Abstract
Background
Modulation of N-methyl-D-aspartate receptor subunits NR1 and NR2 through phosphorylation mediates opioid-induced hyperalgesia, and activations of protein kinase C and extracellular signal-regulated kinase 1/2 potentiate while activation of calcium/calmodulin-dependent protein kinase II inhibits opioid-induced hyperalgesia. However, the mechanism of opioid-induced hyperalgesia development and in particular the potential interplay between N-methyl-D-aspartate receptors and protein kinase C or calcium/calmodulin-dependent protein kinase II or extracellular signal-regulated kinase 1/2 in the development of remifentanil-induced hyperalgesia is unclear.
Methods
Remifentanil (1 µg ċ kg−1 ċ min−1) was given intravenously over 60 min in rats, followed by the infusion of either vehicle solution or the respective inhibitors of protein kinase C (chelerythrine), extracellular signal-regulated kinase II (KN93), or extracellular signal-regulated kinase 1/2 (PD98059). Thereafter, the pain behaviors were evaluated by the paw withdrawal mechanical threshold and paw withdrawal thermal latency. In in vitro studies, fetal spinal cord dorsal horn neurons were primary cultured in the presence of 4 nM remifentanil for 60 min, and then the remifentanil was washed out and replaced immediately by culturing in the absence or presence of chelerythrine, KN93 or PD98059, respectively for up to 8 h. The expressions of N-methyl-D-aspartate receptors subunits and their phosphorylation (NR1, NR2B, p-NR1, p-NR2B) were analyzed by Western blotting after the completion of treatments. Functional changes of N-methyl-D-aspartate receptors were evaluated by electrophysiologic recordings of N-methyl-D-aspartate currents.
Results
Remifentanil induced significant thermal and mechanical hyperalgesia, which were significantly attenuated by Chelerythrine or KN93 but not PD98059. The expressions of NR1, NR2B, p-NR1, and p-NR2B were increased significantly and progressively over time after remifentanil administration, and these increases were all significantly attenuated by either chelerythrine or KN93 but not PD98059. Intriguingly, N-methyl-D-aspartate receptor functional enhancement induced by remifentanil was attenuated by Chelerythrine, KN93, and PD98059.
Conclusions
It is concluded that the enhancements in function and quantity of N-methyl-D-aspartate receptor via phosphorylation of its subunits through protein kinase C and calcium/calmodulin-dependent protein kinase II activation may represent the major mechanism whereby remifentanil induced hyperalgesia.
Keywords: Remifentanil, hyperalgesia, N-methyl-D-aspartate, protein kinase C, calcium/calmodulin-dependent protein kinase II, extracellular signal-regulated kinase 1/2
Introduction
Opioids are the most commonly used drugs for the treatment of moderate to severe surgical and chronic pain. But opioid therapy intended to alleviate pain may potentially make patients more sensitive to pain and may even aggravate preexisting pain, and this phenomenon is termed opioid-induced hyperalgesia (OIH).1 Remifentanil is routinely used intraoperatively due to its pharmacokinetic properties of rapid and predictable recovery that is unaffected by the dose and duration of infusion.2 However, many clinical evidence manifests that remifentanil could enhance mechanical pronociception, shorten the time of first post-operative analgesic requirement, and elevate cumulative opioid consumption during the postoperative period, a phenomenon referred to as remifentanil-induced hyperalgesia (RIH).3–5 While the precise mechanisms underlying OIH or RIH are poorly understood, abnormal activation of N-methyl-D-aspartate (NMDA) receptors in the central nervous system and long-term potentiation (LTP) of synapses between nociceptive C fibers and neurons in the spinal dorsal horn (DH) have been suggested to play critical roles.6 Initially, studies about RIH was conducted in animal (rat) pain models of plantar incision.7 However, more recent studies showed that administration of remifentanil without incision also induced hyperalgesia8,9 with mechanisms yet to be elucidated. Given that remifentanil without incision might be more suitable to solely study the mechanism of the drug action per se without the influence of surgery, for instance, injured tissue can produce endogenous analgesic mediators to counteract pain, such as opioid peptides, somatostatin, end cannabinoids, and anti-inflammatory cytokines.10 Among these mediators, endogenous opioid-mediated anti-nociception has been most extensively studied and its physiologic and clinical relevance has been established in postoperative pain in humans and in animal models.11 Therefore, in this study, we choose the model of no plantar incision in rats to determine whether remifentanil itself affects pain behaviors in rats and whether RIH without surgery is associated with NMDA receptor (NMDAR) phosphorylation/activation. In other words, we believe that the model of no plantar incision and no inflammation in rats is suitable to explore the possible mechanism of RIH from another important angle.
The NMDAR is a glutamate receptor, and ion channel protein exists in nerve cells.12 The NMDAR forms a heterotetramer between two obligatory NMDARs NR1 subunits and two regionally localized NR2 subunits (NR2A and NR2B).13 NMDAR is activated through a specific agonist-binding to its NR2 subunit. And, following NMDAR activation, a non-specific cation channel is opened, which can allow the passage of Ca2+ and Na+ into the cell and K+ out of the cell.14 The excitatory postsynaptic potential produced by the activation of NMDAR also increases the concentration of Ca2+ in the cell. The Ca2+ can in turn function as a second messenger in various signaling pathways.12,15 This property of the NMDAR explains many aspects of LTP and synaptic plasticity,16 including those activated by opioids.17
Studies have showed that activation of presynaptic or postsynaptic NMDAR is a crucial mechanism underlying the development and maintenance of different models of pain.18,19 Phosphorylation is the mechanism by which ionic glutamate receptor is activated. The subunit of NMDAR has multiple amino acid residues in their C-terminals.20 Serine/threonine21 or/and tyrosine22 kinase activation-induced phosphorylation of NR1/NR2 subunit have been shown to play crucial roles in the occurrence and progress of synaptic plasticity, and those C terminal domains are the substrates of protein kinase A, protein kinase C (PKC), calcium/calmodulin-dependent protein kinase II (CaMKII), and extracellular signal-regulated kinase (ERK).23 Some studies showed that the PKC inhibitor chelerythrine,24 CaMKII inhibitor KN93,25 and the ERK1/2 inhibitor PD9805926 could decrease allodynia and hyperalgesia by regulating phosphorylation of NMDA subunits. However these experiments only studied one particular subunit of NMDAR, for example, NR1 or NR2B without assessing NMDAR channel function. So in this study, we investigated the effects of chelerythrine, KN93, and PD98059 on remifentanil-induced phosphorylation of NR1 and NR2B subunits and their potential relationship with RIH, incorporating the application of rat in vivo models of RIH and in vitro models of remifentanil-induced NMDA functional changes in rat embryonic DH neurons.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 250 ∼ 300 g and pregnant female Sprague-Dawley rats were provided by Chongqing Medical University Medical Animal Center (Chongqing, China). Animals were housed two per cage and maintained in a room under a 12-h light/dark cycle (lights on at 7:00 a.m.), at controlled temperature (22 ± 1℃). Food and water were available ad libitum except during behavioral evaluation. This study was conducted with the approval of the institutional animal experimental ethics committee of Chongqing Medical University (Chongqing, China). The experimental protocol was approved by institutional animal care and use committee of Chongqing Medical University.
