Health disparities are ubiquitous, but could be eliminated for preventable conditions by ensuring equitable access to and use of disease prevention, detection, and treatment services. Screening is an established tool for preventing premature death for many health conditions, including colorectal cancer (CRC).1–3 The Affordable Care Act (ACA), in part, aimed to increase access to CRC screening by mandating coverage without cost sharing (effective September 23, 2010). However, ACA the did not address provisions in section 1834(d)(3)(D) of the Balanced Budget Act (BBA) of 1997, which disallows Medicare from waiving the beneficiary’s share of coverage for the cost of screening (usually 20%) when a diagnostic procedure such as biopsy or polypectomy is performed during the course of a screening endoscopy. This provision also applies if endoscopy is performed because of a positive result on another screening test.
These legal restrictions hinder the goal of eliminating (and may exacerbate) longstanding disparities in mortality from CRC for Medicare beneficiaries. For low-income individuals without supplemental coverage for the coinsurance, cost-sharing may be an insurmountable barrier.4 A disproportionately high percentage of Medicare beneficiaries from low-income background lack Medigap or supplemental insurance, even among retirees.5–7
Public Health Benefits of Screening for CRC
CRC is the second leading cause of cancer death in the United States. An estimated 49,190 people will die of CRC in the United States in 2016, and people in low socioeconomic status bear a disproportionate share of this burden.8 The burden of CRC is greatest in the Medicare population. About 70% of CRC deaths occur in Medicare age-eligible people and the average age of people dying of CRC is 73 years.9
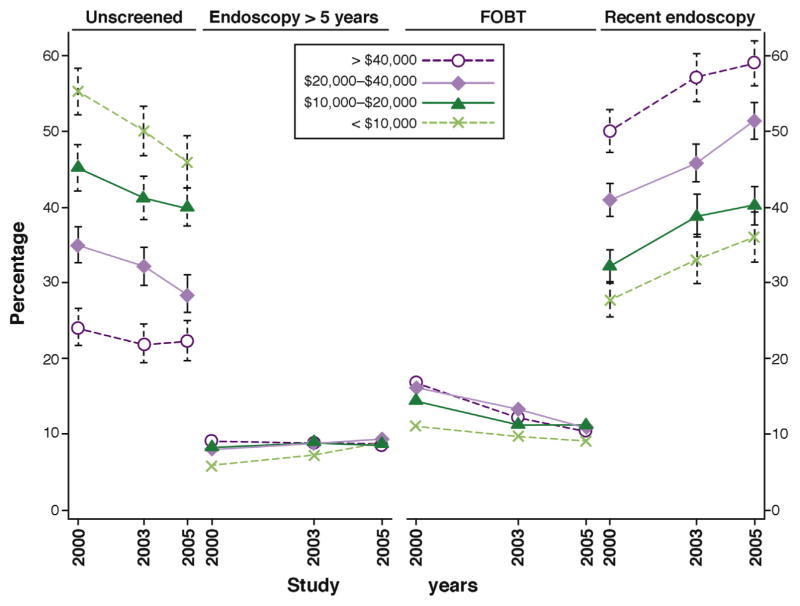
Screening is estimated to have prevented more than one-half million new cases of CRC between 1987 and 2010.10 Importantly, about 63% of the deaths from CRC in 2010 were owing to not having been screened. A study estimated that increasing screening uptake from 58% in 2013 to 80% by 2018 in the United States could further reduce disease incidence by 17% and mortality rates by 19%.11 Among Medicare beneficiaries, those from low-income backgrounds have half the rate of screening of high-income groups (Figure 1).12 Thus, increasing screening uptake in low-income populations is critical to public health goals to decrease persistent disparities in CRC.
Figure 1.
