Abstract
Few current studies compare the outcomes of islet transplantation alone (ITA) and pancreas transplantation alone (PTA) for type 1 diabetes (T1D). We examined these two beta cell replacement therapies in nonuremic patients with T1D with respect to safety, graft function and cost. Sequential patients received PTA (n = 15) or ITA (n = 10) at our institution. Assessments of graft function included duration of insulin independence; glycemic control, as measured by hemoglobin A1c; and elimination of severe hypoglycemia. Cost analysis included all normalized costs associated with transplantation and inpatient management. ITA patients received one (n = 6) or two (n = 4) islet transplants. Mean duration of insulin independence in this group was 35 mo; 90% were independent at 1 year, and 70% were independent at 3 years. Mean duration of insulin independence in PTA was 55 mo; 93% were insulin independent at 1 year, and 64% were independent at 3 years. Glycemic control was comparable in all patients with functioning grafts, as were overall costs ($138 872 for ITA, $134 748 for PTA). We conclude that with advances in islet isolation and posttransplant management, ITA can produce outcomes similar to PTA and represents a clinically viable option to achieve long-term insulin independence in selected patients with T1D.
Introduction
The most physiological method of achieving normoglycemia without the associated risk of hypoglycemia in patients with type 1 diabetes (T1D) is currently to restore islet function by vascularized pancreas transplantation or transplantation of isolated pancreatic islets. Although combined solid organ pancreas and kidney transplantation in uremic patients with diabetes is a complex procedure, advances in immunosuppressive regimens and surgical technique have made it increasingly successful, particularly because these patients enjoy the benefits of independence from dialysis (1,2). In contrast, solitary pancreas transplantation in nonuremic patients has received limited acceptance, primarily because of the associated surgical complications, the high risk of rejection and the nephrotoxic side effects of current immunosuppressive regimens in a patient population that is already at risk for renal dysfunction (1,3,4). This perception continues despite advances in immunosuppression and patient management that have improved 5-year graft survival rates in this patient population to >50% (2,5,6).
Islet transplantation offers a promising minimally invasive approach to restore insulin independence in patients with T1D without the surgical complications associated with whole-organ transplantation. Success rates for islet transplant alone (ITA) have traditionally been much lower than for pancreas transplant alone (PTA); however, this is changing with improvements in islet processing and immunosuppressive management. Consequently, islet transplantation is increasingly being considered as a realistic beta cell replacement option for patients with T1D who are not candidates for whole-organ transplant (7–13). Despite these innovations, there have not been any recent comparisons of these two therapies with respect to graft and patient outcomes. In the current study, we addressed this issue by comparing patient safety, graft survival and costs of ITA and PTA in nonuremic patients with T1D at our center.
Materials and Methods
Participants and study duration
Ten sequential nonuremic patients with T1D who received islet transplants from 2007 to 2010 as part of a clinical trial (13) were compared with 15 sequential nonuremic patients with T1D who underwent PTA from 2002 to 2011. Patients were followed until December 2012. All patients had undetectable baseline C-peptide levels and poor glycemic control manifested by elevated HbA1c levels, severe hypoglycemia and hypoglycemia unawareness. Complications related to the transplant were included if they occurred ≤4 years after transplantation because this allowed comparable follow-up for the majority of patients. The patient characteristics are listed in Table 1. All study procedures were reviewed and approved by the institutional review board at the University of California, San Francisco (UCSF), and all participants signed informed consent after extensive discussions with the transplant staff.
Table 1.
Baseline recipient characteristics
| Recipient characteristics (mean SD) | PTA (n = 15) | ITA (n = 10) | p-value* |
|---|---|---|---|
| Age, years | 42.5 ± 10.45 | 51.8 ± 8.3 | 0.02 |
| Duration of T1D, years | 29.9 ± 8.12 | 40.29 ± 11.10 | 0.014 |
| BMI | 24.9 ± 4.6 | 23.6 ± 3.3 | 0.41 |
| Male:female | 6:9 | 1:9 | |
| HbA1c, % | 7.3 ± 0.9 | 7.2 1 ± | 0.92 |
| Serum GFR#, mL/min/1.73m2 | 86.3 ± 18.0 | 79.0 ± 13.7 | 0.26 |
| T1D comorbidities | |||
|
| |||
| Autonomic neuropathy | 7 | 4 | |
| Retinopathy | 6 | 4 | |
| Proteinuria | 2 | 1 | |
| Gastroparesis | 4 | 3 | |
| CAD | 3 | 1 | |
| PVD | 1 | 0 | |
| Charcot arthropathy | 0 | 1 | |
CAD, coronary artery disease; HbA1c, hemoglobin A1c; ITA, islet transplant alone; PTA, pancreas transplant alone; PVD, peripheral vascular disease; SD, standard deviation.
The p-values were calculated using the Student’s t-test.
Calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (PTA) or measured directly using iohexol clearance (ITA).