Behavioral testing
Many studies about rat pain model were model of plantar incision with 1-cm longitudinal incision.7 Different from most studies reported in existing literature, several studies showed that administration of remifentanil without incision also induced hyperalgesia.8,9 In this study, we choose the model of no plantar incision in rats to determine whether remifentanil itself affects pain behaviors in adult rats. Each rat’s tail vein was cannulated with a 26-gauge catheter at the root of the tail and flushed with heparinized saline. For behavioral studies, paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) of the rats were tested.27 The changes of rat behavior were measured at 24 h before the administration of drugs and then 1 h, 2 h, 4 h, 8 h after the stop of remifentanil infusion in the five study groups stated below. Remifentanil (YiChang Humanwell, China) was dissolved in saline and systemically administered with an infusion syringe pump at a rate of 1 µg ċ kg−1 ċ min−1.8,28 KN93, chelerythrine, and PD98059 were dissolved in 0.5% dimethelsulfooxide (DMSO). Rats were divided into five groups of five rats each according to different drug administration. Control group (C group, saline 1 µg ċ kg−1 ċ min−1, 60 min, iv and then 0.5% DMSO, 10 min, iv), remifentanil group (R group, remifentanil 1 µg ċ kg−1 ċ min−1, 60 min, iv and then 0.5% DMSO, 10 min, iv), remifentanil plus KN93 group (R + K group, remifentanil 1 µg ċ kg−1 ċ min−1, 60 min, iv and then KN93 0.02 mg ċ kg−1 ċ min−1,10 min, iv), remifentanil plus chelerythrine group (R + C group, remifentanil 1 µg ċ kg−1 ċ min−1, 60 min, iv and then chelerythrine 0.2 mg ċ kg−1 ċ min−1, 10 min, iv), and remifentanil plus PD98059 group (R + P group, remifentanil 1 µg ċ kg−1 ċ min−1, 60 min, iv and then PD 98059 0.2 mg ċ kg−1 ċ min−1, 10 min, iv).
Rats were habituated to the test environment (behavior testing room) daily (a 60-min session) for two days before baseline testing. To measure tactile sensitivity threshold, rats were placed into a plastic cage with a wire mesh bottom and examined by applying a von Frey filament to the plantar surface of each hind paw. The von Frey filament set has a calibrated range of bending force (26, 15, 10, 8, 6, 4, 2, 1.4, 1, .6, .4, .16, and .007 g). A single filament was applied to the plantar surface for five times with an inter-stimulation interval of 5 s. A positive response was defined as at least one clear withdrawal response in the five applications. The filament was applied in an up-and-down fashion according to a negative or positive response.
Rats were placed in clear plastic cages on an elevated glass plate, and the radiant thermal stimulator (BME410A, Institute of Biological Medicine, and Academy of Medical Science, China) was focused onto the plantar surface of the left and right hind paw through the glass plate. The nociceptive end-points in the radiant heat test were the characteristic lifting or licking of the hind paw. The cut off time of 25 s was used to avoid tissue damage.29
In vitro study in primarily cultured DH neurons
Studies by others also showed that remifentanil induced increasing of NMDARs expression in spinal cord.30–32 This was verified in our preliminary experiment that remifentanil 1 µg ċ kg−1 ċ min−1 over 60 min infusion induced increasing of p-NR1 and p-NR2B in spinal cord of rats (data not showed). We decided to investigate the effects of chelerythrine, KN93, and PD98059 on remifentanil-induced phosphorylation of NR1 and NR2B subunits in DH neurons. Primary DH neurons cultures were prepared as previously described in literature.33 In brief, pregnant female SD rats (embryonic day 14 or 15) were anesthetized by sevoflurane and then the fetal SD rats were removed immediately after cervical dislocation. The fetuses were decapitated, and their spinal cords were surgically extracted by an anterior approach and placed in recently thawed DMEM/F12 solution with 10% fetal bovine serum. Each spinal cord was laid open by a longitudinal incision along the dorsal commissure down through the central canal. This allowed the dissection of the dorsal half of the cord by cutting along the lateral funiculus bilaterally. Then, the dorsal halves of the spinal cords were pooled, minced, and incubated in DMEM/F12 solution containing 0.25% trypsin (Gibco, USA) at 37℃ for 20 min. The trypsin was inactivated by rinsing the dorsal cords in minimum essential medium (Sciencell, USA) with 10% fetal bovine serum. The tissue was then mechanically dissociated by trituration with Pasteur pipettes. The cells were plated onto poly-lysine-coated six-wells plates, and the culture medium was no serum-free medium (consists of 500 ml of basal medium, 5 ml of neuronal growth supplement, and 5 ml of penicillin/streptomycin solution) (Sciencell, USA). All electrophysiological recordings and protein western blotting assay were undertaken in the DH neurons after 14 to 21 days of incubation when mature complements of NMDARs are expressed.34 The DH neurons were divided into four groups: remifentanil group (R group, remifentanil 4 nM for 60 min, and then washed out, and then 0.1%DMSO for up to 8 h), remifentanil plus KN93 group (R + K group, remifentanil 4 nM for 60 min, and then washed out, and incubate with KN93 10 µM for up to 8 h), remifentanil plus chelerythrine group (R + C group, remifentanil 4 nM for 60 min, and then washed out, and then chelerythrine 100 µM for up to 8 h), and remifentanil plus PD 98059 group (R + P group, remifentanil 4 nM for 60 min, and then washed out, and then PD98059 100 µM for up to 8 h). Cultured DH neuron samples were collected at baseline and at 0, 1, 2, 4, and 8 h after remifentanil washout in respective subgroups for assays of signaling proteins and their status of phosphorylation by Western blotting. And electrophysiological recordings were detected at before, during remifentanil treatment (20, 40, and 60 min) and after remifentanil washout (20 min, 40 min, 1 h, 2 h, 4 h, and 8 h).
