Abstract
Neuropathic pain induced by chemotherapy drugs such as oxaliplatin is a dose-limiting side effect in cancer treatment. The mechanisms underlying chemotherapy-induced neuropathic pain are not fully understood. KCNQ2 channels are low-threshold voltage-gated K+ channels that play a role in controlling neuronal excitability. Downregulation of KCNQ2 channels has been proposed to be an underlying mechanism of sensory hypersensitivity that leads to neuropathic pain. However, it is currently unknown whether KCNQ channels may be downregulated by chemotherapy drugs in trigeminal ganglion neurons to contribute to the pathogenesis of chemotherapy-induced orofacial neuropathic pain. In the present study, mechanical sensitivity in orofacial regions is measured using the operant behavioral test in rats treated with oxaliplatin. Operant behaviors in these animals show the gradual development of orofacial neuropathic pain that manifests with orofacial mechanical allodynia. Immunostaining shows strong KCNQ2 immunoreactivity in small-sized V2 trigeminal ganglion neurons in controls, and the numbers of KCNQ2 immunoreactivity positive V2 trigeminal ganglion neurons are significantly reduced in oxaliplatin-treated animals. Immunostaining is also performed in brainstem and shows strong KCNQ2 immunoreactivity at the trigeminal afferent central terminals innervating the caudal spinal trigeminal nucleus (Vc) in controls, but the KCNQ2 immunoreactivity intensity is significantly reduced in oxaliplatin-treated animals. We further show with the operant behavioral test that oxaliplatin-induced orofacial mechanical allodynia can be alleviated by the KCNQ2 potentiator retigabine. Taken together, these findings suggest that KCNQ2 downregulation may be a cause of oxaliplatin-induced orofacial neuropathic pain and KCNQ2 potentiators may be useful for alleviating the neuropathic pain.
Keywords: Trigeminal ganglion neurons, chemotherapy-induced peripheral neuropathy, neuropathic pain, mechanical allodynia, orofacial pain, voltage-gated K+ channels, KCNQ2 channels, retigabine, operant test, oxaliplatin
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a major clinical problem and a dose-limiting side effect for cancer patients treated with several types of essential chemotherapy drugs including oxaliplatin.1 Patients with CIPN commonly experience sensory dysfunctions in extremities including fingers and toes.2 However, clinical reports have also indicated that sensory dysfunctions of CIPN can occur in proximal body parts including the orofacial regions.3,4 The most severe sensory dysfunction of CIPN is neuropathic pain. Patients with neuropathic pain often experience exaggerated pain sensations triggered by innocuous mechanical stimuli (mechanical allodynia) and also by innocuous or mild noxious cooling temperatures (cold allodynia/hyperalgesia). Neuropathic pain in orofacial regions constitutes a significant clinical problem because of its severity, special location, and resistance to conventional treatment.5 There is an imperative need to better understand mechanisms underlying neuropathic pain in orofacial regions and identify therapeutic targets for its effective management.
The trigeminal nerves, arising from the trigeminal ganglia (TG), convey thermal, mechanical, and nociceptive signals from peripheral tissues including those in orofacial areas to the central nervous system in the brainstem. The trigeminal nerves have three main branches that innervate the ophthalmic (V1), maxillary (V2), and mandibular (V3) areas. Orofacial regions are innervated by the maxillary (V2) branch of the trigeminal nerves. Nociceptive signals in orofacial areas are mainly conveyed by V2 trigeminal nerve fibers that have small calibers including c- and Aδ-trigeminal nerve fibers, and these nociceptive trigeminal fibers largely project to the Vc in the brainstem. Nociceptive nerve fibers may undergo membrane hyperexcitability under pathological conditions that lead to exaggerated pain induced by innocuous thermal and mechanical stimuli. Since voltage-gated K+ channels play a critical role in regulating excitability of peripheral afferents,6 a change in the expression and/or functions of voltage-gated K+ channels in V2 nociceptive trigeminal neurons can be a potential mechanism underlying neuropathic pain such as orofacial mechanical and cold allodynia in CIPN.7
KCNQ channels are a subfamily of voltage-gated K+ channels that can be activated at low voltages near resting membrane potentials.8 Five KCNQ subunits including KCNQ1 to KCNQ5 have been identified, and four of them (KCNQ2-5) are expressed in the neurons of the central nervous system and the peripheral nervous system.8 In the peripheral nervous system, functional KCNQ channels in dorsal root ganglion (DRG) neurons are thought to be mainly formed by KCNQ2 and KCNQ3 subunits in a heteromeric form (KCNQ2/3 channels), and these KCNQ2/3 channels are believed to underlie the M-type outward K+ currents (M-currents) recorded in DRG neurons.9–11 KCNQ2/3 channels have been shown to play a role in regulating excitability of nociceptive DRG neurons.8 Importantly, studies have shown that KCNQ2 and KCNQ3 subunits are downregulated in nociceptive DRG neurons after nerve injury and in bone cancer in animals.9–12 These studies have further shown that KCNQ channel downregulation is associated with the increase of the excitability of nociceptive DRG neurons.11,12 In TG neurons, we have recently shown that KCNQ2 channels are expressed in nociceptive TG neurons including those that sense cooling temperatures, and we have also found that pharmacological inhibition of these channels leads to TG neuron hyperexcitability.13 The previous studies from both DRG and TG neurons raise a possibility that functional KCNQ channels may also undergo down regulation in TG neurons under pathological conditions including oxaliplatin-induced trigeminal neuropathy, which may lead to TG neuron hyperexcitability and trigeminal neuropathic pain.