Vascularized pancreas transplantation
Donor pancreata were procured from suitable deceased donors. Vascular preparation of the donor pancreas used a donor iliac artery Y-graft anastomosis to the splenic and superior mesenteric arteries, as described previously (1). Pancreata were implanted through a midline intraperitoneal approach with anastomosis to the right iliac vessels. Exocrine drainage was established by anastomosis of the graft duodenal segment to the recipient proximal ileum. One patient initially had bladder drainage and subsequently required enteric conversion. All patients received anticoagulation with heparin for 4–5 days after transplant.
Islet preparation and transplantation
Pancreatic islets were purified from pancreata procured from deceased donors, as described previously (14). Islets were maintained by in vitro culture for 36–48 h prior to transplantation, assessed for sterility and viability, and suspended in transplant medium supplemented with heparin (70 U/kg recipient body weight). Suitability for clinical islet transplantation was determined according to standard criteria (15). Islets were infused by percutaneous transhepatic portal vein catheterization with periodic portal pressure monitoring (16). All recipients received intravenous heparin infusions for 48 h after transplantation, followed by 5 days of twice-daily subcutaneous enoxaparin injections. Patients who were not insulin independent 2–3 mo after transplantation but who had detectable C-peptide received second islet transplants.
Immunosuppressive protocols
Pancreas recipients
Induction immunosuppression in all PTA recipients consisted of anti–thymocyte globulin (thymoglobulin, 6 mg/kg) and methylprednisolone. Maintenance therapy consisted of mycophenolate mofetil (MMF), tacrolimus and prednisone (5 mg/day). Sirolimus was added 3–4 weeks after transplant in 12 of 15 patients, with target serum levels ranging from 8–12 ng/mL.
Islet recipients
Induction immunosuppression in all ITA recipients consisted of thymoglobulin (4 mg/kg) and was initiated 2 days prior to islet transplant. A single dose of methylprednisolone was used as premedication prior to the first dose of thymoglobulin. Patients were then maintained on belatacept (BELA; LEA29Y; Bristol-Myers Squibb, New York, NY), a costimulatory signal-blocking fusion protein that binds ligands CD80 and CD86 on antigen-presenting cells, or efalizumab (EFA; Raptiva; Genentech, Inc., South San Francisco, CA), an antileukocyte functional antigen-1 antibody. BELA-treated patients (n = 5) received BELA at a dose of 10 mg/kg intravenously (IV) on days 0, 4, 14, 28, 56, and 75 after transplant, followed by 5 mg/kg IV every 4 weeks until 18 mo after final transplant, at which time the dosing frequency was further reduced to 5 mg/kg every 8 weeks. This regimen corresponds to the “less intensive” regimen used in published kidney transplant studies (17). EFA-treated patients (n = 5) received EFA at a dose of 1 mg/kg per week subcutaneously, starting 1 day prior to transplant and continuing for 3 mo after transplant, after which the dose was reduced to 0.5 mg/kg per week. Both groups also received sirolimus (target levels 4–8 ng/mL) with or without MMF (13). Patients who required a second islet transplant received induction immunotherapy with basiliximab (20 mg IV on days 0 and 4 relative to transplant), had EFA or BELA dosing recycled according to the dosing guidelines for the first islet transplant, and continued their other immunosuppressive medications. EFA was discontinued in all patients in May 2009 due to safety concerns, as described previously (18). These patients continued on a combination of sirolimus and mycophenolate, with the addition of low-dose tacrolimus in one patient not able to tolerate therapeutic levels of sirolimus.
Assessment of graft function
Blood glucose levels in ITA recipients were measured three to five times per day with a glucometer throughout the study, and all patients kept diaries of carbohydrate intake and insulin requirements. They underwent mixed meal tolerance testing (MMTT) and continuous glucose monitoring for 24 h every 3 mo during the first year after transplantation and every 6 mo thereafter (19,20). PTA patients had comprehensive blood tests (including fasting glucose levels) drawn at least monthly, were instructed to monitor blood glucose periodically at home, and had hemoglobin A1c (HbA1c) measured and reviewed every 3 mo. All patients were considered to be insulin independent if they remained off insulin with Hba1c values within the normal range of our laboratory (4.3–6.5%) and fasting blood glucose levels <126 mg/dL.
Assessment of renal function
Serum creatinine (Cr) levels were measured at baseline and at regular intervals in both the PTA and ITA groups. In PTA recipients, estimated GFRs (eGFRs) were calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. In ITA recipients, GFRs were determined using iohexol clearance before transplantation and biannually thereafter (21). Formal urine protein and albumin excretion rates with 24-h or spot urine collections were performed at regular intervals in the ITA group, per study protocol, but not in the PTA group.
Cost analysis
All costs associated with the transplant process and hospitalization were collected for up to 4 years following transplant for each recipient. These included the costs of organ acquisition and processing, transplant procedure, and all other aspects of the related hospitalization. In addition, the analysis included costs of any complications that required readmission during the follow-up period. The availability of data on the costs of outpatient follow-up visits and treatments was limited; therefore, these costs were not included in the present analysis. All costs were normalized to 2012 data using the standard consumer price index available from Oregon State University to account for inflation and changes in costs over the years (22).