Protein extract and Western blotting assays
DMSO vehicle (0.1% final concentration) was added to control cultures as described in literature.35 The protein extracts were subjected to centrifugation at 30,000 g for 15 min at 4℃, and the supernatant was removed to a fresh tube. The protein concentration was determined using Bio-Rad DC protein assay kit. The protein samples were loaded on sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene difluoride membranes (Trans-blot SD semi-dry transfer cell, BioRad) for 1 h at 15 V in transfer buffer (48 mM Trizma, 39 mM glycine, 20% methanol, and 0.25% sodium dodecyl sulfate). After transfer, the nitrocellulose membranes were incubated in blocking solution for 1 h (tris buffered saline (TBS)/T plus 5% defatted dried milk). After incubation, the blot was washed twice for 5 min with TBS plus 0.05% Tween-20 (TBS/T) and then incubated overnight at 4℃ in blocking solutions containing the following monoclonal antibodies: anti-NR1(1:1000; Abcam, Cambridge, UK), anti-NR2B(1:1000; Abcam, Cambridge, UK), anti-phospho-NR1Ser896(1:1000; Abcam, Cambridge, UK), anti-phospho-NR2BY1336 (1:1000; Abcam, Cambridge, UK), and GAPDH(1:1000;Cell Signaling, USA). After incubation with the primary antibody, membranes were washed three times for 15 min each with TBS/T. Membranes was incubated for 1 h with anti-rabbit IgG HRP antibody with gentle agitation at room temperature. After washing three times for 15 min with TBS/T, chemiluminescence (Pierce, Rockford, IL) was used to detect the immune complex. Western blots were analyzed by densitometric scanning of X-ray films using an image analysis system.
Electrophysiological recording
All electrophysiological recordings were made at room temperature (20℃–22℃). Patch electrodes were pulled from thin-walled borosilicate glass using a two-stage vertical puller (WPI Pul-100; USA) with a series resistance of 3 to 8 MΩ. Whole cell potentials and currents were recorded, and data were filtered (2 kHz), digitized using the Digidata 1322A (Axon Instruments Inc.), and acquired on-line at a sampling frequency of 10 kHz using the pCLAMP8 program (Axon Instruments Inc.). Tonic-firing, small-sized DH neurons with capacitance less than 22 pF were previously shown to have an increased likelihood for the co-expression of NMDA and opioid receptors by demonstrating enhanced NMDA-evoked current amplitude after chronic morphine treatment.34 Therefore, only DH neurons with these electrophysiological properties were used in the current study.
Recording electrodes were filled with intracellular solution consisting of 140 mM KCl, 10 mM HEPES, 2 mM MgCl2, 10 mM EGTA, and 4 mM MgATP. This solution was buffered to a pH of 7.4 using KOH. Culture media from dishes of DH neurons were gently replaced with an extracellular recording solution containing 140 mM NaCl, 1.3 mM CaCl2, 5.4 mM KCl, 25 mM HEPES, and 33 mM glucose, buffered to a pH of 7.4 with NaOH.34 At a holding potential of −60 mV, the selected DH neurons were perfused continuously with the extracellular solution containing 3 µm glycine. This solution was delivered via a three-barrel capillary tube system with each barrel attached to a 5-ml reservoir, and the height of which was adjusted to deliver solutions at a rate of 1 ml/min. Rapid exchange of solutions between barrels by lateral movement of the capillary tube system allowed exposure of the neuron to a 1-s application of NMDA at a saturating concentration of 1 mM. NMDA-evoked currents were recorded at 20 min intervals after remifentanil co-perfusion at 4 nM concentrations and other study drugs described above (n = 10). NMDA-evoked currents were recorded from 10 randomly chosen cells at each time point, and the current altitudes were thus averaged from 10 recordings at each time.
Statistics
Data are presented as mean with standard deviation in parentheses unless indicated otherwise. After testing for normal distribution using the D’Agostino-Pearson omnibus K2 test, data were analyzed by two-way analysis of variance followed by a post hoc Bonferroni comparison of individual means. The behavioral responses to mechanical stimuli over time, alteration of expression of the proteins, and NMDA peak current amplitude detected among groups were tested with one-way and two-way analysis of variance with repeated measures followed by Bonferroni post hoc tests, respectively. Differences are considered statistically significant when p value is less than 0.05.
Result
CaMKII inhibition with KN93 and PKC inhibition with chelerythrine but not ERK1/2 inhibition with PD98059 attenuated remifentanil-induced mechanical and thermal hyperalgesia
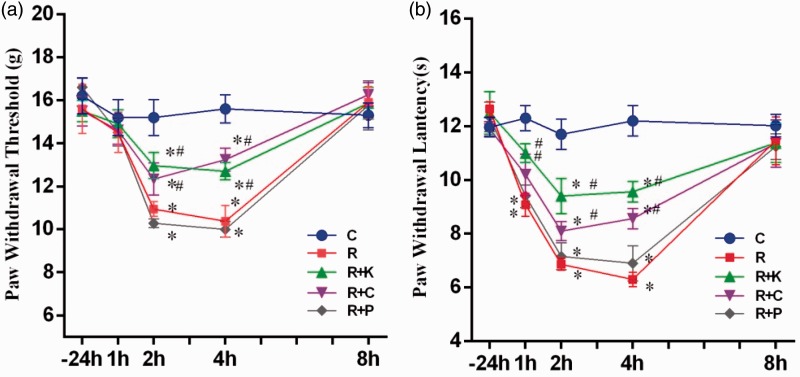
As shown in Figure 1, values of PWT or PWL did not differ among groups at baseline (−24 h) (p > 0.05; Figure 1(a) and (b)). Remifentanil infusion alone (group R) caused significant decrease in PWT and PWL, respectively, starting at 2 h and 1 h after the stop of remifentanil infusion as compared to the control group (all p < 0.05, group R vs. group C Figure 1(a) and (b)), while the values of PWT and PWL in group R all returned to baseline values at 8 h after stopping remifentanil infusion (p > 0.05. group R vs. group C). Animals in group R + K and group R + C exhibited significantly higher mechanical threshold (g) at 2 h, 4 h and higher thermal latency (s) at 1 h, 2 h, and 4 h after the stop of remifentanil infusion as compared to those in group R (all p < 0.05). By contrast, the values of PWT and PWL in the group of rats receiving PD98059 did not significantly differ from those in the group R at any time point (all p > 0.05, group P vs. group R).
Figure 1.
Mechanical and thermal hyperalgesia in remifentanil-induced hyperalgesia. The time course of paw withdrawal threshold (PWT, (a)) and paw withdrawal latency (PWL, (b)) on the hind paws in the normal saline (Group C), remifentanil (Group R), remifentanil + CaMKII inhibitor KN93 (Group R + K), remifentanil + PKC inhibitor chelerythrine (Group R + C), and remifentanil + ERK1/2 inhibitor PD98059 (Group R + P), respectively; −24 h means baseline; 1 h, 2 h, 4 h, and 8 h, respectively, means 1 h, 2 h, 4 h, and 8 h after the stop of remifentanil infusion. Data are expressed as means ± SD (n = 5 per group). *p < 0.05 compared with group C, #p < 0.05 compared with group R.