KCNQ channels in DRG and TG neurons may serve as therapeutic targets for alleviating pain because of the presence of small molecule compounds that can potentiate KCNQ channel functions to suppress neuronal hyperexcitability in nociceptive neurons.14 Retigabine, one of the best characterized KCNQ channel potentiators approved for clinical use to treat seizure in human patients,15 has been found to relieve carrageenan-induced inflammatory pain in animals.9 We have recently shown that retigabine can alleviate cold allodynia/hyperalgesia in animals following chronic trigeminal nerve injury or in animals treated with oxaliplatin.13 In the present study, we examined the expression of KCNQ2 subunits in V2 TG neurons and determined whether oxaliplatin-induced orofacial mechanical allodynia is associated with downregulation of KCNQ2 expression. We further determined whether potentiation of KCNQ activity by retigabine may alleviate orofacial mechanical allodynia induced by oxaliplatin.
Materials and methods
Orofacial operant behavioral test and animal models
Behavioral assessments were conducted on male Sprague-Dawley rats (300–450 g) as described in our previous studies using the orofacial operant test.16,17 In brief, animals initially underwent four to six sessions of adaptation training in two weeks using the Ugo Basile Orofacial Stimulation Test System® (Comerio, VA, Italy). For each training session, animals were first fasted for a 12-h period. Each rat was then placed in a cage in which there was an Orofacial Stimulation Test System. The Orofacial Stimulation Test System had a drinking window for the rat head to enter and acquire a reward (30% sweetened condensed milk, Nestle Carnation®). The milk was placed in a cylindrical plastic container with a metal nipple drinker being located inside the drinking window. The Orofacial Stimulation Test System also consisted of a mechanical stimulation module custom made with five NiTi filaments (0.009” diameter, SmallParts, IN, USA) on each side of the drinking window. The filaments were spaced at the distance of 3 mm and each filament had a bendable length of 8 mm. An infrared beam was built in the drinking window and wired to a computer to automatically detect and record the head accessing the nipple drinker. A training session was started by placing a rat in the cage. After the rat was given 10 min to familiarize itself with its environment, the drinking window was opened and the testing rat was subsequently timed for 10 min to allow drinking the milk.
After two weeks of the adaptation training, the rats were divided into two experimental groups: the neuropathic pain group induced by oxaliplatin (oxaliplatin-treated group) and the control group. For the oxaliplatin-treated group,18 rats were injected with oxaliplatin intraperitoneally at a dose of 2 mg/kg (200 µl for each rat) for five consecutive days (an accumulative dose of 10 mg/kg). The rats in the control group were injected with the same amount of saline. For both the oxaliplatin-treated group and the control group, one day before the experiments, the testing rats’ facial areas were shaved. Subsequent experiments were performed for orofacial operant tests at different days for up to six weeks. To determine the effects of retigabine on oxaliplatin-induced mechanical allodynia, retigabine was administered intraperitoneally at the doses of 2 mg/kg on day 30 following oxaplatin treatment and orofacial operant tests were performed between 60 and 90 min after retigabine administration. No attempt was made to examine effects of retigabine at multiple time points because orofacial operant tests could only be performed once every two days.
Hind paw withdrawal responses to static and dynamic mechanical stimulation
The hind paw withdrawal responses to static mechanical stimulation were determined using the von Frey test. Ten von Frey filaments with bending forces ranging from 0.16 to 10.0 g were used to determine the paw withdrawal threshold. The 50% likelihood of a paw withdrawal response (50% threshold) was determined using the up–down method.19 Rats were placed in testing chambers that were situated on wire mesh platforms and habituated for 10 min. The series of von Frey filaments was then applied perpendicularly from below the platform to the central region of the plantar surface of hind paws in ascending order beginning with the hair of the lowest bending force (0.16 g). The filament was applied when the rat was standing on all four paws and not moving. The upward application of von Frey filament continued until buckling of the filament occurred and was maintained for approximately 2 s. A withdrawal response was considered positive when the hind paw was fully moved away from the platform. A trial consisted of application of a von Frey filament to the hind paw five times at intervals of 30 s. When the hind paw was withdrawn from a particular filament more than three times out of the five applications, the value of that filament bending force in grams was defined as the withdrawal threshold.
The hind paw withdrawal responses to dynamic mechanical stimulation were assessed by lightly striking the plantar surface of the hind paw with a paint brush in the same manner as described by a previous study.20 Each test included five brush strokes separated by 30 s intervals. For each application, the brush was applied longitudinally through the paw in a front to back manner. The hind paw withdrawal responses were graded as follows: 0, no response or moving of the stimulated paw aside; 1, lifting of the stimulated paw toward the abdomen; 2, flinching or licking of the stimulated paw. Total scores in five strokes were used as measures of the withdrawal responses to the dynamic mechanical stimulation.
