Abstract
Objectives
We examined the long-term effects of everolimus in patients with renal angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis.
Methods
Following favorable results from the double-blind core phase of EXIST-2 (NCT00790400), patients were allowed to receive open-label everolimus (extension phase). Patients initially randomly assigned to everolimus continued on the same dose; those who were receiving placebo crossed over to everolimus 10 mg/day. Dose modifications were based on tolerability. The primary end point was angiomyolipoma response rate, defined as a ≥50% reduction from baseline in the sum volume of target renal angiomyolipomas in the absence of new target angiomyolipomas, kidney volume increase of >20% from nadir, and angiomyolipoma-related bleeding grade ≥2. The key secondary end point was safety.
Results
Of the 112 patients who received ≥1 dose of everolimus, 58% (95% CI, 48.3% to 67.3%) achieved angiomyolipoma response. Almost all patients (97%) experienced reduction in renal lesion volumes at some point during the study period. Median duration of everolimus exposure was 46.9 months. Sixteen (14.3%) patients experienced angiomyolipoma progression at some point in the study. No angiomyolipoma-related bleeding or nephrectomies were reported. One patient on everolimus underwent embolization for worsening right flank pain. Subependymal giant cell astrocytoma lesion response was achieved in 48% of patients and skin lesion response in 68% of patients. The most common adverse events suspected to be treatment-related were stomatitis (42%), hypercholesterolemia (30.4%), acne (25.9%), aphthous stomatitis and nasopharyngitis (each 21.4%). Ten (8.9%) patients withdrew because of an adverse event. Renal function remained stable, and the frequency of emergent adverse events generally decreased over time.
Conclusions
Everolimus treatment remained safe and effective over approximately 4 years. The overall risk/benefit assessment supports the use of everolimus as a viable treatment option for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis.
Trial registration
ClinicalTrials.gov NCT00790400
Introduction
Tuberous sclerosis complex (TSC) is a genetic disorder with a birth incidence of 1 in 6000 and results in growth of hamartomas in organs such as the brain, kidneys, skin, lungs, eyes, and heart [1,2]. In the brain, subependymal giant cell astrocytomas (SEGAs) develop in up to 20% of patients and their growth can potentially lead to ventricular obstruction, acute hydrocephalus, and death [3,4]. Skin lesions of various types including hypopigemented macules, ungual fibromas, and facial angiofibromas are the most readily visible TSC manifestation with more than 90% of patients possessing one or more lesions [4].
Angiomyolipomas are the most common renal lesions associated with TSC, occurring in up to 80% of patients [5–8]. They are also seen in 33%–50% of patients with sporadic lymphangioleiomyomatosis (LAM) [9]. Angiomyolipomas usually manifest as multifocal, bilateral, asymptomatic disease, but as the lesions exceed 3 to 4 cm, they may become symptomatic [10–12] and carry an increased risk for aneurysms that can lead to hemorrhage [11,13]. Renal angiomyolipomas associated with TSC or sporadic LAM (sLAM) exhibit upregulated activity of mammalian target of rapamycin (mTOR) resulting from mutations in either TSC1 or TSC2 genes [4,14]. The TSC consensus guidelines recommend mTOR inhibitors as first-line therapy for asymptomatic growing angiomyolipomas measuring >3 cm in diameter, whereas selective embolization and kidney-sparing resection are acceptable second-line therapies [15]. The EXIST-2 (EXamining everolimus In a Study of Tuberous sclerosis complex) study demonstrated superiority of everolimus over placebo in angiomyolipoma response rate, time to angiomyolipoma progression, and skin lesion response rate in patients with angiomyolipoma associated with either TSC or sLAM [16,17]. Results from an interim analysis of the open-label extension phase demonstrated stability of everolimus effects over time [18]. Here we report data from the end of the EXIST-2 extension phase to evaluate the longer-term efficacy and safety of everolimus in treating renal angiomyolipoma.
