Abstract
Background:
Allograft healing (ligamentization) after reconstruction of the anterior cruciate ligament (ACL) is dependent on multiple factors, including tissue processing, host biologic environment, and biomechanical stressors. Magnetic resonance imaging (MRI) can be used to assess graft maturation after ACL reconstruction.
Hypothesis:
A significant difference will exist in the MRI analysis between 2 distinct allograft constructs. Specifically, the MRI signal-to-noise quotient (SNQ) value will be smaller in quadrupled hamstring tendon (HT) allografts compared with doubled tibialis anterior (TA) allografts due to the difference in graft geometry (surface area–to-volume ratio).
Study Design:
Cohort study; Level of evidence, 2.
Methods:
Prospectively collected data from a subset of patients who participated in a randomized controlled trial at a single center from July 2010 to April 2012 were reviewed. Patients underwent ACL reconstruction using either HT or TA allografts. Six months postoperatively, 32 patients underwent noncontrast MRI to assess ligamentization. The SNQ was calculated for the allograft using sagittal noncontrast T2-weighted MRI as follows: SNQ = (S graft − S qaudriceps)/S backgroud. Graft properties including sagittal and coronal angle as well as tibial and femoral tunnel location were measured. All participants completed validated patient-reported outcome measures preoperatively and at 2 years postoperatively.
Results:
The mean MRI SNQ for the HT and TA allografts was 2.56 ± 2.41 and 3.15 ± 3.38, respectively (P = .57). For the entire group, there was a significant correlation between MRI SNQ and both sagittal graft angle (P = .02) and sagittal tibial tunnel position (P < .001). We did not find a significant correlation between the tibial tunnel location in the coronal plane, coronal graft angle, or location of the femoral tunnel and the MRI SNQ.
Conclusion:
Allograft ligamentization 6 months postoperatively, as assessed by MRI, is dependent on position of the tibial tunnel in the sagittal plane as well as sagittal graft orientation. We did not detect a difference in graft maturation at 6 months between the tibialis anterior and hamstring tendon allografts. This is the only study to our knowledge that directly compares quadrupled HT allografts and doubled TA allografts using postoperative MRI.
Keywords: anterior cruciate ligament reconstruction, allograft, magnetic resonance imaging, tibialis anterior, semitendinosus, hamstring tendon
The use of soft tissue allografts for anterior cruciate ligament (ACL) reconstruction has increased over the past 20 years, with recent estimates suggesting 20% of ACL reconstructions are now performed using allograft sources.3 Head-to-head comparisons of soft tissue allograft constructs are limited.5,6,9,14,15 In a larger prospective randomized controlled trial, we directly compared clinical and patient-reported outcomes of hamstring tendon (HT) and tibialis anterior (TA) allografts.12 In a subset of these participants, we obtained magnetic resonance images at 6 months postoperatively to assess allograft remodeling (ligamentization). Theoretically, the rate of ligamentization after reconstruction of the ACL is dependent on multiple factors, including allograft processing and biomechanical stressors. Additionally, a difference in graft geometry and surface area–to-volume ratio potentially affects the rate at which host fibroblasts are able to migrate, infiltrate, and remodel the donor tendon. Histologic analysis has shown that grafts undergo a process of increased cellularity and vascularization between 6 and 12 weeks postoperatively. At 6 months, organized fascicles similar to the native ACL are present.16 However, routine biopsy of reconstructed ACLs is not possible. Therefore, magnetic resonance imaging (MRI) has emerged as a means to assess graft maturation after ACL reconstruction.2 Specifically, MRI can be used to quantify graft remodeling,1,4,10 and graft signal is inversely correlated with biomechanical properties.16 The most common method reported in the literature to assess graft healing via MRI is to calculate a signal-to-noise quotient (SNQ), where the smaller the value the more “mature” the reconstructed graft is assumed to be.1,4,16
We hypothesized that a significant difference will exist in the MRI analysis between quadrupled HT and doubled TA allografts. Specifically, the MRI SNQ value will be smaller in quadrupled HT allografts compared with doubled TA allografts due to the difference in graft geometry (increased surface area–to-volume ratio). Secondary outcomes included differences in sagittal and coronal graft angle, tibial and femoral tunnel placement, arthrometric testing, and patient-reported outcome (PRO) measures.