Patterns of colo-rectal cancer screening by income among Medicare enrollees ages 65 to 80 years, 2000–2005. Revised with permission from Doubeni et al.12
Definition of Screening and the CRC Screening Episode
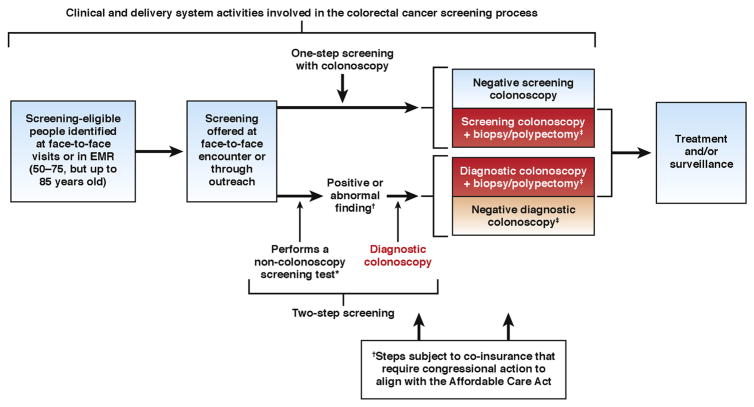
The goal of screening is to prevent CRC or enable more effective management through early detection, before symptoms that portend advanced, less curable, disease develop. Until recently, screening was thought of as a one-time clinical activity and many health policy groups recommended individual tests in their executive summaries and not the interrelated activities involved in the screening process. In practice, screening is a series of clinical activities involved in identifying and testing asymptomatic screen-eligible people, and performing diagnostic confirmation when necessary (Figure 2). Screening involves many tests and multiple steps.3 Timely diagnostic workup of abnormal results is a central tenet of safe patient care,13 and is essential for effective CRC screening. Colonoscopy allows for diagnostic procedures such as removal of precancerous lesions or biopsy to confirm cancer diagnosis to be performed at the time of screening or during workup for a positive result on another screening test. However, out-of-pocket costs may make diagnostic evaluation unaffordable for low-income patients.4
Figure 2.
The colorectal cancer screening process and steps affected by coinsurance requirement.
*Non-colonoscopy tests include: high-sensitivity fecal occult blood test, fecal immunochemical test, multitarget fecal DNA test, and flexible sigmoidoscopy
†Critical steps in the screening process that require co-insurance and out-pocket expenses for Medicare and Medicaid beneficiaries who do not have supplemental coverage
Medicare’s Authority to Cover CRC Screening
Authority for Medicare’s coverage for CRC screening under Part B is provided in Section 4104 of BBA. Medicare currently reimburses for the fecal occult blood test (FOBT), multitarget stool DNA test, sigmoidoscopy, barium enema, and colonoscopy. Medicare first implemented screening in January 1998 with coverage for FOBT, sigmoidoscopy, and barium enema for routine screening, and colonoscopy for high-risk populations. Amendments in the Medicare, Medicaid, and SCHIP (State Children’s Health Insurance Program) Benefits Improvement and Protection Act of 2000 that authorize coverage for screening colonoscopy in average-risk persons became effective July 1, 2001. Coverage for immunoassay FOBT and stool DNA testing became effective November 4, 2003, and October 9, 2014, respectively.
Confusion about the Patchwork of Laws and Policies
The patchwork of CRC screening coverage laws and policies has resulted in considerable confusion for the public and providers. For Medicare beneficiaries, out-of-pocket costs depend on the type of facility in which the procedure is performed, supplemental insurance coverage status, and the provider accepting Medicare assignment.
Recommendations
Because the ACA did not address BBA provisions, the Centers for Medicare and Medicaid Services currently lacks the authority to waive the 20% or 25% coinsurance for diagnostic or therapeutic procedures done in the context of a screening episode, which was about $163 or $203 on average in 2014. This puts Medicare coverage at odds with the practice of many private insurers, who comply with ACA provisions even though private insurers may weigh the upfront cost of screening as greater than the benefits that accrue years later. Because there are no reliable tools to predict beforehand when a polyp or cancer would be detected,14 a patient expecting free-of-charge screening may be either surprised with a bill or asked to provide the coinsurance and copay before she or he can undergo the procedure. Therefore, we recommend the following.
Congress should amend, without further delay, BBA provisions to waive the coinsurance and copay for all diagnostic procedures arising during the course of screening. This would improve the affordability of screening for low-income people and advance the US public health policy objective to remove barriers to disease prevention, detection, and treatment.
Congress should waive the coinsurance for colonoscopy done as a result of a positive result on another screening test. This measure would promote timely diagnostic workup of positive screening; diagnostic delay owing to cost sharing undermines the benefits of screening. Waiving coinsurance could also enable greater choice of screening tests, which has been shown to increase uptake. It also avoids unreasonably penalizing beneficiaries whose negative colonoscopy that was done for a positive result on a noncolonoscopy screening test.
Congress should without delay remove the requirement to reclassify screening test as diagnostic on the basis of findings at the time of testing. Discontinuing the current practice of recoding screening tests as diagnostic would enable research to increase understanding of the true patterns of screening and their effectiveness.