Methods
Study participants
A complete description of study participants, design, and outcomes has been published [16,18]. In short, patients ≥18 years of age with a definitive diagnosis of TSC per consensus criteria or sLAM confirmed by biopsy or chest computed tomography (CT) with ≥1 angiomyolipoma measuring ≥3 cm on longest diameter using CT/magnetic resonance imaging (MRI) were eligible. Patients were excluded if their angiomyolipoma necessitated surgery at randomization or if they had angiomyolipoma-related bleeding or embolization within 6 months before randomization. Patients who had carbon monoxide diffusion capacity (DLCO) ≤35%, below-normal oxygen saturation at rest, or oxygen saturation ≤88% on a 6-minute walking test (6MWT, with up to 6 L/min of oxygen) were also excluded [16].
The study was reviewed by an independent ethics committee or institutional review board for each center (see S1 Table for list of committees/review boards), and was conducted in compliance with Good Clinical Practice and under the principles of the Declaration of Helsinki. Informed written consent was obtained from each patient or the patient’s guardian before randomization. The protocol of this trial and supporting CONSORT checklist are available as supporting information (see S1 Protocol and S1 CONSORT Checklist).
Study design and treatment
EXIST-2 was a prospective, double-blind, randomized, parallel group, placebo-controlled, multicenter phase 3 study of everolimus 10 mg/day versus placebo [16,18]. During the double-blind core phase, patients were unblinded if they had any evidence of angiomyolipoma progression, and those receiving placebo were allowed to cross over to receive open-label everolimus. The study was unblinded on September 9, 2011, after a planned interim review showed everolimus to be highly effective and safe, while patients receiving placebo experienced continued deterioration [18]. An extension phase ensued in which patients who had been on everolimus during the core phase continued to receive the same dose and those who had been on placebo were switched to open-label everolimus (10 mg/day) (S1 Fig). Dose reductions to 5 mg/day or 5 mg/every other day were permitted based on safety. The extension phase continued until 4 years after the last patient was randomly assigned, ensuring patient follow-up of 4 to 5 years. Any patient who discontinued everolimus had a follow-up visit 28 days after the last dose to assess safety.
This analysis from EXIST-2 presents all data from patients who received everolimus during either the core or the extension phase of the study up to the data cutoff date of February 4, 2015.
Study outcomes
The primary efficacy outcome was the angiomyolipoma response rate, defined as the proportion of patients with a confirmed angiomyolipoma response (≥50% reduction in the sum volume of target renal angiomyolipomas [≥1 cm in longest diameter] relative to baseline in the absence of new target angiomyolipomas, kidney volume increase of >20% from nadir [to account for possible growth in non-target lesions], and angiomyolipoma-related bleeding grade ≥2) [16]. Initial response required confirmation by second MRI. All MRI scans were analyzed in a central institute by independent radiologists. Additional end points included response duration, time to angiomyolipoma response and progression, proportion of patients with ≥30% reduction in angiomyolipoma volume, proportion of patients with angiomyolipoma-related surgery, skin lesion response rate (≥50% improvement on skin lesions, based on Physicians Global Assessment), SEGA response rate (≥50% reduction in sum volume of target SEGA lesions in the absence of new target SEGA lesion with no worsening of nontarget SEGA lesions and no new or worsening hydrocephalus). Pulmonary function tests were monitored in patients with LAM. Skin lesion types included in the assessment for skin lesion response were facial angiofibromas, shagreen patches, periungual or subungual fibromas, hypomelanotic macules, and forehead plaques.
Safety assessments included collection of all adverse events (AEs) and serious AEs, with their severity. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [19]. Serious AEs were defined as those that were fatal or life-threatening, resulted in persistent or signifnicant disability/incapacity, constituted a congenital anomaly/birth defect, or required inpatient hospitalization or prolongation of existing hospitalization. Clinically notable AEs, defined as those AEs eliciting specific clinical interest in connection with everolimus, were also reported. Renal function was assessed using glomerular filtration rate (GFR), which was calculated using the Modification of Diet in Renal Disease formula [20]. Severe renal impairment was defined as a GFR <30 mL/min/1.73 m2 (chronic kidney disease stage ≥4).