KN93 and chelerythrine but not PD98059 significantly attenuated remifentanil-induced activation/phosphorylation of NMDAR subunits NR1 and NR2B
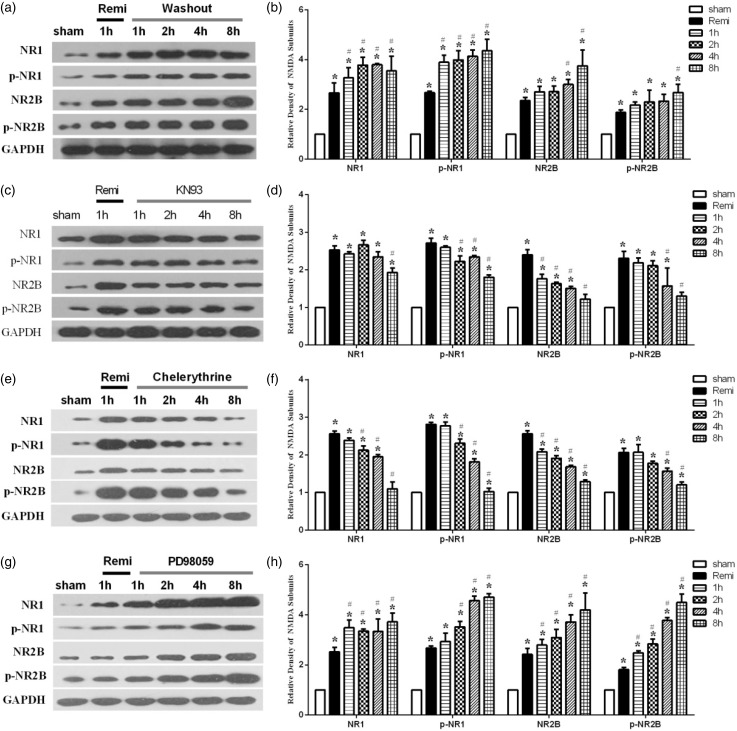
The embryonic DH neurons were treated with 4 nM remifentanil for 60 min and then washed out. As shown in Figure 2(a) and (b), exposure to 4 nM remifentanil for 60 min caused significant increases in NR1, NR2B, p-NR1, and p-NR2B protein expression (all p < 0.05 Rem. vs. sham), and all these receptor proteins and their phosphorylation continued to increase until up to 8 h after remifentanil washout (i.e., the experimental completion time). Application of the CaMKII inhibitor KN93 at the time of remifentanil washout significantly decreased remifentanil washout induced increases in NR1, NR2B, p-NR1, and p-NR2B expression and returned the magnitude of p-NR1 and p-NR2B expression to baseline values (Figure 2(c) and (d)). Given that KN93 also attenuated RIH as shown above, it is likely that KN93 inhibited RIH through decreasing phosphorylation/activation of NR1 and NR2B. Treatment with chelerythrine for 8 h after remifentanil washout also significantly reduced NR1, NR2B, p-NR1, and p-NR2B expression (Figure 2(e) and (f)), with effects similar to those seen with CaMKII inhibitor KN93. Therefore, PKC inhibition with chelerythrine also seems to have attenuated RIH through decreasing phosphorylation NR1 and NR2B. By contrast, ERK1/2 inhibition with PD98059 did not decrease remifentanil washout induced increases in NR1, NR2B, p-NR1, and p-NR2B expression. PD98059 also did not attenuate RIH. Thus, PKC and CaMKII, but not ERK1/2 played important roles in RIH through phosphorylating/activating NR1 and NR2B subunits.
Figure 2.
Changes of the NR1, NR2B, p-NR1, and p-NR2B expression after drugs administration in DH neurons. In (a) and (b), sham represents baseline, no drugs treatment; Remi represents 4 nM remifentanil treatment 60 min; 1 h, 2 h, 4 h, and 8 h, respectively, represents 1 h, 2 h, 4 h, and 8 h remifentanil washout ((a) and (b)). In (c) and (d), sham represents baseline, no drugs treatment; Remi represents 4 nM remifentanil treatment 60 min; 1 h represents 60 min remifentanil washout and KN93 treatment 1 h; 2 h represents 60 min remifentanil washout and KN93 treatment 2 h; 4 h represents 60 min remifentanil washout and KN93 treatment 4 h; 8 h represents 60 min remifentanil washout and KN93 treatment 8 h; In (e) and (f) and (g) and (h), treatment protocols of chelerythrine and PD98059 are the same to that in (c) and (d). Data are expressed as means ± SD; n = 5 per group per time point.*p < 0.05 vs. Control, #p < 0.05 vs. Remi. Values for NMDA subunits expression were presented as fold increase over group sham and normalized to the expression of GAPDH.