Immunohistochemistry
Animals used for immunochemistry were adult male rats that were treated with oxaliplatin (2 mg/kg, i.p. for five consecutive days, oxaliplatin-treated group) or saline (control group). Tissues including TGs and brainstems were harvested from these animals 28 to 30 days following the oxaliplatin or saline treatment. Animals were anesthetized with ketamine/xylazine (100 mg/kg:10 mg/kg, i.p.), transcardially exsanguinated with 0.1 M phosphate-buffered solution, and perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered solution. TGs and brainstems were removed and placed in 30% sucrose for cryoprotection for two nights. The TGs and brainstems were then separately embedded in OCT® compound (Baxter Scientific) and cut on a cryostat (Leica Biosystems, Buffalo Grove, IL, USA) into 10 and 16 µm sections, respectively. The sections of TGs and brainstems were thaw-mounted onto separate slides and allowed to air-dry. For each slide, three sections from saline-injected animals (control) and three sections from oxaliplatin-treated animals were mounted on the same slide. These sections were then encircled together with hydrophobic resin (PAP Pen—The Binding Site) so that the two groups were paired in immunostaining. The slide-mounted sections were rinsed with the BupHTM Modified Dulbecco’s Phosphate Buffered Saline (PBS, ThermoFisher Scientific, Waltham, MA, USA) three times, and then sequentially incubated at room temperature with ethanol solutions at the concentrations of 50% for 10 min, 70% for 10 min and 50% for 10 min. The slides were rinsed three times with PBS and sections incubated with 10% normal goat serum in PBS for 1 h at room temperature. The sections were incubated with a polyclonal rabbit anti-KCNQ2 antibody (1:2000 diluted with 5% normal goat serum in PBS; Abcam, Cambridge, MA, USA) at 4℃ for two nights. Following three rinses with PBS solution, the sections were incubated with a secondary antibody for one night at room temperature. The secondary antibody (1:1000 in 5% normal goat serum in PBS) was a goat anti-rabbit IgG conjugated with Alexa-594 (ThermoFisher Scientific, Waltham, MA, USA). The sections were rinsed three times with PBS solution, cover-slipped with the Prolong Diamond Antifade Mountant medium (ThermoFisher Scientific). Slices were viewed under an upright fluorescent microscope (BX-43, Olympus, Tokyo, Japan), and images were captured using a CCD camera (DP80, Olympus, Tokyo, Japan).
Data analysis
For orofacial operant tests, the events of head assessing nipple drinker were detected by the infrared beam, recorded by a computer, and analysed by the Oro Software (Ugo Basile, Comerio, VA, Italy). This computer software recorded and analyzed several variables of the rat’s behavior including total contact time and total contact numbers. Unless otherwise indicated, total contact time in a 10-min experimental session was used as orofacial operant behavioral parameters. For the data of immunostaining on TG sections, KCNQ2 immunoreactivity (KCNQ2-ir) positive neurons in TG sections were visually identified from the acquired fluorescent images, and total neurons in TG sections were also counted from the same images. The percent of KCNQ2-ir positive neurons was then calculated. For the data of immunostaining on brainstem sections, fluorescent intensity in arbitrary units (a.u.) was integrated from superficial to deep layers using the Image-J software (https://imagej.nih.gov/ij/). Data were presented as Mean ± SEM, analyzed by the unpaired Student’s t test or one-way AMOVA with the Tukey post hoc test, *p < .05, **p < .01, and ***p < .001.
Results
We made the CIPN animal model by treating rats with the chemotherapy drug oxaliplatin at daily dose of 2 mg/kg for five consecutive days. To assess the development of mechanical allodynia in orofacial regions following the oxaliplatin regimen, we performed the orofacial operant test to examine how operant behaviors are altered following the oxaliplatin treatment. The orofacial operant test device used in the present study (Figure 1(a), left) had a mechanical stimulation module that was positioned in front of the nipple of a milk feeding bottle. Animals received mechanical simulation on their orofacial areas by mechanical stimulation filaments when their head entered through the stimulation module to access the nipple and drink milk (Figure 1(a), right). The operant behaviors of the animals were recorded by an automated detection/acquisition system, which registered time spent by animals to contact stimulation filaments with their orofacial areas while drinking milk (Figure 1(b)). For control animals that were injected with saline for five consecutive days, the total time spent to drink milk (total contact time) was about 300 s in a 10-min session of operant test, and there was no significant difference in total contact time among different testing days for up to 40 days (Figure 1(b), top and (c)). For example, the total contact time was 323 ± 14 s (n = 9) on day 0 (before saline injection), 303 ± 31 s (n = 9) on day 19, and 282 ± 44 s (n = 5) on day 40 following saline injections (Figure 1(c)). For animals treated with oxaliplatin, the total contact time was 310 ± 15 s (n = 12) on day 0 before oxaliplatin treatment and was gradually decreased following the oxaliplatin regimen to 182 ± 15 s (n = 12, p < .01) on day 19 and 132 ± 20 s (n = 10, p < .001) on day 30. This was followed by a partial recovery with total contact time of 221 ± 39 s (n = 9, day 40). These results suggested the development of mechanical allodynia in orofacial regions in animals treated with oxaliplatin.
Figure 1.
