People with autism spectrum disorder (ASD) show atypical attention to social stimuli [1] and gaze at faces [2] and complex images [3] in unusual ways. But all studies to date are limited by the experimenter’s selected stimuli, which are generally photographs taken by people without autism. What might participants with ASD show us if they were the ones taking the photos? We gave participants a digital camera and analysed the photos they took: images taken by participants with ASD had unusual features and showed strikingly different ways of photographing other people.
Sixteen participants with ASD and 21 matched controls were instructed to take photos freely with the same camera under three blocked conditions: indoors and of people in our laboratory; indoors in the lab but not of people; and outdoors. Participants with ASD took more photos of other people than did controls (mean 16 versus 10), but similar numbers of indoor and outdoor photos not containing people. They also spent slightly longer than controls on the entire photography session (41 versus 31 minutes), and in particular spent significantly more time taking photos of other people (12 versus 4 minutes) because it took them nearly twice as long as controls for each person photographed (see Supplemental Information for full details).
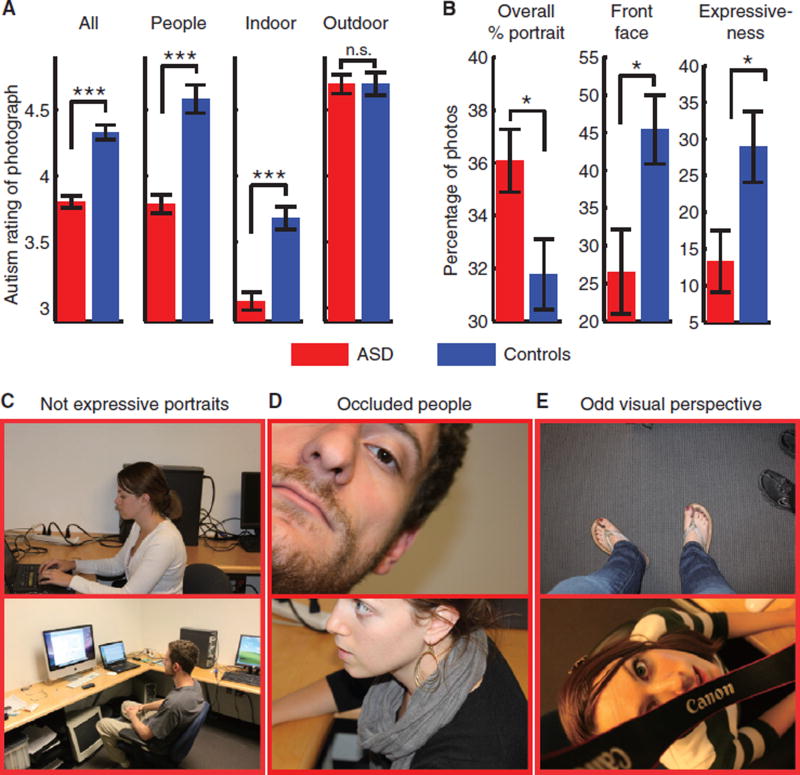
Three independent raters familiar with the clinical presentation of ASD and reliable on the ADOS-2 [4] were asked to judge whether each photo was taken by a subject with ASD or a control (1–9 scale; 1 = ASD, 9 = control; photos were shown in random order blocked by photography session, and raters were blind to the identity of the subject). Raters successfully distinguished photos taken by subjects with ASD from those taken by controls at the group level (Ps < 0.05 for every rater; Figures 1A, S1), but ratings were not correlated with any other individual differences. Raters noted repetitions of specific objects, photos of bodies without faces, photos of other people’s faces that lacked engagement with the photographer, focus on single and often unusual objects, and odd visual perspective and occluded objects (Figures 1C–1E, S2C–S2H for examples).
Figure 1. Photographs taken by participants with ASD and controls.
(A) Ratings from three ADOS-reliable professionals on their confidence that the photo was taken by a person with ASD (1 = ASD, 9 = control). Data are shown for all photos (ASD: 3.80 ± 1.43 (mean ± SD), controls: 4.33 ± 1.46; two-tailed t-test across photos: t(1668) = 7.32, P < 0.0001, effect size in Hedges’g (standardized mean difference): g = 0.36, permutation test with 1000 runs, P < 0.001), photos containing people (ASD: 3.79 ± 1.48, controls: 4.58 ± 1.55; t(702) = 6.44, P < 0.0001, g = 0.53, permutation P < 0.001), indoor photos (ASD: 3.05 ± 1.09, controls: 3.68 ± 1.26; t(483) = 5.91, P < 0.0001, g = 0.54, permutation P < 0.001), and outdoor photos (ASD: 4.69 ± 1.10, controls: 4.70 ± 1.35; t(479) = 0.029, P = 0.98, g = 0.0026, permutation P = 0.96). Ratings were not correlated with subject age, FSIQ, AQ, SRS-2 Adult Self Report, nor any ADOS-2 severity scores (all Ps > 0.05). Error bar denotes one SEM across photos. ***P < 0.001; n.s., not significant. (B) Compared to controls, participants with ASD had a higher percentage of photos containing other people (ASD: 36.1 ± 4.75%, controls: 31.8 ± 6.16%; two-tailed t-test across subjects: t(35) = 2.32, P = 0.027, g = 0.75, permutation P = 0.030), a lower percentage of portrait photos with front faces of other people (ASD: 26.6 ± 22.4%; controls: 45.4 ± 20.8%; t(35) = 2.63, P = 0.013, g = 0.86, permutation P = 0.004), and a lower percentage of photos in which people were expressive or posing (ASD: 13.3 ± 16.8%; controls: 28.9 ± 22.1%; t(35) = 2.34, P = 0.025, g = 0.76, permutation P = 0.026). Interestingly, when taking self-portraits, participants with ASD took a similar proportion of front-facing and expressive photos as controls did when taking photos of other people (front-facing: ASD: 56.4 ± 34.9%, controls: 45.4 ± 20.8%; t(32) = 1.16, P = 0.26; expressive: ASD: 31.9 ± 34.8%; controls: 28.9 ± 22.1%; t(32) = 0.31, P = 0.76). *P < 0.05. (C–E) Example photos. (C) Subjects in the portrait photos taken by participants with ASD did not pose or look at the camera and were not expressive. Photos from participants with ASD (D) often were partially occluded, and (E) had odd visual perspective.