Methods
Approval was obtained from the Oregon Health and Science University institutional review board, and the study was registered with the National Institutes of Health on ClinicalTrials.gov before enrollment began. All patients aged 18 to 70 years who presented to the principal investigator’s clinic for a primary ACL tear were considered for the study and consented to participate in a prospective longitudinal database (SOCRATES, Ortholink Pty Ltd). To be included in this study, participants had to have received their MRI and completed patient-reported outcome (PRO) measures at 2 years postoperatively. Patients were excluded if they had multiple ligamentous injuries to the knee, uncorrected instability of the ACL on the contralateral knee, or were unable to obtain an MRI postoperatively or adhere to study protocol.
Patients were randomized in blocks of 4 by a research assistant to either HT or TA allograft and blinded for the duration of follow-up. Reconstruction of the ACL was performed with a transtibial single-tunnel technique.11 A modified short oblique tibial tunnel orientation starting approximately 2 cm medial to the standard tibial tunnel position at the level of the tibial cortex was used to allow for anatomic femoral tunnel placement. Allografts were cryopreserved, low-level irradiated (<20 kGy), treated with a proprietary detergent, Allowash, and were of a minimum diameter of 7.0 mm when bundled (Community Tissue Services). Fixation was performed with an Endobutton (Smith & Nephew) on the femoral cortex and a biointerference screw with sheath (DePuy Mitek) in the tibia (sized to nearest 0.5 mm).
Six months postoperatively, 32 patients underwent a noncontrast MRI of the reconstructed knee (2.5-T, repetition time 3000 ms, echo time 40 ms). MRI sequences were analyzed in a blinded fashion by a senior orthopaedic surgery resident. Graft signal was calculated automatically by the Impax markup tool (Afga HealthCare) via noncontrast T2/spectral presaturation with inversion recovery–weighted MRI sequences. A standard ellipse demarcating the intra-articular portion of the graft was used to analyze the sagittal cut containing the largest portion of the reconstructed ACL (showing the majority of the graft from origin to insertion) (Figure 1). The SNQ was calculated using the following equation: SNQ = (S graft − S qaudriceps)/S backgroud.1,4,16 The signals of the tibial insertion, femoral insertion, quadriceps tendon, and background were also measured on T2-weighted sequences via standard circles with a 2.5-mm diameter (Figure 1).
Figure 1.
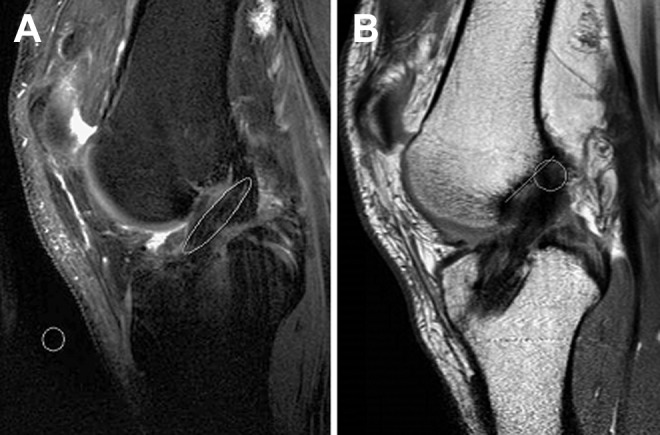
Representative magnetic resonance imaging (MRI) of reconstructed anterior cruciate ligament (ACL). (A) Sagittal noncontrast T2-weighted MRI cut containing the largest portion of the reconstructed ACL. Measurement of the signal-to-noise quotient (SNQ) was performed with a standard ellipse demarcating the intra-articular portion of the graft: SNQ = (S graft − S qaudriceps)/S backgroud. A standard circle was used anterior to the patellar tendon for the signal of the background (measurement of quadriceps signal not shown). (B) Sagittal proton density MRI cut showing the femoral insertion point of the allograft. A perpendicular line was used to measure the distance (mm) from the center of the circle to the Blumensaat line.