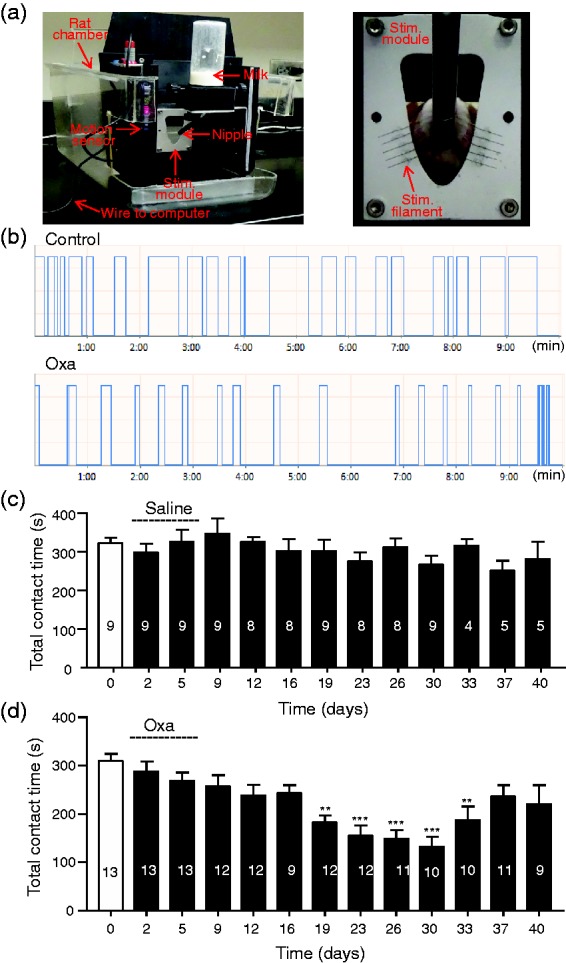
Orofacial operant test of orofacial mechanical sensitivity in animals of control and oxaliplatin-treated groups. (a) Left, the main parts of orofacial operant test device used to assess mechanical sensitivity in orofacial regions of rats. A mechanical stimulation module is placed in front of the nipple of a milk bottom. Right, enlarged image shows the mechanical stimulation module and a rat whose orofacial area is contacting mechanical stimulation filaments while drinking milk. (b) Two traces show contact events recorded over a 10-min session of operant test in a control animal injected with saline (top trace) and a different animal 30 days following oxaliplatin treatment (Oxa). Oxaliplatin is injected in five consecutive days at 2 mg/kg each injection. Saline is injected in the same manner as oxaliplatin injections. (c) Summary data of total contact time during a 10-min session orofacial operant test in different testing days in control group. Dashed line indicates the saline injections in five consecutive days. (d) Similar to (c) except data are obtained from oxaliplatin-treated animals. Dashed line indicates oxaliplatin injections in five consecutive days. In both (c) and (d), the number in each bar indicates the numbers of animals tested. Data represent Mean ± SEM, **p < .01, ***p < .001, compare with the data before the injections of saline or oxaliplatin.
Since oxaliplatin was administered systemically to the animals, we determined whether mechanical allodynia was concurrently developed in the other parts of the body. This was done by examining hind paw withdrawal threshold to mechanical stimulation using the von Frey test (Figure 2(a)) and the paint brush test (Figure 2(b)). As shown in Figure 2(a), the hind paw withdrawal threshold was gradually decreased following the treatment of animals with oxaliplatin. Hind paw withdrawal threshold was 4.67 ± 0.62 g (n = 12) before oxaliplatin treatment, significantly decreased to 2.77 ± 0.37 g (n = 15, p < .05) on day 11 and 1.9 ± 0.25 g (n = 10, p < .001) on day 30 following oxaliplatin treatment. The time course of the reduction of hind paw withdrawal threshold was similar to the time course of the reduction of total contact time in the orofacial operant test in these oxaliplatin-treated animals (Figures 1(d) and 2(a)). In addition to the von Frey test, we also determined hind paw withdrawal responses to gently striking the plantar surface of the hind paws using a soft paint brush (Figure 2(b)). Total scores of hind paw withdrawal responses were 2.5 ± 0.5 (n = 11) before oxaliplatin treatment, significantly increased to 5.7 ± 0.7 (n = 14, p < .05) on day 16, 7 ± 0.9 (n = 11, p < .001) on day 26 following oxaliplatin treatment, and on day 43, the responses returned to the level before oxapliplatin treatment.
Figure 2.
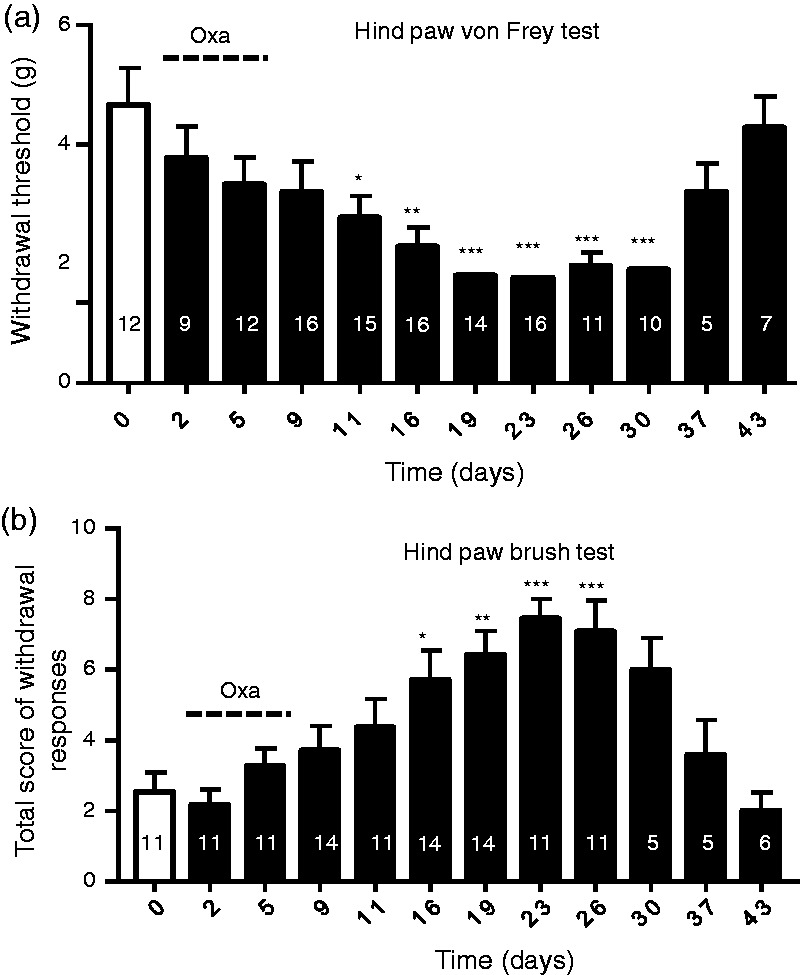
Hind paw mechanical sensitivity assessed in animals treated with oxaliplatin. (a) Hind paw withdrawal thresholds measured in different testing days using the von Frey test in animals before (open bar) and following oxaliplatin treatments (closed bars). Dashed line indicates oxaliplatin injections in five consecutive days. (b) Total scores of hind paw withdrawals in responses to paint brush strokes onto the plantar surface of the hind paws. The paint brush test is performed in animals before (open bar) and following (closed bars) oxaliplatin treatment. Dashed line indicates oxaliplatin injections in five consecutive days. In both (a) and (b), the number in each bar indicates the numbers of animals tested. Data represent Mean ± SEM, * p < .05, ** p < .01, *** p < .001, compare with the data before oxaliplatin treatment.