Once BBA constraints are removed, Medicare should next consider value-based and evidence-based benefit design for CRC screening coverage. This could involve differential payment for screening colonoscopy based on performance on quality measures that directly impact screening effectiveness, such as pathologically confirmed adenoma detection.15 This could improve quality and outcomes of screening for beneficiaries and also enable research on secular trends in adenoma prevalence by linking verifiable performance data to payments.
National policy groups should align their recommendations with the current state of the science and practice of CRC screening. For instance, colonoscopy could be recommended as “colonoscopy every 10 years with biopsy or polypectomy when necessary” and stool-based tests as “FOBT annually plus diagnostic colonoscopy with biopsy or polypectomy, as needed, when positive.”
Value for Medicare and Gaps in Evidence
Medicare spent about $2 billion on an estimated 3.8 million colonoscopies in 2013, but this is dwarfed by CRC treatment costs, about $7.3 billion in 2010, for this potentially preventable cancer.16 Many Medicare beneficiaries are overdue for screening (Figure 1) and some may remain screen-eligible up to age 85. Removing cost sharing for screening colonoscopy, whether as a primary screening test or for diagnostic workup of another positive screening test, may increase screening uptake in low-income populations and thus decrease the net cost to Medicare,16 but this needs further research. Removing BBA restrictions could promote high-quality screening practices through performance-based reimbursement for colonoscopy to maximize benefits and reduce treatment-related costs from greater disease prevention.17 Studies could also inform the impact and value of alternative benefit designs on downstream treatment costs and lives saved from averted cancers.
Acknowledgments
The authors are grateful to Reinier G. S. Meester of Erasmus MC in Rotterdam, the Netherlands, for his helpful comments.
Funding
Supported in part by funding (number U01CA151736, and U54 CA163262 - Population-based Research Optimizing Screening through Personalized Regimens) from the National Cancer Institute of the National Institutes of Health. The views expressed here are those of the authors only and do not represent any official position of the National Cancer Institute or National Institutes of Health.
Contributor Information
CHYKE A. DOUBENI, University of Pennsylvania, Philadelphia, Pennsylvania
DOUGLAS A. CORLEY, Kaiser Permanente Division of Research Oakland, California
ANN G. ZAUBER, Memorial Sloan Kettering Cancer Center, New York, New York
References
- 1.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Wharam JF, Graves AJ, Landon BE, et al. Two-year trends in colorectal cancer screening after switch to a high-deductible health plan. Med Care. 2011;49:865–871. doi: 10.1097/MLR.0b013e31821b35d8. [DOI] [PubMed] [Google Scholar]
- 5.Wilensky GR. The future of Medicare supplemental insurance. JAMA Intern Med. 2013;173:1669–1670. doi: 10.1001/jamainternmed.2013.9947. [DOI] [PubMed] [Google Scholar]
- 6.Pourat N, Rice T, Kominski G, et al. Socioeconomic differences in Medicare supplemental coverage. Health Aff (Millwood) 2000;19:186–196. doi: 10.1377/hlthaff.19.5.186. [DOI] [PubMed] [Google Scholar]
- 7.McArdle F, Neuman T, Huang J. Retiree health benefits at the crossroads, publication #8576. The Henry J. Kaiser Family Foundation; 2014. Available: https://kaiserfamilyfoundation.files.wordpress.com/2014/04/8576-retiree-health-benefits-at-the-crossroads.pdf. [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 9.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: 2008. Available: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 10.Doubeni CA. The impact of colorectal cancer screening on the US population: is it time to celebrate? Cancer. 2014;120:2810–2813. doi: 10.1002/cncr.28789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281–2285. doi: 10.1002/cncr.29336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doubeni CA, Laiyemo AO, Reed G, et al. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18:2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med. 2012;27:1334–1348. doi: 10.1007/s11606-011-1949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doubeni CA. Precision screening for colorectal cancer: promise and challenges. Ann Intern Med. 2015;163:390–391. doi: 10.7326/M15-1677. [DOI] [PubMed] [Google Scholar]
- 15.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goede SL, Kuntz KM, van Ballegooijen M, et al. Cost-savings to Medicare from pre-Medicare colorectal cancer screening. Med Care. 2015;53:630–638. doi: 10.1097/MLR.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Variation in adenoma detection rate and the lifetime benefits and cost of colorectal cancer screening: a microsimulation model. JAMA. 2015;313:2349–2358. doi: 10.1001/jama.2015.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]