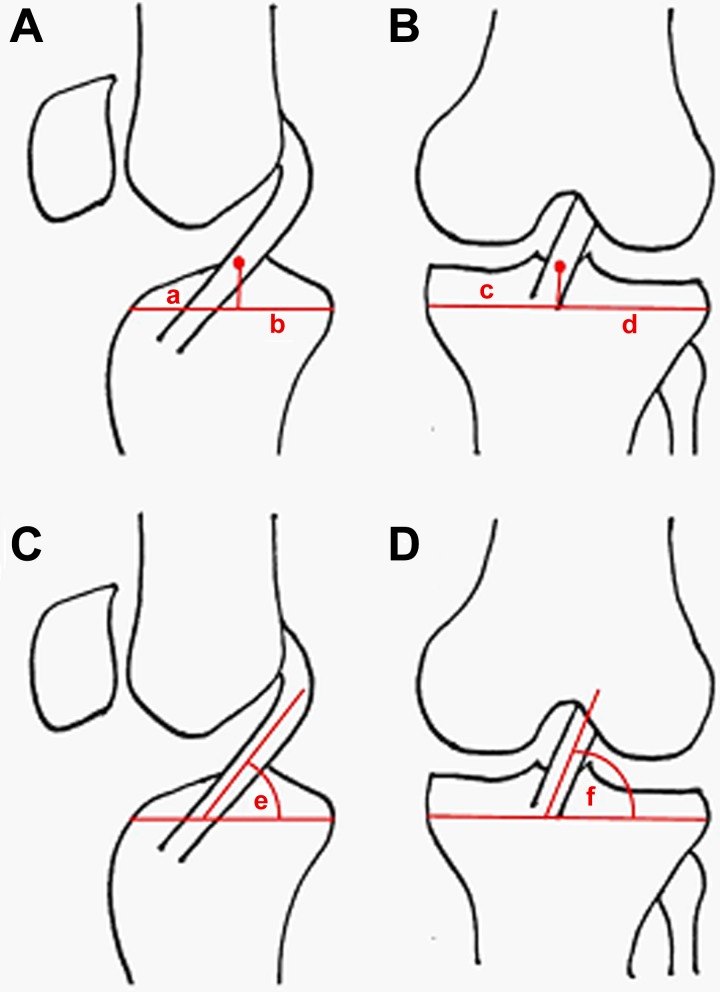
Graft parameters, including angles and tunnel positions, were measured on proton density sequences as described by Ahn et al2 (Figure 2). For sagittal and coronal graft measurements, the tibial plateau served as the reference. Tibial tunnel locations were calculated as a ratio, where the distance to the center of the tunnel, anterior (sagittal plane), or lateral (coronal plane) served as the numerator and the distance of the entire plateau served as the denominator. The posterior cruciate ligament insertion served as the posterior landmark of the tibial plateau in the sagittal plane. Femoral tunnel position was measured on sagittal proton density sequences with the Blumensaat line serving as the reference point. The distance (in millimeters) from the center of the femoral tunnel, measured perpendicular to the reference line, was recorded (see Figure 1). Average surface area–to-volume ratio for the allograft constructs was calculated using the following assumptions: average graft diameter 8 mm, average intra-articular graft length 20 mm, average HT strand radius (1 mm), average TA strand radius (2 mm).
Figure 2.
Calculation of graft parameters. (A) Sagittal tibial tunnel location. Ratio calculated as the distance from the anterior aspect of the plateau to a line drawn from the center of the tunnel, a, over the entire plateau length, b. The posterior cruciate ligament (PCL) insertion served as posterior landmark of tibial plateau. (B) Coronal tibial tunnel location. Ratio calculated as the distance from the lateral plateau, d, to the center of the tunnel over the entire plateau length, c. (C) Sagittal graft angle, e, with the tibial plateau serving as the reference. (D) Coronal graft angle, f, with the tibial plateau serving as the reference.