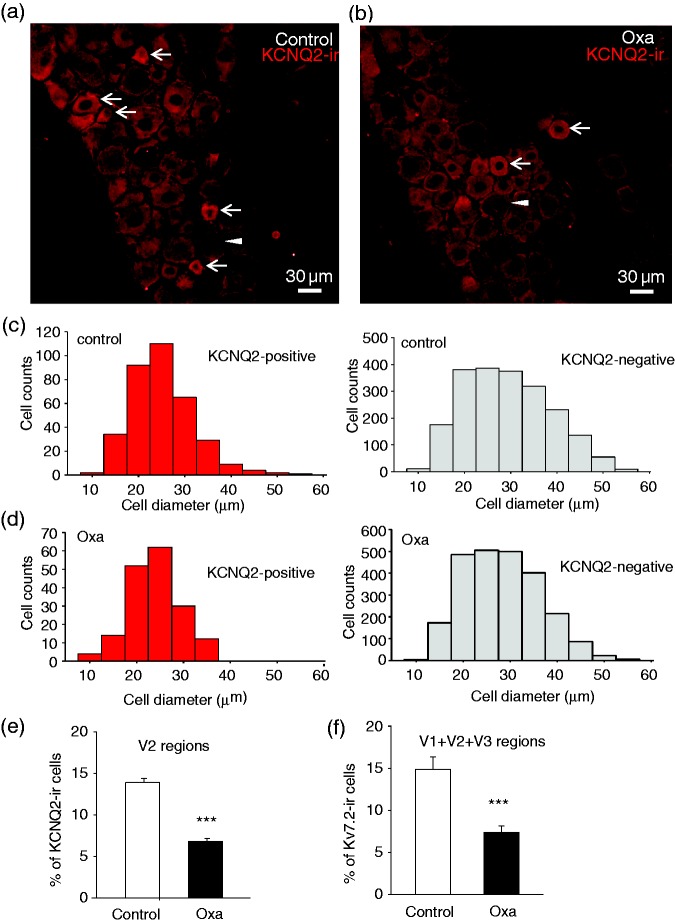
We explored whether downregulation of KCNQ2 channel expression might be associated with oxaliplatin-induced trigeminal neuropathic pain that manifested with mechanical allodynia. Strong KCNQ2-ir was observed mainly in small-sized neurons in TG sections of both control animals (Figure 3(a) and (c)) and animals treated with oxaliplatin (Figure 3(b) and (d)). Since orofacial regions are innervated by V2 parts of TGs, we focused on KCNQ2-ir in the V2 regions of TGs (Figure 3(a) to (e)). As sampled from V2 regions of TGs in control animals, KCNQ2-ir positive neurons were mostly small-sized neurons with mean diameter of 23 µm (22.8 ± 0.4, 348 positive neurons out of 2432 total neurons in 16 TG sections of 4 rats, Figure 3(c), left panel). In contrast, KCNQ2-ir negative neurons from these TG sections of control animals showed broader cell size distribution with mean diameter of 27 µm (26.9 ± 0.2 µm, 2084 negative neurons out of 2432 total neurons, Figure 3(c), right panel). As sampled from V2 regions of oxaliplatin-treated animals, KCNQ2-ir positive neurons also showed to be mostly small-sized neurons with mean diameter of 21 µm (21.4 ± 0.4 µm, 175 positive neurons out of 2396 total neurons of 17 TG sections of 4 rats, Figure 3(d), left panel), and KCNQ2-ir negative neurons displayed wider cell size distribution with mean diameter of 26 µm (25.8 ± 0.2 µm, 2396 negative neurons out of 2571 total neurons, Figure 3(d), right panel). We compared percentage of KCNQ2-ir positive neurons of V2 regions between control and oxaliplatin-treated animals (Figure 3(e)). In the control group with a total of 2507 V2 TG neurons examined, about 14% (13.7 ± 0.5%, 16 TG sections of 4 rats) of them were KCNQ2-ir positive. In oxaliplatin-treated animals with a total of 2588 V2 TG neurons examined, KCNQ2-ir positive V2 TG neurons were about 7% (6.7 ± 0.3%, 17 TG sections of 4 rats), significantly less than that of the control group (p < .001). Since oxaliplatin was administered systemically and their effect would not be limited to V2 regions, we also examined KCNQ2-ir in V1 and V3 regions of TG and pooled neurons of all three regions (V1, V2, and V3) of TGs together (Figure 3(f)) and observed similar reductions of KCNQ2-ir positive neurons, from control of 14.9 ± 4.4% (9 sections of 4 control rats, total 4956 cells) to 7.4 ± 2.2% following oxaliplatin treatment (9 sections of 4 oxaliplatin-treated rats, total 7948 neurons, p < .001).
Figure 3.