Statistical analysis
Data are expressed as means plus or minus standard deviation unless otherwise stated. Differences between groups were determined using the Student’s t-test. The p-values <0.05 were considered significant. Kaplan-Meier curves were created to display insulin independence in the ITA and PTA recipients, and the Cox proportional hazards model was used for univariate analysis of the time-to-event data for graft failure. Data analysis was performed using Stata 12.0 statistical software (Stata Corp, College Station, TX).
Results
Recipient characteristics
The baseline clinical characteristics and posttransplant courses of the 10 ITA recipients and 15 PTA recipients are shown in Table 1. The mean age at the time of transplant and the duration of diabetes were lower in PTA recipients than in ITA recipients. There were proportionally more male patients in the PTA group. The other characteristics did not show statistically significant differences.
Posttransplant graft function
The islet doses and infusions for each ITA recipient are listed in Table 2. All preparations intended for clinical use met the clinical release criteria. The length of follow-up, timing of islet transplantation, duration of insulin independence, and timing of EFA discontinuation are depicted in Figure 1. The follow-up duration for the ITA group ranged from 38 to 66 mo. All five BELA patients became insulin independent after the first transplant; patients BELA-4 and -5 resumed exogenous insulin (days 645 and 300, respectively) and became insulin independent after second transplants. BELA-1 and -3 remained insulin independent after one transplant, and BELA-2 resumed insulin use (day 720), although she continued to make C-peptide. All five EFA patients achieved insulin independence after their first islet transplant. Two remained independent, and three resumed partial insulin use and became independent after a second transplant. Patients EFA-3 and -5 resumed insulin use (days 645 and 240, respectively) after discontinuation of EFA but continued to make C-peptide. Mean duration of insulin independence in the ITA group was 35 mo at most recent follow-up, with 90% maintaining insulin independence at 1 year and 70% maintaining insulin independence at 3 years (Figures 1 and 3).
Table 2.
Islet equivalents used in ITA recipients
| Patient | IEQ Tx 1 | IEQ Tx 2 | Total IEQ/kg |
|---|---|---|---|
| EFA-1 | 672 070 | 600 900 | 18 720 |
| EFA-2 | 661 409 | 11 023 | |
| EFA-3 | 482 050 | 8 034 | |
| EFA-4 | 630 165 | 11 670 | |
| EFA-5 | 574 950 | 351 195 | 13 620 |
| BELA-1 | 507 660 | 7 577 | |
| BELA-2 | 645 500 | 10 940 | |
| BELA-3 | 691 500 | 12 805 | |
| BELA-4 | 608 400 | 695 193 | 17 381 |
| BELA-5 | 557 500 | 419 000 | 17 754 |
| Mean | 595 459 | 516 572 | 12 952 |
BELA, belatacept; EFA, efalizumab; IEQ, islet equivalents; ITA, islet transplant alone; Tx, transplant.
Figure 1.
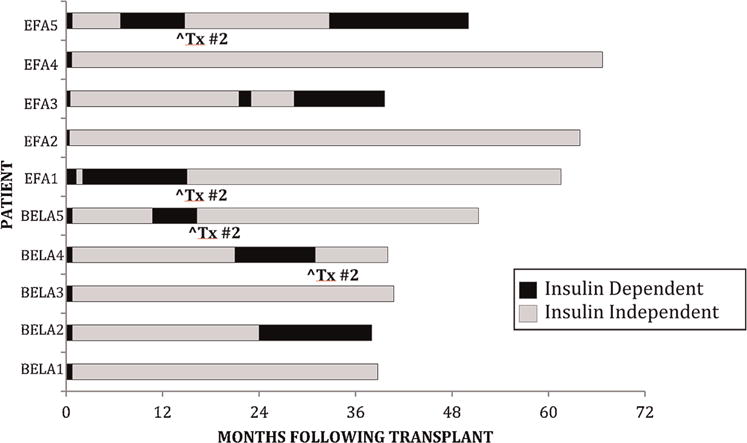
Duration of follow-up and insulin independence in islet transplant recipients. ^Tx#2 shows time of second islet infusion.
Figure 3.