KN93 and chelerythrine but not PD98059 prevented remifentanil-induced enhancement of NMDAR function in spinal DH neurons
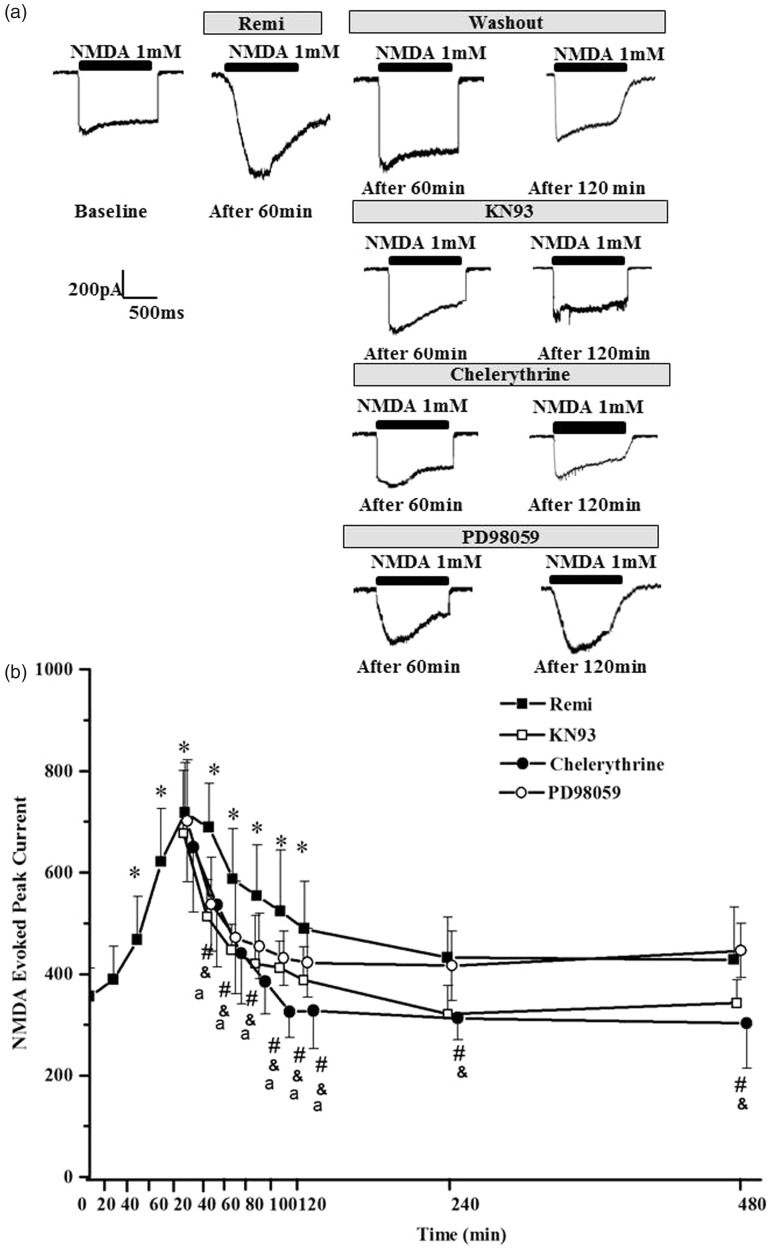
The embryonic DH neurons were treated with 4 nM remifentanil for 60 min and then washed out. As shown in Figure 3, NMDA evoked a significant increase in peak current amplitude in the subset of spinal DH neurons after 60 min of 4 nM remifentanil perfusion as compared to baseline values (Figure 3(a) top lane). The increases in NMDA-evoked peak current amplitude were significant as early as 40 min after the start of remifentanil perfusion and reached its maximum value at 20 min after remifentanil washout (Figure 3(b)). These enhanced NMDA responses persisted for 120 min during remifentanil washout. The peak current amplitude of NMDA responses resumed normal at 240 min and 480 min after remifentanil washout compared with baseline. CaMKII antagonist KN93 (10 µM), PKC antagonist chelerythrine (100 µM), and the ERK1/2 inhibitor PD98059 (100 µM) attenuated the increases in NMDA-evoked peak current from 40 min to 120 min after remifentanil washout (Figure 3(a) lower panels and (b)). KN93 and chelerythrine also significantly decreased NMDA peak current 240 min and 480 min after remifentanil washout. These results indicated that PKC, CaMKII, and ERK regulate the formation of the NMDA-induced current enhancement. The results indicate that remifentanil induced hyperalgesia both through enhancement of NMDAR function and more importantly through up-regulation of NMDAR phosphorylation/activation.
Figure 3.
N-Methyl-D-aspartate (NMDA)-evoked current responses in remifentanil and inhibitors treated dorsal horn neurons. Significant increases in NMDA peak current amplitude occurred with remifentanil exposure. There was a significant decrease in NMDA-evoked current after chelerythrine, KN93, and PD98059 treatment.*p < 0.05 versus time 0 in group Remi, #p < 0.05 group KN93 versus group Remi, &p < 0.05 group chelerythrine versus group Remi, ap < 0.05 group PD98059 versus group Remi.
Discussion
In the present study, we showed that intravenous infusion of remifentanil could induce mechanical and thermal hyperalgesia in adult rats, which was prevented by PKC and CaMKII inhibitions. Moreover, remifentanil infusion increased the NR1, NR2B, p-NR1, and p-NR2B expression in spinal DH neurons, which were prevented by PKC and CaMKII inhibitions. In patch-clamp study, we found that remifentanil enhanced the NMDAR-mediated peak current amplitude, which were also attenuated by inhibition of PKC, CaMKII, or ERK1/2, respectively. These results suggest that PKC and CaMKII inhibition may have prevented RIH via regulating NMDAR activation and function in spinal DH neurons.
Remifentanil induced acute hyperalgesia in no incisional rats
Remifentanil is widely used as an analgesic during surgery. Some studies have indicated that intraoperative infusion of remifentanil is associated with postoperative hyperalgesia and increases postoperative analgesic requirements in both animal models and human clinical trials.36 Conversely, some studies have reported that remifentanil did not induce hyperalgesia.37,38 The results showed that remifentanil induced acute mechanical hyperalgesia and thermal hyperalgesia, respectively, at 2 h and 1 h after the stop remifentanil infusion and did not last too long. Interestingly, the pain threshold returned to baseline at 8 h after the stop of remifentanil infusion in the no incisional pain models in our current study. Wang et al.8 showed that remifentanil infusion (1 µg ċ kg−1 ċ min−1 for an hour) without surgery induced mechanical and thermal hyperalgesia which lasted for 48 h. While in another study, Ishida et al.28 showed that remifentanil had a dose-dependent antinociceptive effect which rapidly diminished; 10 - or 30-min remifentanil infusion did not induce hyperalgesia, while the tail-flick latency and mechanical pain threshold after termination of 120-min remifentanil infusion were significantly lower than those in the control group regardless of remifentanil dose, but duration of hyperalgesia lasted no longer than 60 min. Findings of these studies suggest that the duration of RIH may vary in different studies. While the underlying mechanism of this phenomenon is not clear, differences in animal origin as well as feeding and experimental environments might be the potential factors that affected animal reactivity. In our current study, the behavioral change disappeared within 8 h, which is in contrast to the findings (48 h) of Wang et al., despite that the dosage and duration of remifentanil infusion were the same in rat models of remifentanil infusion without incision. In addition to the differences in animal origin or feeding/experimental environments, the potential differences of manufacturer and production batches of remifentanil could also be a cause.