Sample means, standard deviations, and regression analyses were performed using Microsoft Excel (Microsoft Corp). The Student t test was used to analyze continuous data. Categorical data were statistically analyzed by means of a chi-square test or Fisher exact test (n < 10). Regression analysis was performed by calculating the Spearman correlation coefficient (rho) for MRI parameters. A P value of <.05 was considered significant.
Results
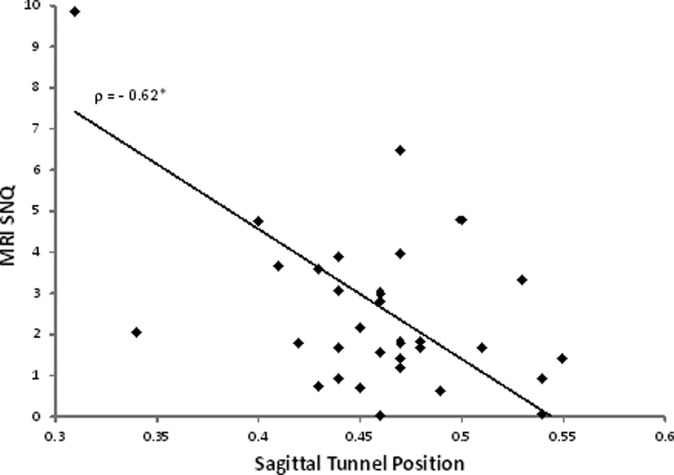
Thirty-two patients (16 in each group) underwent noncontrast MRI at 6 months postoperatively (mean ± SD, 193.8 ± 30.8 days). The mean age of the cohort was 41.0 ± 12.7 years, and the mean body mass index (BMI) was 27.8 ± 5.7 kg/m2. There were no significant differences in age, surgical side, graft diameter, sex, height, weight, BMI, or smoking status between the groups (Table 1). The mean MRI SNQs for the HT and TA allografts were 2.56 ± 2.41 and 3.15 ± 3.38, respectively (P = .57) (Table 2). For the entire cohort, there was a moderate but significant correlation in the sagittal graft angle and the MRI SNQ (R = −0.41, P = .02) (Table 3). The correlation between sagittal tunnel position and the MRI SNQ was stronger (R = −0.62, P = .0002) (Figure 3). Specifically, the signal in the graft increased as the tibial tunnel moved more anterior in the sagittal plane. We did not find a significant correlation between the coronal grant angle, coronal tunnel position, or femoral tunnel position and the MRI SNQ. We detected a very strong and significant correlation between the MRI SNQ at the femoral insertion versus midsubstance (R = 0.82, P < .001) as well as the MRI SNQ at the tibial insertion versus midsubstance (R = 0.83, P < .001). When analyzing patient variables, we did not find any correlation between MRI SNQ and age or BMI for the cohort (P = .20 and P = .74, respectively).
TABLE 1.
Patient Demographics (N = 32)a
| Hamstring Tendon | Tibialis Anterior | P Value | |
|---|---|---|---|
| Patients | 16 | 16 | |
| Age, y | 37.0 ± 12.0 | 45.0 ± 12.5 | .08 |
| Height, cm | 173.7 ± 9.0 | 170.8 ± 10.0 | .41 |
| BMI, kg/m2 | 26.5 ± 3.8 | 29.1 ± 7.0 | .20 |
| Graft diameter, mm | 7.91 ± 0.80 | 7.94 ± 0.51 | .90 |
| Meniscal pathology | 7 (43.8) | 8 (50) | .50 |
| Chondral pathology (MFC, LFC, tibia) | 3 (18.8) | 6 (37.5) | .43 |
| Surgical side (right) | 8 (50) | 10 (62.5) | .72 |
| Male sex | 11 (68.8) | 7 (43.8) | .39 |
| Smoking status (current or previous) | 5 (31.3) | 2 (12.5) | .39 |
aData are represented as mean ± SD or as number of patients (percentage). Difference between means calculated via 2-sample t test, α = .05. Difference between proportions calculated via Fisher exact test if n ≤ 10 or χ2 test. BMI, body mass index; LFC, lateral femoral condyle; MFC, medial femoral condyle; MRI, magnetic resonance imaging.