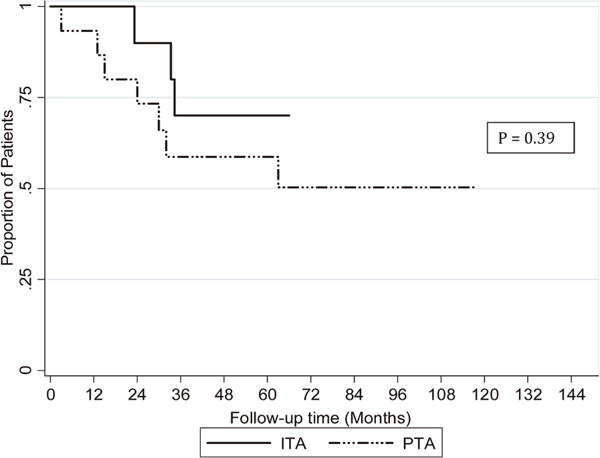
Kaplan Meier estimate of insulin independence in ITA and PTA recipients. ITA, islet transplant alone; PTA, pancreas transplant alone
The follow-up duration for the 15 PTA recipients ranged from 12 to 118 mo. The mean duration of insulin independence, based on normal HbA1c values in the absence of exogenous insulin use, was 55 mo, with a range of 3–117 mo. Fourteen of 15 (93%) maintained insulin independence at 1 year, and 9 of 14 (64%) maintained insulin independence at 3 years (Figures 2 and 3).
Figure 2.
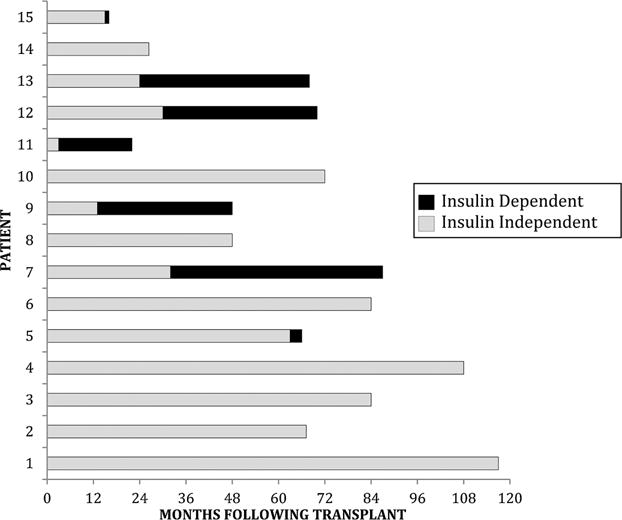
Duration of follow-up and insulin independenceinpancreastransplantrecipients.
Glycemic control after transplantation
Glycemic control improved and severe hypoglycemic episodes resolved in all ITA recipients after transplantation. Importantly, this effect persisted in recipients who resumed insulin use. Mean pretransplant HbA1c levels in the ITA group were elevated (7.2%) and decreased to 5.7% at the time of most recent follow-up (mean 54 mo after transplant). MMTTs in BELA-treated patients performed at least 180 days after final islet transplant showed appropriate C-peptide responses, with mean fasting and stimulated C-peptide levels of 1.2±0.3 and 7.3±5.6 ng/mL, respectively. MMTTs in the insulin-independent EFA-treated patients also showed appropriate fasting and stimulated C-peptide responses (1.2±0.3 and 6.1±2.0 ng/mL, respectively). In contrast, MMTT results in the two EFA-treated patients who resumed insulin showed significantly reduced fasting and stimulated C-peptide responses (patient EFA-3: 0.4 and 2.1 ng/mL, respectively; patient EFA-5: 0.4 and 0.8 ng/mL, respectively).
As expected, the PTA group also experienced dramatically improved glycemic control. Those with successful graft function had complete resolution of severe hypoglycemia and reduction of mean HbA1c levels from 7.3 ± 0.9% to 5.5 ± 0.9% at time of most recent follow-up (mean 62 mo after transplant). Incontrast to the ITA group, the PTA recipients did not undergo routine fasting C-peptide measurements or MMTTs.
Renal function
The mean GFR in the ITA group was 79±13.7 mL/min per 1.73 m2 before transplant and 72.9±20.4 mL/min per 1.73 m2 (p = 0.5 compared with pretransplant values) at the most recent follow-up (mean 54 mo after transplant). One patient (EFA-4) developed a transient increase in Cr from 0.9 to 2.88 mg/dL, but this level returned to baseline during the study period. The remainder of the islet recipients maintained normal serum Cr after transplantation. The 24-h urinary protein and albumin excretion rates were normal in the islet recipients prior to transplant and remained normal in six recipients after transplant. Four patients developed mild microalbuminuria (mean excretion rate of 82.4 mg per 24 h) at the time of most recent follow-up.
The mean pretransplant eGFR in the PTA group was 86.3 ± 18 mL/min per 1.73 m2 and decreased to 67.9 ± 25.4 mL/min per 1.73 m2 at the most recent post-transplant measurement (p=0.025 compared with pre-transplant measurement). =Four PTA recipients had reduced eGFRs (25.8–48.8 mL/min per 1.73 m2; mean: 36.7 ± 7.4 mL/min per 1.73 m2) at most recent follow-up. One patient developed renal failure requiring dialysis. The remaining PTA recipients maintained baseline Cr levels. Surveillance urine microanalyses on PTA recipients with stable GFRs were negative for proteinuria after transplantation, although formal testing for microalbuminuria was not routinely performed.