Impacts of CaMKII on the development of RIH and NMDAR activation
The excitatory neurotransmitter NMDA plays a central role in the development of OIH. NMDARs become activated after the administration of opioids, and inhibition of NMDARs prevented the development of tolerance and OIH.3,39 Phosphorylation is a fundamental and pervasive mechanism widely known to regulate the functions of proteins.40 NMDAR function and its cell surface expression depend on phosphorylation of the subunits of the NMDAR.41 NMDAR mediated signaling pathways that subsequently activate downstream effectors such as CaMKII. CaMKII is abundant in postsynaptic densities (PSD), and Ca2+ can be activated through NMDAR in PSD. Ca2+ activates CaMKII and causes the auto-phosphorylation of CaMKII at site Thr286 that is associated with increased NMDAR activity and the subsequently increased Ca2+ in flux.42 Oh et al.43 showed that the acute intrastriatal injection of the CaMKII inhibitor KN93 attenuated serine phosphorylation of NR2A and NR2B subunits. Li et al.44 showed that administration of KN93 in the central nucleus of amygdala dose-dependently reversed fentanyl-induced hyperalgesia in rats. Intrathecal administration of KN93 down-regulated p-CaMKII and NR2B expression and attenuated bone cancer pain in a dose- and time-dependent manner.25 Intrathecal injection of KN93 also reduced mechanical allodynia and reduced phosphorylation of CaMKII at Thr286 and phosphorylation of GluR1 at Ser831 in the spinal cord seven days after spared nerve injury.45 Luo et al.46 showed that acute treatment with KN93 dose-dependently reversed SNL-induced thermal hyperalgesia and mechanical allodynia. These findings support a critical role of CaMKII in OIH and allodynia. In our remifentanil infusion without incision model, KN93 reversed remifentanil-induced reduction of PWT values at 2 and 4 h and PWL values at 1, 2, and 4 h. KN93 (10 µM) not only decreased the NMDA subunits (phospho-NR1 and phospho-NR2B) expression during 8-h treatment but also attenuated the increases in NMDA peak current during washout of remifentanil. Findings from us and others collectively suggest that activation of CaMKII is one of the major mechanisms whereby remifentanil induces hyperalgesia.
Impacts of PKC on the development of RIH and NMDAR activation
An intense stimulation of primary afferent fibers initially activates AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic) and peptide receptors and, when frequency of stimulation exceeds threshold, the voltage-dependent Mg-block of NMDARs is removed, allowing activation of these receptors to take place.47 As a consequence, the opening of the NMDAR channel leads to an influx of calcium (Ca2+) into the neurons, triggering a cascade of intracellular events such as PKC activation.48 PKC in turn potentiates NMDA responses.49 This positive feedback would enhance PKC activity and intracellular Ca2+ excessively, leading to neuronal excitotoxicity and cell death.50 Some studies showed that PKC could affect the function of NMDARs, and selective PKC inhibitors have been found to block the development of behavioral hyperalgesia.51 PKC can phosphorylate NMDARs, resulting in changes in their properties. Direct phosphorylation of NMDARs may not be the only mechanism by which PKC regulates the function of NMDARs.52 PKC has been shown to potentiate NMDA responses indirectly by activation of the tyrosine kinase (Src) signaling cascade.53 Increasing evidence also suggests that PKC modulates the distribution and function of NMDARs by participating in the interactions between NMDARs and postsynaptic density and cytoskeletal proteins.54 Studies showed that PKC phosphorylated the NR1 subunit at Ser890 and Ser896,55 and PKC also phosphorylated the NR2A and NR2B subunits and subsequently induced enhancement of NMDA currents.56 PKC inhibitor chelerythrine significantly inhibited the morphine-evoked enhancement of glutamate-induced currents.57 Activation of PKC has also been shown to enhance NMDA-induced currents in a variety of experimental systems.58 Chelerythrine chloride, a potent and specific inhibitor of PKC, reversed remifentanil-induced hyperalgesia in rats. In vitro, 100 µM chelerythrine not only reduced the protein expression and activation of NMDA subunits (NR1, NR2B phospho-NR1, and phospho-NR2B) during 8 h of treatment but also attenuated the increases in NMDA peak current during washout of remifentanil.
Diverse effects of ERK1/2 on NMDAR activation and NMDA-evoked currents
Stimulation of NMDARs can lead to a Ca2+-dependent activation of the MAP kinase (ERK1/2) cascade.59 The ERK1/2 cascade has been implicated in a number of glutamate receptor-dependent physiological functions. ERK1/2, as a member of MAPA family, may modulate transient LTP through regulating voltage-gated potassium channels60 and modulate late LTP through phosphorylation of the transcription factors cAMP response element binding protein and Erk-1.61 ERK1/2 activation during neuropathological stimulation of NMDARs may contribute to neuronal death in the adult brain.62 It has been shown that the MEK inhibitor PD98059 is a specific blocker of ERK1/2 activation.63 Pre-incubation of striatal neurons with PD98059 (50 IM) completely prevented glutamate-mediated ERK1/2 activation.63 Perkinton et al. also showed that treatment of striatal neurons with NMDA (100 IM) for 5 min induced a robust activation of ERK1/2 and pre-incubation of striatal neurons with the NMDAR blocker MK801 (2 IM) completely prevented glutamate-evoked activation of ERK1/2.63 Earlier studies demonstrated NR1 phosphorylation on Ser897 via the Erk and PKC pathways in isolated spinal DH.59 Since ERK/MAPK is localized in spines and the PSD fractions, the activation of ERK/MAPK may phosphorylate tyrosine of NR2B as shown in the case of estradiol-induced MAPK signaling.64 However, studies showed that the ERK kinase (MEK) inhibitor PD98059 (5 µM) had no effect on endogenous brain-derived neurotropic factor-induced NR1 phosphorylation. Thus, ERK/MAPK pathway was not involved in brain-derived neurotropic factor-induced NR1 phosphorylation.65 Further study showed that application of PD98059 (50 µM) for 1 h did not affect the magnitude of NMDA-excitatory postsynaptic potential.66 While Cao et al.67 showed that intrathecal injection of PD98059 (10 µg) 1 h before Freund’s adjuvant (CFA) injection, partly prevented CFA-induced heat hyperalgesia.67 Wang et al.26 showed that PD98059 (10 µg) did not affect morphine-induced NR1 up-regulation but suppressed the increases in NR2A and NR2B expression.26 In our study, 2 mg/kg PD98059 intravenous injection did not reverse remifentanil-induced hyperalgesia on rats, nor did it decrease the expression and activation of NMDA subunits (NR1, NR2B p-NR1, and p-NR2B). However, 100 µM PD98059 attenuated the increases in NMDA peak current during washout of 4 nM remifentanil. The reason why ERK1/2 participated in the enhancement of NMDA function without affecting NMDA subunit expression is unclear. Similar to the findings of our study, Ishida et al.28 showed that remifentanil infusion induced transient withdrawal hyperalgesia soon after its termination in rats without incision, while intrathecal administration of the MEK inhibitor U0126 could not suppress RIH. The results observed by us and Ishida et al. may collectively suggest that ERK1/2 by itself may not be the essential factor involved in RIH at least in models without surgery, despite the underlying mechanism has yet to be elucidated.