TABLE 2.
MRI Signal to Noise Quotient and Patient-Reported Outcomesa
| Hamstring Tendon | Tibialis Anterior | P Value | |
|---|---|---|---|
| MRI SNQb | |||
| Midsubstance | 2.56 ± 2.41 | 3.15 ± 3.38 | .57 |
| Tibial insertion | 2.61 ± 4.40 | 4.52 ± 10.2 | .50 |
| Femoral insertion | 2.86 ± 2.23 | 4.93 ± 8.47 | .35 |
| Sagittal graft angle, deg | 56.5 ± 8.3 | 54.6 ± 6.4 | .47 |
| Sagittal tibial tunnel position | 0.46 ± 0.05 | 0.45 ± 0.06 | .60 |
| Coronal graft angle, deg | 66.8 ± 7.3 | 64.9 ± 4.2 | .38 |
| Coronal tibial tunnel position | 0.58 ± 0.03 | 0.59 ± 0.04 | .48 |
| Femoral tunnel position,c mm | 3.52 ± 2.75 | 5.06 ± 1.52 | .06 |
| KT-1000 differenced | 0.88 ± 1.42 | 0.40 ± 1.73 | .32 |
| KOOS change | 18.8 (9.1-28.4) | 32.5 (23.9-41.0) | .03e |
| IKDC change | 24.4 (14.0-34.8) | 38.1 (28.9-47.3) | .05e |
| Lysholm change | 11.6 (1.5-21.7) | 31.5 (18.6-44.3) | .01e |
| Tegner change | 2.8 (2.0-3.6) | 2.7 (1.4-4.0) | .88 |
| VR-12 physical change | 9.9 (4.0-15.7) | 19.2 (11.8-26.5) | .04e |
aData are represented as mean ± SD or mean (95% CI). Difference between means calculated via 2-sample t test, α = .05. IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; SNQ, signal-to-noise quotient; VR-12, Veterans RAND 12-Item Health Survey.
bIncreased SNQ indicates decreased ligamentization in the reconstructed anterior cruciate ligament.
cDistance from the center of the femoral tunnel to the Blumensaat line.
dDifference in KT-1000, a positive value indicates that the operative side scored higher when compared with the contralateral side.
eStatistically significant difference between groups (P < .05).
TABLE 3.
Interrelationship of Graft Parameters Measured via Magnetic Resonance Imaging (Spearman Correlation Coefficients, Rho)
| MRI SNQ Midsubstance | Sagittal Tunnel Location | Sagittal Graft Angle | Coronal Tunnel Location | Coronal Graft Angle | Femoral Tunnel Position | MRI SNQ Tibial Tunnel | MRI SNQ Femoral Tunnel | |
|---|---|---|---|---|---|---|---|---|
| MRI SNQ mid-substance | — | −0.62b | −0.41c | 0.27 | −0.27 | 0.25 | 0.83b | 0.82b |
| Sagittal tunnel location | — | 0.46d | −0.20 | −0.003 | −0.09 | −0.46d | −0.46d | |
| Sagittal graft angle | — | −0.33 | 0.47d | −0.17 | −0.26 | −0.16 | ||
| Coronal tunnel location | — | −0.41b | 0.39c | 0.29 | 0.17 | |||
| Coronal graft angle | — | −0.42c | −0.09 | −0.15 | ||||
| Femoral tunnel location | — | 0.09 | 0.22 | |||||
| MRI SNQ tibial tunnel | — | 0.81b | ||||||
| MRI SNQ femoral tunnel | — |
aMRI, magnetic resonance imaging; SNQ, signal-to-noise quotient.
bP < .001.
cP < .05.
dP < .01.
Figure 3.
Magnetic resonance imaging (MRI) signal to noise quotient (SNQ) versus sagittal tibial tunnel position. Rho (ρ) is the Spearman correlation coefficient. *P < .001.