Adverse events
In the ITA group, complications included self-limited bleeding from the liver puncture site that resolved without need for transfusion or intervention (n = 1), partial portal vein thrombosis that was successfully treated with oral anticoagulation therapy (n = 1), and posttransplant lympho-proliferative disorder (PTLD) that resolved after reduction of immunosuppression and outpatient treatment with rituximab (n = 1) (Table 3). One patient developed pulmonary aspergillosis that was successfully treated with systemic antifungal agents. Symptomatic oral ulcers developed in four patients taking sirolimus and improved with topical therapy and reduced sirolimus dosing or substitution with MMF. Additional side effects included nausea (n = 3), diarrhea (n = 4), transient neutropenia (n = 4), transient anemia (n = 10) and transient thrombocytopenia (n = 2).
Table 3.
Complications following transplantation
| Complications* | PTA (n = 15) |
ITA (n = 10) |
|---|---|---|
| Surgical | ||
| Graft thrombosis requiring pancreatectomy | 3 | 0 |
| Anastomotic leak requiring pancreatectomy | 1 | 0 |
| Enteric conversion | 1 | 0 |
| Small bowel obstruction | 1 | 0 |
| Incisional hernia | 2 | 0 |
| Intra-abdominal abscess | 1 | 0 |
| Medical | ||
| Acute rejection, treated pharmaceutically | 1 | 0 |
| Posttransplant lymphoproliferative disorder | 1 | 1 |
| Gastric ulcer | 1 | 0 |
| Vascular | ||
| Partial portal vein thrombosis | 0 | 1 |
| Pulmonary embolism | 1 | 0 |
| Self-limited bleed during islet infusion | 0 | 1 |
| Infectious | ||
| Readmission for surgical site infection | 1 | 0 |
| CMV viremia | 2 | 0 |
| Pulmonary aspergillosis | 0 | 1 |
| Renal | ||
| Renal insufficiency | 5 | 1 |
| New onset proteinuria | 1 | 3 |
| Renal failure due to acute tubular necrosis | 1 | 0 |
CMV, cytomegalovirus; ITA, islet transplant alone; PTA, pancreas transplant alone.
Complications were included if they developed within 4 years of initial transplant.
Complications in the PTA group included pancreatectomy for graft thrombosis (n = 3) or anastomotic leak (n = 1), small bowel obstruction requiring surgical intervention (n = 1), conversion from bladder to enteric drainage for recurrent dehydration or bladder infections (n = 1), and symptomatic incisional repair (n = 2). Infectious complications included a surgical site infection requiring an additional hospitalization (n = 1) and readmission for percutaneous abscess drainage (n = 1). Medication side effects included oral ulcerations related to sirolimus (n = 4), dose which resolved with reduction and topical therapy. Two patients developed cytomegalovirus viremia that required outpatient. One patient developed PTLD, which necessitated discontinuation of all immunosuppressive medications and resulted in graft loss. One developed renal failure and dialysis dependence (Table 3). Additional adverse events related to medications were similar to the ITA group. None of the PTA patients who lost graft function were retransplanted during the study period.
Hospital duration and cost analysis
The mean hospital duration for each islet transplant was 5.75 days, and the mean normalized cost for each first transplant was $99 194.48 ± 4310.80. Four of 10 recipients required a second islet transplant, doubling their mean transplant costs to $198 388.96 ± 4658.00 per patient. When the additional expense of the second transplants was included in the entire ITA group costs, the final mean cost per patient increased to $138 872.27. There were no inpatient hospitalizations for transplant-related complications in the first 4 years after transplant in the ITA group.
Total inpatient hospital cost records were available for 14 of 15 PTA recipients. Cost records for one patient who died in the first year after transplantation, for reasons unrelated to the transplant, were incomplete and thus were not included in the analysis. The mean duration of hospitalization for this group was 12 days, and the mean normalized transplant and hospitalization cost was $109 041.44 ± 26 147.04. Surgical and medication-related complications requiring inpatient treatment occurred in eight of 14 patients within 4 years following transplantation, giving an additional mean cost of $25 706.64 ± 16 059.98 per patient. Incorporating this additional expense increased the overall mean cost per patient to $134 748.08 ± 42 207.02. The normalized itemized and total costs of both types of transplant hospitalizations are listed in Table 4.
Table 4.
Cost analysis
| Itemized expenses | PTA, mean costs, $ | ITA, mean costs, $ |
|---|---|---|
| Operating room | 16 475.20 | – |
| Interventional radiology | – | 4272.22 |
| Anesthesia care | 1025.37 | – |
| Hospital room/nursing | 15 907.99 | 7887.24 |
| Medications (including induction immunosuppressive therapy) | 12 693.34 | 5025.67 |
| Imaging | 695.89 | 810.61 |
| Laboratory | 3405.17 | 1837.65 |
| Organ and procurement costs | 37 847.00 | 37 847.00 |
| Islet processing | – | 30 621.00 |
| GMP facility fee | – | 7000.00 |
| Surgical recovery fee | 1950.00 | 1950.00 |
| Organ transport fee | 5982.00 | 5982.00 |
| Additional miscellaneous costs | 13 059.48 | 3848.33 |
| Mean total cost for a single uncomplicated procedure | 109 041.44 | 99 194.48 |
| Mean total cost incorporating complications and second islet transplants (ITA) | 134 748.08 | 138 872.27 |
GMP, good manufacturing practice; ITA, islet transplant alone; PTA, pancreas transplant alone.