In addition, studies have showed that activation of presynaptic or postsynaptic NMDAR is a crucial mechanism underlying the development and maintenance of different models of pain.18,19 Remifentanil mediated enhancement of NMDAR function increases intracellular Ca2+, Ca2+ influx in turn as a second messenger directly activate CaMK or indirectly via ERK1/2 signaling to regulate nuclear transcriptional machineries. While the nuclear CaMK pathway is fast-acting, the ERK1/2 pathway is slower acting but longer lasting although both of which are promoted by activation of synaptic NMDAR.68 Therefore, it is possible that inhibition of the ERK1/2 signaling does not affect non-ERK1/2-induced NMDAR activations, such as those induced by PKC or CaMKII. It should be noted that ERK1/2 has been shown to activate the putative osmolyte channel transient receptor potential vanilloid 4 (TRPV4),69 and the activation of TRPV4 can potentiate NMDA-induced currents.70 It is thus plausible that, in our in vitro studies, inhibition of ERK1/2 reduced NMDA-activated currents via mechanisms other than NMDAR activation (e.g., TRPV4).
In summary, the present study first examined and confirmed the effect of PKC/CaMKII/ERK on NMDAR phosphorylation and NMDA function during and following remifentanil stimulation in embryonic DH neurons in vitro and the impacts of PKC, CaMKII, or ERK inhibition on remifentanil-induced hyperalgesia in vivo. It was concluded that the enhancements in function and quantity of NMDAR via phosphorylation of its subunits through PKC and CaMKII activation may represent the major mechanism whereby remifentanil induced OIH. Insights gained from the current study may trigger further in-depth studies to simultaneously study NMDA function using spinal cord slice and primarily cultured neurons in order to elucidate the roles of NMDAR and its signaling in remifentanil-induced hyperalgesia.
Acknowledgments
The authors thank Shenzhen Ivy-Valued Biotechnology Co. Ltd. for the editorial assistance on the paper.
Author Contributions
CongYu and Zhengyuan Xia share senior authorship for this article. This study was presented at Experimental Biology 2016 at San Diego, USA, April 2 to 6, 2016 and was published in abstract form at FASEB Journal vol. 30 no. 1 Supplement lb510.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Research Project of Health Bureau of Chongqing (2013-1-031, Chongqing, China) and the Chongqing Science & Technology Commission (cstc2014yykfB10010, Chongqing, China and cstc2017jcyjAX0093,Chongqing, China).
References
- 1.Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg 2001; 93: 204–209. [DOI] [PubMed] [Google Scholar]
- 2.Egan TD. Remifentanil pharmacokinetics and pharmacodynamics. A preliminary appraisal. Clin Pharmacokinet 1995; 29: 80–94. [DOI] [PubMed] [Google Scholar]
- 3.Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology 2005; 103: 147–155. [DOI] [PubMed] [Google Scholar]
- 4.Hwang WJ, Moon YE, Cho SJ, et al. The effect of a continuous infusion of low-dose esmolol on the requirement for remifentanil during laparoscopic gynecologic surgery. J Clin Anesth 2013; 25: 36–41. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita S, Yokouchi T, Tanaka M. Effects of intraoperative high-dose vs. low-dose remifentanil for postoperative epidural analgesia after gynecological abdominal surgery: a randomized clinical trial. J Clin Anesth 2016; 32: 153–158. [DOI] [PubMed] [Google Scholar]
- 6.Drdla R, Gassner M, Gingl E, et al. Induction of synaptic long-term potentiation after opioid withdrawal. Science 2009; 325: 207–210. [DOI] [PubMed] [Google Scholar]
- 7.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Li Y, Wang H, et al. Inhibition of DOR prevents remifentanil induced postoperative hyperalgesia through regulating the trafficking and function of spinal NMDA receptors in vivo and in vitro. Brain Res Bull 2015; 110: 30–39. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Wang JY, Yuan F, et al. Glycogen synthase kinase-3beta contributes to remifentanil-induced postoperative hyperalgesia via regulating N-methyl-D-aspartate receptor trafficking. Anesth Analg 2013; 116: 473–481. [DOI] [PubMed] [Google Scholar]
- 10.Coutaux A, Adam F, Willer JC, et al. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine 2005; 72: 359–371. [DOI] [PubMed] [Google Scholar]
- 11.Busch-Dienstfertig M, Stein C. Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain–basic and therapeutic aspects. Brain Behav Immun 2010; 24: 683–694. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa H, Singh SK, Mancusso R, et al. Subunit arrangement and function in NMDA receptors. Nature 2005; 438: 185–192. [DOI] [PubMed] [Google Scholar]
- 13.Moriyoshi K, Masu M, Ishii T, et al. Molecular cloning and characterization of the rat NMDA receptor. Nature 1991; 354: 31–37. [DOI] [PubMed] [Google Scholar]
- 14.Dingledine R, Borges K, Bowie D, et al. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61. [PubMed] [Google Scholar]
- 15.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 2007; 7: 39–47. [DOI] [PubMed] [Google Scholar]
- 16.France G, Fernandez-Fernandez D, Burnell ES, et al. Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus. Neuropharmacology 2017; 112: 76–83. [DOI] [PMC free article] [PubMed]
- 17.Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, et al. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338: 160–182. [DOI] [PubMed] [Google Scholar]
- 18.van Amerongen G, de Boer MW, Groeneveld GJ, et al. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br J Clin Pharmacol 2016; 82: 903–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu XX, Cai J, Li MJ, et al. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol 2009; 215: 298–307. [DOI] [PubMed] [Google Scholar]
- 20.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 2010; 62: 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AM, Messing RO. Protein kinases and addiction. Ann N Y Acad Sci 2008; 1141: 22–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo XQ, Cai QY, Chen Y, et al. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res 2014; 1542: 167–175. [DOI] [PubMed] [Google Scholar]
- 23.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007; 53: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bu F, Tian H, Gong S, et al. Phosphorylation of NR2B NMDA subunits by protein kinase C in arcuate nucleus contributes to inflammatory pain in rats. Sci Rep 2015; 5: 15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Liang Y, Hou B, et al. The inhibitor of calcium/calmodulin-dependent protein kinase II KN93 attenuates bone cancer pain via inhibition of KIF17/NR2B trafficking in mice. Pharmacol Biochem Behav 2014; 124: 19–26. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Ma W, Chabot JG, et al. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFkappaB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain 2010; 151: 194–205. [DOI] [PubMed] [Google Scholar]
- 27.Zhai X, Sun C, Rong P, et al. A correlative relationship between chronic pain and insulin resistance in Zucker fatty rats: role of downregulation of insulin receptors. J Pain 2016; 17: 404–413. [DOI] [PubMed] [Google Scholar]