When we analyzed the groups independently, we found that for the HT group there remained a significant correlation between both MRI SNQ and sagittal graft angle (R = −0.71, P = .002) and MRI SNQ and sagittal tunnel position (R = −0.59, P = .02). For the TA group, there remained a significant correlation between MRI SNQ and the sagittal tunnel position (R = −0.63, P = .009). The estimated surface area for the HT allograft constructs was 527.8 mm2, average volume 251.3 mm3, and surface area–to-volume ratio was 2.1. The estimated surface area for the TA allograft constructs was 552.9 mm2, average volume 502.66 mm3, and surface area–to-volume ratio was 1.1.
Arthrometric data were collected for 75% of patients who received HT allograft as compared with 81% of patients who were randomized to TA allograft. There were no significant differences in the degree of anterior tibial translation between the 2 allograft constructs 6 months postoperatively as determined by KT-1000 testing (P = .32) (Table 2). We did not find any significant differences in the average baseline values for the Knee injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Lysholm, International Knee Documentation Committee (IKDC), Veterans RAND 12-Item Health Survey (VR-12), or Tegner scales between the 2 groups (data not shown). However, when we looked at the change from baseline, we found a significant difference in the groups, with the TA cohort improving significantly more than the HT cohort in 4 of 5 PROs measured (Table 2). The absolute values for the PRO scores at 2 years were not significantly different between groups (data not shown). We did not find any significant correlations between arthrometric testing or PROs (all time points) and MRI SNQ (data not shown).
Discussion
Theoretically, incorporation of allografts after ACL reconstruction should be dependent on numerous factors, including host environment, surgical and rehabilitation technique, and allograft processing and quality, with the spectrum of available allograft types representing a heterogeneous sample of biologic tissue. Our group was specifically interested in the role that graft geometry, specifically surface area–to-volume ratio, plays in remodeling. The TA construct is a single tendon doubled to create 2 bundles compared with the HT construct, which is 2 tendons doubled to create 4 bundles. We theorized that smaller-caliper hamstring tendons potentially lend themselves to more rapid remodeling given their larger surface area–to-volume ratio. Therefore, we elected to use postoperative MRI to assess the graft healing (ligamentization) between the 2 constructs.
In our study, the average SNQ was higher for the TA, although a significant difference was not detected. Analysis showed that MRI SNQ was independent of patient variables, including age, sex, BMI, and smoking status. However, SNQ was dependent on sagittal graft angle and tibial tunnel position in the sagittal plane. As the sagittal tunnel location moved more anterior, the MRI SNQ increased. We surmise this is because a more anterior tunnel location places more strain on the reconstructed ACL, causing increased inflammation and delayed healing. Interestingly, we did not see a correlation between MRI SNQ and femoral tunnel location, as would be expected based on previously reported studies.7 In the absence of 3-dimensional remodeling, determining the location of the femoral tunnel is extremely difficult. Therefore, we may have been limited in our ability to correlate the location with SNQ data. We also compared the SNQ of the intra-articular graft to the tibial and femoral insertions due to the role bony integration plays in graft remodeling after ACL reconstruction. We found very strong correlations (R > 0.8) between intra-articular signal and signal at both insertion points. Therefore, we can assume graft strain during the remodeling process is fairly evenly distributed throughout all portions of the graft. Finally, we showed in this subgroup that the individuals in the TA group on average improved their PRO scores significantly more than the HT group at 2 years. This statistical difference was not seen in the larger prospective randomized controlled trial.12
Limitations to our study include the fact that there was no autograft group for comparison, and we used noncontrast MRI. Studies have shown autografts incorporate faster than allografts after ACL reconstruction, and allograft maturation as assessed histologically or by MRI can continue as long as 18 months postoperatively.8,13 Additionally, the absolute value for the MRI SNQ in allografts is higher at 6 months when contrast is used.8 However, we decided to forgo contrast due to safety and cost considerations. Since we were comparing 2 allograft constructs, we were more interested in the difference between the 2 and not the absolute value of the MRI SNQ. Additionally, MRIs were not obtained at multiple time points to assess rate of healing. However, previous studies have shown substantial maturation of grafts at 6 months when analyzed histologically and by MRI.8,16 Consequently, we felt that the 6-month time point was sufficient to make inferences on overall healing of our allograft constructs. This is commonly the point at which patients are released to full or sport-specific activities; therefore, having information on allograft maturity at this time may assist with clinical decision making. Finally, while MRI analysis was done by a single senior resident, the values used for SNQ calculations were calculated automatically by the MRI software. Therefore, we feel advanced training is not required for proper graft analysis.