Discussion
This retrospective single-center analysis provides evidence showing that for selected patients with T1D, beta cell replacement therapy with islet transplantation can have efficacy and cost outcomes comparable to vascularized pancreas transplantation. We found similar graft survival rates of 60–70% at 3 years and similar average costs in the two groups, even if two islet infusions were required in some ITA patients. PTA patients routinely achieved insulin independence after the first transplant, which reduced organ acquisition-related costs but had longer hospitalizations and more complications requiring readmission. In contrast, some ITA recipients required two transplants but had shorter hospitalizations and fewer serious complications.
These results contrast with a 2004 study by Frank et al, who reported that vascularized pancreas transplantation was more efficacious than islet transplantation, with 100% of whole-pancreas recipients versus 56% of islet recipients maintaining insulin independence at 2 years (3). The study, however, did not evaluate patients who had received PTA but rather those who had received simultaneous pancreas and kidney (SPK) or pancreas after kidney (PAK) transplants. A more recent study reported significantly better initial insulin independence rates in PTA versus ITA recipients (75% vs. 57%, respectively) after a follow-up period of 1–7 years. A possible explanation for the worse outcomes in the ITA group in that study is that the majority of patients received daclizumab induction therapy rather than thymoglobulin-based induction. A number of recent publications have demonstrated that thymoglobulin-based induction regimens achieve higher insulin independence rates in islet transplantation, and this is now considered the preferred approach (23). Another study comparing a group of SPK and PAK recipients with a group of simultaneous islet and kidney (SIK) and islet after kidney (IAK) transplant recipients found that the rate of severe hypoglycemia improved by >90% in both the islet and pancreas transplant groups, and HbA1c levels declined from 7.8 ± 1.3% to 5.9 ± 1.1% in the SPK/PAK group and from 8.0 ± 1.3% to 6.5 ± 1.1% in the SIK/IAK group; however, the 5-year insulin independence rates in the SPK/PAK group were significantly higher than in the SIK/IAK group (73.6% vs. 9.3%, respectively) (24). In the current study, we also found similar improvements in glycemic control in ITA and PTA recipients and significantly higher rates of serious complications in PTA recipients, as reported by others (4).
In contrast to the previously mentioned studies, we observed higher rates and longer durations of insulin independence. Our ITA independence rates were higher than those reported in national databases (Collaborative Islet Transplant Registry), in which the insulin independence rate at 3 years was ≈44% (23). We believe several factors contributed to the higher success rates, including the use of novel immunosuppressive medications, improved donor selection and optimization of the islet manufacturing protocol. Recently, independence rates comparable to ours were reported by several other institutions that have made similar modifications in processing and immunosuppression (25,26). In contrast, our PTA group demonstrated graft survival rates comparable to those observed nationally, with 100% insulin independence at 1 year after transplant and a long-term graft survival rate of 53% at most recent follow-up (3.5–8.8 years) based on normal HbA1c values in the absence of exogenous insulin use (2).
As one would expect, the causes and the tempo of graft failure were quite different between groups. In general, ITA patients lost graft function slowly, and the vast majority who resumed insulin use continued to have residual islet function, as demonstrated by the presence of C-peptide and lower insulin requirements. In contrast, PTA recipients typically lost their grafts due to technical complications, such as thrombosis or anastomotic leaks. Such graft loss was rapid and complete, resulting in loss of all islet function, and frequently required prolonged hospitalization. The relatively short follow-up and small size of our study did not allow a comparison of long-term glycemic control and other outcomes between ITA and PTA patients who resumed insulin use, but such a comparison would be important in a larger prospective study to quantify the benefits of partial islet function in ITA recipients.
Although overall renal function was comparable between the ITA and PTA groups, there was a significant decrease in posttransplant renal function in the PTA group. This was not seen in the ITA group. A possible reason for this difference is that the antibody-based immunosuppressive protocols used in the ITA group were less nephrotoxic because they minimized calcineurin inhibitor use. Additional larger studies in both islet and pancreas transplantation are needed to further characterize the potential benefits of these regimens.
An important goal of this study was to compare the financial impact of each procedure. Not surprisingly, we found the overall cost of a single ITA to be comparable to a single uncomplicated PTA, with the greatest costs attributable to organ acquisition and operative procedure (PTA) or islet manufacture (ITA). The normalized costs presented in this study are similar to those reported by Guignard et al, who found that the average cost of a single islet transplant was $101 845 (converted from euros to US dollars at the 2012 exchange rate) in a Swiss–French consortium (27). More recently, Gerber et al compared hospitalization costs of SPK transplants to those of SIK transplants in Switzerland and reported average costs of hospitalization of $73 619 versus $70 337, respectively, when converted from euros to US dollars at the 2012 exchange rate (28). Although these results may not be comparable to ours due to national differences in organ-acquisition costs, they nevertheless demonstrate that the costs of the two procedures are relatively similar.