KCNQ2 immunoreactivity in V2 areas of TG of control and oxaliplatin-treated animals. (a) Image shows KCNQ2 immunoreactivity (KCNQ2-ir) in V2 part of a TG section (V2 TG) from a control rat. Arrows indicate KCNQ2-ir positive neurons. A negative neuron is indicated by an arrowhead. (b) Similar to (a) except the TG is harvested from an animal on day 28 following oxaliplatin injections. Arrows indicate KCNQ2-ir positive neurons and a negative neuron is indicated by an arrowhead. (c) Histograms of KCNQ2-ir positive (left panel) and negative (right panel) V2 TG neurons in control rats. (d) Histograms of KCNQ2-ir positive (left panel) and negative (right panel) V2 TG neurons in oxaliplatin-treated rats. (e) Summary data of the percent of KCNQ2-ir positive V2 TG neurons in control (open bar) and in oxaliplatin-treated group (closed bar). (f) Summary data of the percent of KCNQ2-ir positive neurons in all TG areas (V1 + V2 + V3) in control (open bar) and oxaliplatin-treated group (closed bar). Data in (e) and (f) represent Mean ± SEM, ***p < .001, compare with the data of control group.
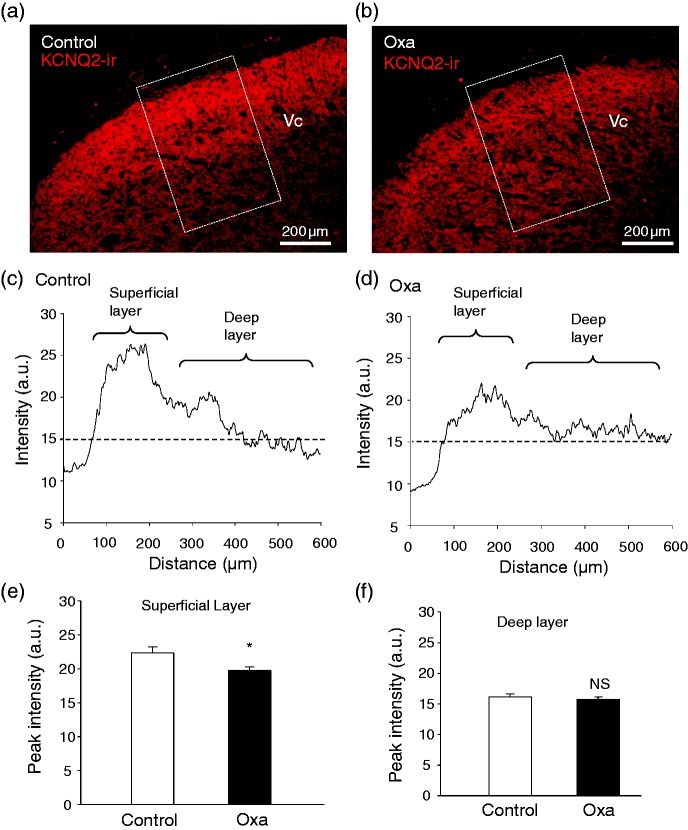
We examined KCNQ2-ir in the Vc, a primary region in the brainstem that is mainly innervated by nociceptive trigeminal afferent central terminals. As shown in Figure 4(a) and (c), strong KCNQ2-ir could be observed in the superficial layer of the Vc region of the control group. In contrast to the control group, KCNQ2-ir was weakened in the Vc region in animals treated with oxaliplatin (Figure 4(b) and (d)). We integrated fluorescent intensity along the directions from superficial to deep layers of Vc regions (Figure 4(c) and (d)). Higher fluorescent intensity of KCNQ2-ir was measured in the superficial layer of Vc regions in the brainstem section of both control animals and oxaliplatin-treated animals (Figure 4(c) and (d)). However, the peak intensity in the superficial layers was lower in the oxaliplatin-treated group (19.8 ± 0.5 a.u., n = 9) than in the control group (22.3 ± 0.9 a.u., n = 6, p < .05, Figure 4(e)). In contrast to the superficial layer, there was no significant difference in the fluorescent intensity in deeper layers between the control group (n = 9) and the oxaliplatin-treated group (n = 6, Figure 4(f)).
Figure 4.
KCNQ2 immunoreactivity in the caudal spinal trigeminal nucleus of control animals and animals treated with oxaliplatin. (a) Sample image shows KCNQ2 immunoreactivity in the Vc region of a brainstem section from a control rat. (b) Similar to (a) except the brainstem section was from an animal on day 28 following oxaliplatin treatment. (c) Fluorescent intensity integrated from superficial to deep layer of the Vc area in the dashed box in (a). (d) Fluorescent intensity integrated from superficial to deep layer of the Vc area in the dashed box in (b). (e) Summary data of peak intensity of superficial Vc regions of brainstem sections from control animals (open bar) and animals treated with oxaliplatin (closed bar). (f) Summary data of intensity of deep Vc regions (at 400 µm) of brainstem section from control animals (open bar) and animals treated with oxaliplatin. Data represent Mean ± SEM, ns, not significantly different, *p < .05, compare with the data of control group.