Based on the themes noted by our three raters, we had the photographs rated on additional scales (three other blind raters, photos blocked by subject) (Figure S2A), which confirmed that participants with ASD took more repetitive photos (Figure S2C), more photos of people (Figures 1D, S2E) and objects (Figure S2F) that were partly occluded, more blurred photos (Figure S2G), and more tilted photos (Figure S2H). We further asked two different raters to judge the composition and quality of all photos (see Supplemental Experimental Procedures for definitions). Participants with ASD did not differ from controls in how well the foreground object was centered or the number of people shown in person photos (Figure S2B) but took photos of poorer quality overall (Figure S2B). Although they took a higher percentage of photos containing other people, a smaller proportion of these photos were front-facing (Figure 1B,1C) or showed faces that were expressive or posing (Figure 1B–E), suggesting that they did not ask their subjects to attend to the camera when the photo was taken.
Thirteen of 16 participants with ASD were instructed to take photos of themselves. Interestingly, when taking self-portraits, participants with ASD took a similar proportion of front-facing and expressive photos as controls did when taking photos of other people. Finally, image-based analyses (Figure S2A) showed that photos did not differ in image entropy, suggesting equal levels of pixel-level image complexity.
We confirmed that the above findings could not be attributed to group differences in photography experience (Table S1), and in a separate experiment we found qualitatively the same results when photos were taken using a smart phone (see Supplemental Information). Furthermore, in subsequent ratings participants with ASD generally did not prefer photos taken by ASD subjects to photos taken by controls (see Supplemental Information). Additional ratings of the photos by a large online sample of raters who had no training in ASD diagnosis (N = 223) showed that such naive raters could not reliably detect whether photos were taken by participants with ASD (see Supplemental Information), in contrast to the above results obtained from our ADOS-reliable raters.
Our findings provide an initial characterization of the photographs taken by participants with ASD and support some expectations based on salient features of the diagnosis: photos contained atypical aspects of people and repetitions of specific objects, which bear resemblance to the social and restricted-interest components of the clinical presentation. But there were also surprises: participants with ASD actually took more, not fewer, photos of other people, while also spending more time shooting each photo of a person. These findings challenge theories that trace atypical social cognition in ASD to a reduced interest or motivation in people [5], but may support theories focusing on impairments in distinguishing other people from oneself (possibly related to the well-studied deficits in ‘theory of mind’ [6,7]). It is also clear that there are more general difficulties with composing a photograph, as evident from the preponderance of blurred, tilted, occluded, and poorly framed images — aspects that may relate to deficits in global and holistic perception in ASD, or the theory of weak central coherence [6,8]. It should be stressed that all our findings are of course limited by sample size, and that replications, ideally in considerably larger samples and with a variety of approaches (for example, from photos posted on the internet), will be needed to establish the generality of our results — or possibly to discover further details, such as subgroups of people with ASD that might be distinguished by the kinds of photos they take.
Finally, it is important to note that the photographs that comprised our data have an explicitly social communicative aspect: these photos may show more than how ASD participants might see or represent the world, or even what they prefer in photos, and may instead reveal how individuals with ASD attempt to communicate their experiences to others. This is notable in light of the potential therapeutic value of photography and art more generally [9]. Given the wealth of emerging data on eye-tracking and visual attention in ASD, the task and data we present here offer an ecologically important complement by measuring what people find interesting enough to want to capture and share.
Supplementary Material
Acknowledgments
We thank Catherine Holcomb, Tim Armstrong, Remya Nair and Sai Sun for help with running the experiment and providing ratings, Daniel Kennedy and Christina Corsello for judging and rating the photos, and Daniel Kennedy also for comments on the manuscript.
This research was supported by a postdoctoral fellowship from the Autism Science Foundation (S.W.), and a grant from the Simons Foundation (SFARI Award 346839, R.A.).
Footnotes
Supplemental Information includes experimental procedures, two figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.08.055.
The authors declare no conflict of interest.
Author Contributions
R.A. designed and supervised experiments. L.K.P. provided diagnoses and some of the ratings. S.W. and R.A. wrote the paper. All authors analyzed data, discussed the results and provided comments on the manuscript.
References
- 1.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J. Autism Dev. Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 2.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J. Autism Dev. Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Jiang M, Duchesne Xavier M, Laugeson EA, Kennedy Daniel P, Adolphs R, Zhao Q. Atypical visual saliency in autism spectrum disorder quantified through model-based eye tracking. Neuron. 2015;88:604–616. doi: 10.1016/j.neuron.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hus V, Lord C. The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. J. Autism. Dev. Disord. 2014;44:1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn. Sci. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frith U. Autism: Explaining the Enigma. 2. Blackwell; 2003. [Google Scholar]
- 7.Baron-Cohen S. Mindblindness: an Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 8.Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 9.Latta A, Rampton T, Rosemann J, Peterson M, Mandleco B, Dyches T, Roper S. Snapshots refl ecting the lives of siblings of children with autism spectrum disorders. Child Care Health Dev. 2014;40:515–524. doi: 10.1111/cch.12100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.