As expected, the itemized cost distribution was markedly different in the two groups. In the ITA group, some patients went on to require a second transplant to achieve insulin independence, and this contributed to a large portion of the mean cost. In contrast, the PTA group accumulated additional costs primarily as a result of perioperative surgical complications and associated readmissions. Although the surgical complication rates for PTA have remained relatively stable during the past decade, durable insulin independence after a single infusion of islets is becoming more common with improvements in manufacturing techniques, immunosuppression, and recipient clinical management. If this trend continues, it is foreseeable that islet transplantation may eventually become more cost-effective than vascularized pancreas transplantation in selected nonuremic patients with T1D. One recurrent criticism of islet transplantation is that the need for multiple islet infusions will further reduce an already small donor pool and thus may affect organ availability for other types of pancreas transplantation. It is unlikely, however, that significant competition between these groups would occur because donor organs that are most suitable for islet isolation generally come from older patients with higher BMI, whereas organs suitable for vascularized transplantation usually come from younger, leaner donors.
A number of limitations need to be considered when interpreting our findings. First, this is a single-center retrospective trial with relatively few patients and thus may not be representative of current national graft survival and complication rates. Larger prospective studies will be needed to confirm our trends and observations. Second, although this analysis provides some insight into the efficacy, safety and costs of the two modes of transplantation, the two groups of recipients are not directly comparable because they differ in baseline age, duration of diabetes, sex distribution and maintenance immunosuppressive therapies. Moreover, follow-up assessments and monitoring of glucose control differed between the groups. ITA recipients were monitored closely after transplantation because they were all enrolled in study protocols, and metabolic studies and graft dysfunction (loss of insulin independence) were strictly defined. PTA recipients were not enrolled in clinical trials, and thus were monitored per standard of care (monthly lab work). The rigorous metabolic studies performed in the islet patients were not performed in this group. At UCSF, graft survival is defined as insulin independence in the absence of oral agents or insulin. The costs of ambulatory care, including follow-up visits and medications, imaging and laboratories for outpatient complications were not included. Last, cost assessments may differ between the two groups because PTA patients were managed according to the standard of care and ITA patients were managed according to a research protocol.
In conclusion, we provide preliminary evidence that with recent improvements in immunosuppression and islet manufacturing, the efficacy and cost of islet transplantation can approach those of vascularized pancreas transplantation in nonuremic patients with T1D. Furthermore, we demonstrate that ITA has a more favorable safety profile than PTA. Although islet transplantation is currently considered investigational in the United States, several other countries now consider it a clinically appropriate procedure that is reimbursable by health insurance (29,30). If such a view were to be adopted in United States, ITA should be considered as an alternative beta cell replacement therapy for selected patients who may not be appropriate candidates for whole-organ pancreas transplantation.
Acknowledgments
This work was in part supported by a grant from the Juvenile Diabetes Research Foundation (4-2004-372). S. Moassesfar was supported in part by the National Institutes of Health (NIH) training grant T32DK007161-40 and the NIH 5K12DK094726-04 grant. The University of California San Francisco (UCSF) islet facility is supported in part by NIH grants P30 DK63720, UO1 AIO65193, and Clinical Research Center (CRC) grant UL1 RR024131. We are indebted to the many persons without whose enthusiastic participation and help this study would never have been accomplished: the study nurse coordinators, Joan McElroy and Debbie Ramos; the administrative/regulatory personnel, Tina Johnson and Tara Rojas; the islet isolation team, Florinna Dekovic, Jiena Lang and Vihn Nguyen; and the capable staff of the UCSF CRC.