We used the trigeminal neuropathic pain model and orofacial operant test to determine whether orofacial mechanical allodynia in these animals could be alleviated by retigabine, a KCNQ2 channel potentiator. Figure 5(a) shows time course of the development of orofacial mechanical allodynia in animals following oxaliplatin-treatment and also showed the additional treatment with retigabine on day 30. The total contact time during the orofacial operant test was 304 ± 31 s (n = 8) on day 0 before oxaliplatin treatment, reduced to 113 ± 39 s (n = 5) on day 26 after oxaliplatin treatment, and became 305 ± 26 s (n = 7) on day 30 after the additional treatment with retigabine. In a separate group of animals treated with oxaliplatin, orofacial mechanical allodynia developed following oxaliplatin in a similar time course and the injection of the vehicle on day 30 showed no effect (Figure 5(b)). Concurrent with the improvement of orofacial behaviors with the additional treatment with retigabine, the KCNQ2 potentiator was also shown to alleviate mechanical allodynia in hind paws. This was evidenced by the results of the von Frey test that showed the increase of hind paw withdrawal threshold when retigabine was administered to the animals (Figure 5(c)). Since orofacial operant tests were performed at one time point every two days, this limitation prevented us from performing multiple time points for studying time course of the analgesic effect.
Figure 5.
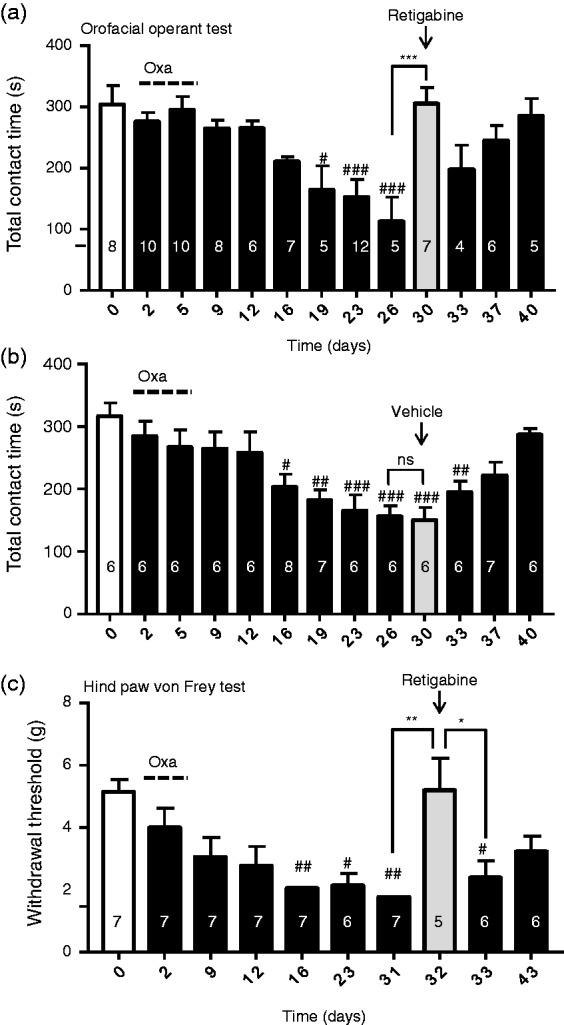
Alleviation of mechanical allodynia in both orofacial and hind paw regions by the KCNQ channel potentiator retigabine. (a) Summary data of total contact time during a 10-min session operant test in different testing days before (open bar) and following oxaliplatin treatment (closed). Oxaliplatin is administered in five consecutive days as indicated by the dashed line. On day 30 (gray bar), retigabine is injected and operant test is performed between 60 and 90 min after the retigabine administration. (b) Similar to (a) except this set of experiments is performed in a different group of rats, and vehicle is administered to the animals on day 30. (c) Hind paw withdrawal thresholds measured by the von Frey test in different days before (open bar) and following oxaliplatine treatment (closed bars). On day 30 (gray bars), retigabine is administered and the von Frey test is performed between 60 and 90 min after retigabine administration. Data represent Mean ± SEM, ns, not significantly different, *p < .05, **p < .01, ***p < .001, compare with the data of adjacent testing days; #p < .05, ##p < .01, ###p < .001, compare with the data before oxaliplatin treatment.
Discussion
The use of the orofacial operant test in the present study has allowed us to show the development of trigeminal neuropathic pain that manifests with orofacial mechanical allodynia in animals treated with the chemotherapy drug oxaliplatin. A main finding of the present study is that oxaliplatin-induced trigeminal neuropathic pain is associated with significant downregulation of KCNQ2 channel expression in V2 TG neurons. Moreover, we have demonstrated that the oxaliplatin-induced trigeminal neuropathic pain can be alleviated by pharmacological potentiation of KCNQ2 function. These findings suggest that downregulation of KCNQ2 channel expression in V2 TG neurons may play a significant pathological role in the development of trigeminal neuropathic pain that manifests with mechanical allodynia in orofacial areas.
We have used the rat model of CIPN induced by oxaliplatin in the present study because oxaliplatin is the first-line chemotherapy for advanced colorectal cancer, and the drug is also an essential chemotherapy drug for several other types of cancers.1 Most cancer patients treated with oxaliplatin develop CIPN with symptoms including mechanical allodynia and cold allodynia/hyperalgesia.21 The use of the operant test in the present study allows us to assess pain in orofacial regions as opposed to nociceptive reflex measured by classical behavioral tests.16,22 Thus, the model of CIPN and the operant behavioral test used here provide clinically relevant assessment for both trigeminal neuropathic pain and alleviation of the pain by retigabine. In the present study, we show that mechanical allodynia occurs in both orofacial areas and hind paws of animals treated with oxaliplatin systemically, indicating that the trigeminal sensory nervous system is equally vulnerable to the side effects of chemotherapy drugs. Consistently, clinical CIPN can also occur in proximal body parts including the orofacial regions3,4 in addition to CIPN in extremities.2 In addition to the development of orofacial mechanical allodynia in oxaliplatin-treated animals shown in the present study, these animals have also been shown to develop orofacial cold allodynia/hyperalgesia in our previous study.13 The development of both symptoms has a similar time courses, raising the possibility that a common molecular mechanism may contribute to both orofacial mechanical allodynia and cold allodynia/hyperalgesia induced by oxaliplatin.