Conclusion
Our data showed there were no significant differences in the postoperative healing of the 2 allograft constructs after ACL reconstruction. Additionally, we have shown sagittal tunnel location has the strongest correlation with graft maturation 6 months postoperatively. This is the only study known to us to directly compare quadrupled HT allografts and doubled TA allografts using postoperative MRI.
Acknowledgment
The authors thank the following individuals for their contribution: Matthew Dehart, Christopher Domes, Michael Durkin, Mehwish Farooqi, and Samantha Quilici.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: D.C. received research support from Community Tissue Services to cover the cost of the postoperative MRI scans.
Ethical approval for this study was obtained from Oregon Health and Science University Research Integrity Office (IRB 00006158).
References
- 1. Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med. 2010;38:1768–1777. [DOI] [PubMed] [Google Scholar]
- 2. Ahn JH, Lee SH, Yoo JC, Ha HC. Measurement of the graft angles for the anterior cruciate ligament reconstruction with transtibial technique using postoperative magnetic resonance imaging in comparative study. Knee Surg Sports Traumatol Arthrosc. 2007;15:1293–1300. [DOI] [PubMed] [Google Scholar]
- 3. Cohen SB, Sekiya JK. Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:597–605. [DOI] [PubMed] [Google Scholar]
- 4. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89:1165–1171. [DOI] [PubMed] [Google Scholar]
- 5. Joyce CD, Randall KL, Mariscalco MW, Magnussen RA, Flanigan DC. Bone–patellar tendon–bone versus soft-tissue allograft for anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2016;32:394–402. [DOI] [PubMed] [Google Scholar]
- 6. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26:41–49. [DOI] [PubMed] [Google Scholar]
- 7. Marchant BG, Noyes FR, Barber-Westin SD, Fleckenstein C. Prevalence of nonanatomical graft placement in a series of failed anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38:1987–1996. [DOI] [PubMed] [Google Scholar]
- 8. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24:1038–1044. [DOI] [PubMed] [Google Scholar]
- 9. O’Brien DF, Kraeutler MJ, Koyonos L, Flato RR, Ciccotti MG, Cohen SB. Allograft anterior cruciate ligament reconstruction in patients younger than 30 years: a matched-pair comparison of bone–patellar tendon–bone and tibialis anterior. Am J Orthop (Belle Mead NJ). 2014;43:132–136. [PubMed] [Google Scholar]
- 10. Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26:50–57. [DOI] [PubMed] [Google Scholar]
- 11. Rose M, Crawford D. Technique for arthroscopic-assisted primary anterior cruciate ligament reconstruction using doubled tibialis anterior tendon. Arthrosc Tech. 2017;6:e87–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rose MB, Domes C, Farooqi M, Crawford DC. A prospective randomized comparison of two distinct allogenic tissue constructs for anterior cruciate ligament reconstruction. Knee. 2016;23:1112–1120. [DOI] [PubMed] [Google Scholar]
- 13. Shino K, Kawasaki T, Hirose H, Gotoh I, Inoue M, Ono K. Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg Br. 1984;66:672–681. [DOI] [PubMed] [Google Scholar]
- 14. Siebold R, Buelow JU, Bos L, Ellermann A. Primary ACL reconstruction with fresh-frozen patellar versus Achilles tendon allografts. Arch Orthop Trauma Surg. 2003;123:180–185. [DOI] [PubMed] [Google Scholar]
- 15. Sun K, Zhang J, Wang Y, et al. A prospective randomized comparison of irradiated and non-irradiated hamstring tendon allograft for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20:187–194. [DOI] [PubMed] [Google Scholar]
- 16. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29:751–761. [DOI] [PubMed] [Google Scholar]