Abbreviations
- BELA
belatacept
- CAD
coronary artery disease
- CMV
cytomegalovirus
- Cr
creatinine
- EFA
efalizumab
- eGFR
estimated GFR
- HbA1c
hemoglobin A1c
- IEQ
islet equivalents
- IAK
islet after kidney
- ITA
islet transplant alone
- IV
intravenously
- MMF
myco-phenolate mofetil
- MMTT
mixed meal tolerance test
- PAK
pancreas after kidney
- PTA
pancreas transplant alone
- PTLD
posttransplant lymphoproliferative disorder
- PVD
peripheral vascular disease
- SD
standard deviation
- SIK
simultaneous islet and kidney
- SPK
simultaneous pancreas and kidney
- T1D
type 1 diabetes
- Tx
transplant
- UCSF
University of California San Francisco
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Lipshutz GS, Wilkinson AH. Pancreas-kidney and pancreas transplantation for the treatment of diabetes mellitus. Endocrinol Metab Clin N Am. 2007;36:1015–1038. doi: 10.1016/j.ecl.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Gruessner ACl, Sutherland DE. Pancreas transplant outcomes for United States (US) cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) Clin Transpl. 2008:45–56. [PubMed] [Google Scholar]
- 3.Frank A, Deng S, Huang X, et al. Transplantation for type 1 diabetes: Comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240:631–643. doi: 10.1097/01.sla.0000140754.26575.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maffi P, Scavini M, Socci C, et al. Risks and benefits of transplantation in the cure of type 1 diabetes: Whole pancreas versus islet transplantation. A single center study Rev Diabet Stud. 2011;8:44–50. doi: 10.1900/RDS.2011.8.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaba R, Garcia-Roca R, Oberholzer J. Pancreatic islet cell transplantation: An update for interventional radiologists. J Vasc Interv Radiol. 2012;23:583–594. doi: 10.1016/j.jvir.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 6.Niclauss N, Morel P, Berney T. Has the gap between pancreas and islet transplantation closed? Transplantation. 2014;98:593–599. doi: 10.1097/TP.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 8.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group [correction published. N Engl J Med. 2000;342:1376. doi: 10.1056/nejm200005043421820. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2000;342:381–389. [Google Scholar]
- 10.Singh RP, Stratta RJ. Advances in immunosuppression for pancreas transplantation. Curr Opin Organ Transplant. 2008;13:79–84. doi: 10.1097/MOT.0b013e3282f2fd91. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland DE, Gruessner AC, Gruessner RW. Pancreas transplantation: A review. Transplant Proc. 1998;30:1940–1943. doi: 10.1016/s0041-1345(98)00489-8. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland DE, Gruessner RG, Humar A, et al. Pretransplant immunosuppression for pancreas transplants alone in nonuremic diabetic recipients. Transplant Proc. 2001;33:1656–1658. doi: 10.1016/s0041-1345(00)02629-4. [DOI] [PubMed] [Google Scholar]
- 13.Posselt A, Bellin M, Tavakol M. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10:1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 15.Szot GL, Lee MR, Tavakol MM, et al. Successful clinical islet isolation using a GMP-manufactured collagenase and neutral protease. Transplantation. 2009;88:753–756. doi: 10.1097/TP.0b013e3181b443ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen RJ, Ryan EA, O’Kelly K, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: Radiologic aspects. Radiology. 2003;229:165–170. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 18.Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: A cautionary tale for dermatologists. Arch Dermatol. 2009;145:937. doi: 10.1001/archdermatol.2009.175. [DOI] [PubMed] [Google Scholar]
- 19.Faradji RN, Monroy K, Messinger S, et al. Simple measures to monitor beta-cell mass and assess islet graft dysfunction. Am J Transplant. 2007;7:303–308. doi: 10.1111/j.1600-6143.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 20.Paty BW, Senior PA, Lakey JR, Shapiro AM, Ryan EA. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther. 2006;8:165–173. doi: 10.1089/dia.2006.8.165. [DOI] [PubMed] [Google Scholar]
- 21.Brandstrom E, Grzegorczyk A, Jacobsson L, Friberg P, Lindahl A, Aurell M. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe Nephrol Dial Transplant. 1998;13:1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 22.Consumer Price Index (CPI) Conversion Factors 1774 to Estimated 2024 to Convert to Dollars of 2012. Available from: http://webcache.googleusercontent.com/search?q=cache:SAut7q7-x2QJ:liberalarts.oregonstate.edu/files/polisci/faculty-research/sahr/inflation-conversion/excel/cv2012xls+&cd=2&hl=en&ct=clnk&gl=us.
- 23.Collaborative Islet Transplant Registry. CITR sixth annual report. [cited 2012 Feb] Available from: https://web.emmes.com/study/isl/reports/CITR%206th%20Annual%20Data%20Report%20120109.pdf.
- 24.Lehmann R, Jessica G, Brockmann J, et al. Glycemic control in simultaneous islet-kidney versus pancreas-kidney transplantation in type 1 diabetes: A prospective 13-year follow-up. Diabetes Care. 2015;38:752–759. doi: 10.2337/dc14-1686. [DOI] [PubMed] [Google Scholar]
- 25.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickels MR, Kong SM, Fuller C, et al. Improvement in insulin sensitivity after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2013;98:E1780–E1785. doi: 10.1210/jc.2013-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guignard A, Oberholzer J, Benhamou P, et al. Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care. 2004;27:895–900. doi: 10.2337/diacare.27.4.895. [DOI] [PubMed] [Google Scholar]
- 28.Gerber PA, Pavlicek V, Demartines N. Simultaneous islet kidney vs pancreas kidney transplantation in type 1 diabetes mellitus: A 5 year single centre follow-up. Diabetologia. 2008;51:110–119. doi: 10.1007/s00125-007-0860-4. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro J. Islet transplantation in type 1 diabetes: Ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9:385–406. doi: 10.1900/RDS.2012.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peixoto E, Alejandro R. Meeting Report: 14th World Congress of the International Pancreas Transplantation and Islet Transplantation Association (IPITA) 2013. CellR4. 2013;1:e625. [Google Scholar]


