus low dose steroids in children with tuberculous meningitis. J Clin Neurosci. 2014;21:761–764. doi: 10.1016/j.jocn.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research, Quality. Healthcare Cost and Utilization Project. http://hcupnet.ahrq.gov. Accessed 6 May, 2016.
- 31.Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methological issues when studying readmissions and revisits using hospital administrative data. U.S. Agency for Healthcare Research and Quality; 2011. (HCUP Methods Series Report #2011-01). http://www.ncupus.ahrq.gov/reports/methods/methods.jsp. Accessed 6 May, 2016. [Google Scholar]
- 32.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 33.Pugh M, Van Cott AC, Cramer JA, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000–2004. Neurology. 2008;70:2171–2178. doi: 10.1212/01.wnl.0000313157.15089.e6. [DOI] [PubMed] [Google Scholar]
- 34.Kee VR, Gilchrist B, Granner MA, Sarrazin NR, Carnahan RM. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):183–193. doi: 10.1002/pds.2329. [DOI] [PubMed] [Google Scholar]
- 35.Walcott BP, Iorgulescu JB, Stapleton CJ, Kamel H. Incidence, Timing, and Predictors of Delayed Shunting for Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. 2015;23:54–58. doi: 10.1007/s12028-014-0072-y. [DOI] [PubMed] [Google Scholar]
- 36.Lam BL, Lee DJ, Gómez-Marín O, Zheng DD, Caban AJ. Concurrent visual and hearing impairment and risk of mortality: the National Health Interview Survey. Arch Ophthalmol. 2006;124:95–101. doi: 10.1001/archopht.124.1.95. [DOI] [PubMed] [Google Scholar]
- 37.Elixhauser A, Steiner C, Harris DR, Coffey RN. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Sedlis SP, Hartigan PM, Teo KK, et al. Effect of pci on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373:1937–1946. doi: 10.1056/NEJMoa1505532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Török ME, Nguyen DB, Tran TH, et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6:e27821. doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010;90:279–292. doi: 10.1016/j.tube.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Dubé MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992;93:520. doi: 10.1016/0002-9343(92)90579-z. [DOI] [PubMed] [Google Scholar]