In the present study, strong KCNQ2-ir is observed in small-sized TG neurons including those innervating orofacial regions (V2 parts of TGs) in the control group. The result is consistent with previous studies showing the expression of KCNQ2 channels on small-sized nociceptive DRG neurons.9 We have further observed in the control group that there is strong KCNQ2-ir in the superficial layer of the Vc region in the brainstem, a region receiving sensory inputs mainly from nociceptive trigeminal afferent fibers. These results suggest that KCNQ2-expressing TG neurons are primarily nociceptive neurons. Our immunostaining results show a significant reduction of KCNQ2-ir positive TG neurons and also a decrease of KCNQ-ir intensity in the Vc regions of brainstems in animals following oxaliplatin treatment. The reduction of the numbers of KCNQ2-ir positive neurons can be interpreted as a decrease of KCNQ2 expression in some neurons to a degree under the detection threshold. These results suggest that KCNQ2 channel expression undergoes downregulation following oxaliplatin treatment. An alternative interpretation is that oxaliplatin might result in the loss of KCNQ2-expressing TG neurons, but this possibility is less likely, and we did not observe decreases of V2 TG neuron numbers in oxaliplatin-treated animals.
Downregulation of KCNQ channel expression has been observed in nociceptive DRG neurons in other pathological conditions such as tissue inflammation. This change has been suggested to be an underlying mechanism leading to hyperexcitability of nociceptive DRG neurons and pathological pain.10–12 In TG neurons, our recent study has shown that suppressing KCNQ channel activity pharmacologically by linopirdine significantly increases excitability of TG neurons, indicating that KCNQ2 channels play a role in controlling the excitability of TG neurons.13 Our recent study using orofacial operant tests has further shown that inhibition of KCNQ channels directly results in behavioral cold hypersensitivity but potentiation of KCNQ channels with retigabine alleviates cold allodynia/hyperalgesia in the oxaliplatin model of trigeminal neuropathic pain.13 Similarly, our present study has shown that the KCNQ channel potentiator retigabine alleviates trigeminal neuropathic pain that manifests with mechanical allodynia. Thus, downregulation of KCNQ2 channels appears to be a common mechanism for both mechanical allodynia and cold allodynia/hyperalgesia in oxaliplatin-induced trigeminal pain. The trigeminal neuropathic pain in our oxaliplatin-treated animals became relieved automatically after 40 days, raising a possibility that the downregulated KCNQ2 channels in oxaliplatin-treated animals may be recovered to normal levels to account for the behavioral recovery.
Author Contributions
JGG conceived and designed the experiments. JGG wrote the paper. JL, FE, VV, and HK performed experiments.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grants DE018661 and DE023090 (to JGG).
References
- 1.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol 2010; 6: 657–666. [DOI] [PubMed] [Google Scholar]
- 2.Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 2017; 7: e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton RB, Apfel SC, Dutcher JP, et al. Taxol produces a predominantly sensory neuropathy. Neurology 1989; 39: 368–373. [DOI] [PubMed] [Google Scholar]
- 4.Iniguez C, Larrode P, Mayordomo JI, et al. Reversible peripheral neuropathy induced by a single administration of high-dose paclitaxel. Neurology 1998; 51: 868–870. [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807–819. [DOI] [PubMed] [Google Scholar]
- 6.Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 2014; 17: 153–163. [DOI] [PubMed] [Google Scholar]
- 7.Busserolles J, Tsantoulas C, Eschalier A, et al. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain 2016; 157: S7–S14. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 2009; 156: 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passmore GM, Selyanko AA, Mistry M, et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 2003; 23: 7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose K, Ooi L, Dalle C, et al. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain 2011; 152: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Q, Fang D, Liu M, et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 2013; 154: 434–448. [DOI] [PubMed] [Google Scholar]
- 12.Duan KZ, Xu Q, Zhang XM, et al. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain 2012; 153: 562–574. [DOI] [PubMed] [Google Scholar]
- 13.Abd-Elsayed AA, Ikeda R, Jia Z, et al. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain 2015; 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Q, Gao Z, Wang W, et al. Activation of Kv7 (KCNQ) voltage-gated potassium channels by synthetic compounds. Trends Pharmacol Sci 2008; 29: 99–107. [DOI] [PubMed] [Google Scholar]
- 15.Splinter MY. Ezogabine (retigabine) and its role in the treatment of partial-onset seizures: a review. Clin Ther 2012; 34: 1845–1856.e1841. [DOI] [PubMed] [Google Scholar]
- 16.Cha M, Kohan KJ, Zuo X, et al. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behav Brain Res 2012; 234: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo X, Ling JX, Xu GY, et al. Operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: effects of menthol and capsazepine. Mol Pain 2013; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling B, Coudore-Civiale MA, Balayssac D, et al. Behavioral and immunohistological assessment of painful neuropathy induced by a single oxaliplatin injection in the rat. Toxicology 2007; 234: 176–184. [DOI] [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki A, Serizawa K, Andoh T, et al. Pharmacological differences between static and dynamic allodynia in mice with herpetic or postherpetic pain. J Pharmacol Sci 2008; 108: 266–273. [DOI] [PubMed] [Google Scholar]
- 21.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 2005; 39: 128–135. [DOI] [PubMed] [Google Scholar]
- 22.Neubert JK, Widmer CG, Malphurs W, et al. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain 2005; 116: 386–395. [DOI] [PubMed] [Google Scholar]