- 28.Ishida R, Nikai T, Hashimoto T, et al. Intravenous infusion of remifentanil induces transient withdrawal hyperalgesia depending on administration duration in rats. Anesth Analg 2012; 114: 224–229. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Liu Y, Hou B, et al. Activation of spinal alpha-7 nicotinic acetylcholine receptor attenuates remifentanil-induced postoperative hyperalgesia. Int J Clin Exp Med 2015; 8: 1871–1879. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Li SS, Zhao N, et al. Phosphorylation of the GluN1 subunit in dorsal horn neurons by remifentanil: a mechanism for opioid-induced hyperalgesia. Genet Mol Res 2015; 14: 1846–1854. [DOI] [PubMed] [Google Scholar]
- 31.Ye L, Xiao L, Bai X, et al. Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats. Neurosci Lett 2016; 634: 79–86. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Y, Cui S, Liu Y, et al. Dexmedetomidine prevents remifentanil-induced postoperative hyperalgesia and decreases spinal tyrosine phosphorylation of N-methyl-d-aspartate receptor 2B subunit. Brain Res Bull 2012; 87: 427–431. [DOI] [PubMed] [Google Scholar]
- 33.Zhao M, Joo DT. Subpopulation of dorsal horn neurons displays enhanced N-methyl-D-aspartate receptor function after chronic morphine exposure. Anesthesiology 2006; 104: 815–825. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 2008; 109: 308–317. [DOI] [PubMed] [Google Scholar]
- 35.de Sa Lima L, Kawamoto EM, Munhoz CD, et al. Ouabain activates NFkappaB through an NMDA signaling pathway in cultured cerebellar cells. Neuropharmacology 2013; 73: 327–336. [DOI] [PubMed] [Google Scholar]
- 36.Lenz H, Raeder J, Draegni T, et al. Effects of COX inhibition on experimental pain and hyperalgesia during and after remifentanil infusion in humans. Pain 2011; 152: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 37.Ishii H, Petrenko AB, Kohno T, et al. No evidence for the development of acute analgesic tolerance during and hyperalgesia after prolonged remifentanil administration in mice. Mol Pain 2013; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahtinen P, Kokki H, Hynynen M. Remifentanil infusion does not induce opioid tolerance after cardiac surgery. J Cardiothorac Vasc Anesth 2008; 22: 225–229. [DOI] [PubMed] [Google Scholar]
- 39.Xia WS, Peng YN, Tang LH, et al. Spinal ephrinB/EphB signalling contributed to remifentanil-induced hyperalgesia via NMDA receptor. Eur J Pain 2014; 18: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawson T, Scott JD. Protein phosphorylation in signaling–50 years and counting. Trends Biochem Sci 2005; 30: 286–290. [DOI] [PubMed] [Google Scholar]
- 41.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology 1995; 34: 1219–1237. [DOI] [PubMed] [Google Scholar]
- 42.Giese KP, Fedorov NB, Filipkowski RK, et al. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 1998; 279: 870–873. [DOI] [PubMed] [Google Scholar]
- 43.Oh JD, Vaughan CL, Chase TN. Effect of dopamine denervation and dopamine agonist administration on serine phosphorylation of striatal NMDA receptor subunits. Brain Res 1999; 821: 433–442. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Li C, Yin P, et al. Inhibition of CaMKIIalpha in the central nucleus of amygdala attenuates fentanyl-induced hyperalgesia in rats. J Pharmacol Exp Ther 2016; 359: 82–89. [DOI] [PubMed] [Google Scholar]
- 45.Katano T, Nakazawa T, Nakatsuka T, et al. Involvement of spinal phosphorylation cascade of Tyr1472-NR2B, Thr286-CaMKII, and Ser831-GluR1 in neuropathic pain. Neuropharmacology 2011; 60: 609–616. [DOI] [PubMed] [Google Scholar]
- 46.Luo F, Yang C, Chen Y, et al. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 2008; 325: 267–275. [DOI] [PubMed] [Google Scholar]
- 47.Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol 1987; 394: 501–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao J, Price DD, Mayer DJ, et al. Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain Res 1992; 588: 144–149. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 1992; 356: 521–523. [DOI] [PubMed] [Google Scholar]
- 50.Mayer DJ, Mao J, Holt J, et al. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A 1999; 96: 7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997; 71: 165–178. [DOI] [PubMed] [Google Scholar]
- 52.Zheng X, Zhang L, Wang AP, et al. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J Neurosci 1997; 17: 8676–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu WY, Xiong ZG, Lei S, et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 1999; 2: 331–338. [DOI] [PubMed] [Google Scholar]
- 54.Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol 2000; 62: 755–778. [DOI] [PubMed] [Google Scholar]
- 55.Tingley WG, Ehlers MD, Kameyama K, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 1997; 272: 5157–5166. [DOI] [PubMed] [Google Scholar]
- 56.Grosshans DR, Browning MD. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. J Neurochem 2001; 76: 737–744. [DOI] [PubMed] [Google Scholar]
- 57.Narita M, Hashimoto K, Amano T, et al. Post-synaptic action of morphine on glutamatergic neuronal transmission related to the descending antinociceptive pathway in the rat thalamus. J Neurochem 2008; 104: 469–478. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Ari Y, Aniksztejn L, Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci 1992; 15: 333–339. [DOI] [PubMed] [Google Scholar]
- 59.Kurino M, Fukunaga K, Ushio Y, et al. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem 1995; 65: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 60.Adams JP, Anderson AE, Varga AW, et al. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem 2000; 75: 2277–2287. [DOI] [PubMed] [Google Scholar]
- 61.Davis S, Vanhoutte P, Pages C, et al. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 2000; 20: 4563–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang Q, Gu Z, Zhang G, et al. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res 2000; 857: 71–77. [DOI] [PubMed] [Google Scholar]
- 63.Perkinton MS, Ip JK, Wood GL, et al. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J Neurochem 2002; 80: 239–254. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez R, Liu R, Baudry M. 17-Beta-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-D-aspartate receptor phosphorylation in cortical synaptoneurosomes. J Neurochem 2007; 101: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M, Kay JC, Shen S, et al. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCgamma, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 2015; 12: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coogan AN, O’Leary DM, O’Connor JJ. P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro. J Neurophysiol 1999; 81: 103–110. [DOI] [PubMed] [Google Scholar]
- 67.Cao DL, Zhang ZJ, Xie RG, et al. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol 2014; 261: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci 2003; 26: 81–89. [DOI] [PubMed] [Google Scholar]
- 69.Hdud IM, Mobasheri A, Loughna PT. Effect of osmotic stress on the expression of TRPV4 and BKCa channels and possible interaction with ERK1/2 and p38 in cultured equine chondrocytes. Am J Physiol Cell Physiol 2014; 306: C1050–C1057. [DOI] [PubMed] [Google Scholar]
- 70.Li L, Qu W, Zhou L, et al. Activation of transient receptor potential vanilloid 4 increases NMDA-activated current in hippocampal pyramidal neurons. Front Cell Neurosci 2013; 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]