Statistical analysis
Combined data from the core and extension phases were analyzed for all patients who received at least one dose of everolimus using an intention-to-treat principle. Descriptive statistics were used to summarize baseline data and efficacy variables. The best percentage change from baseline at any time in the sum volume of target angiomyolipomas was represented by a waterfall plot. A piecewise linear mixed model with repeated measurements was used to link the post-baseline sum volume of angiomyolipoma lesions to the duration on treatment. The model included the sum volume of angiomyolipoma lesions at baseline as a covariate and considered two periods of time: the period in the first 3 months, and the period beyond the first 3 months. Kaplan-Meier curves were constructed for median duration of angiomyolipoma response. Summary statistics determined median time to angiomyolipoma response and progression, with statistics given as point estimates with 95% confidence interval (CI). Exact 95% CIs for angiomyolipoma, skin lesion, and SEGA responses were obtained from the Clopper-Pearson method. For median time to angiomyolipoma, skin lesion, and SEGA responses, 95% confidence interval were calculated with the LIFETEST procedure using the method of Brookmeyer & Crowley, 1982 [21]. Box plots depict GFR and creatinine values over time with mean, median, and interquartile range indicated. Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
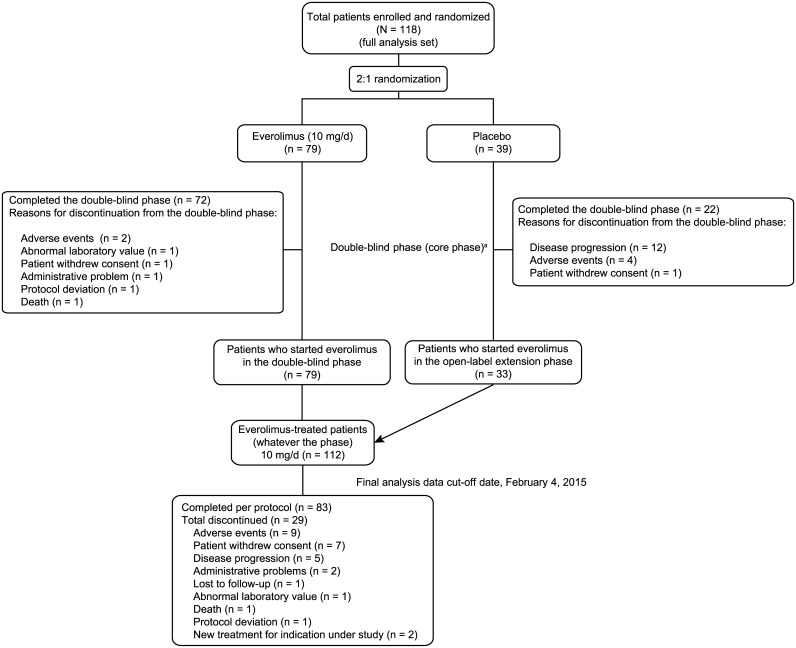
Overall, 112 patients received at least one dose of everolimus and were included in this analysis: 79 patients originally randomly assigned to everolimus and 33 patients who switched to open-label everolimus from placebo. At the data cutoff (February 4, 2015), 83 patients (74.1%) completed the treatment duration as per protocol (Fig 1).
Fig 1. CONSORT flow diagram of patient disposition in the double-blind followed by open-label periods.
aPatients with angiomyolipoma progression were unblinded at the end of the double-blind phase, and patients who were on placebo were allowed to cross over to open-label everolimus.
Most (65.2%) patients were women, and their median age was 32.2 years at baseline (Table 1). More than five target angiomyolipoma lesions at baseline were observed in 59.8% of patients, and 29.5% of patients had renal angiomyolipoma measuring ≥8 cm. The median sum of volumes of target angiomyolipoma lesions was 92.1 (range, 2.8–1611.5) cm3. More than one-third of patients (37.5%) had undergone angiomyolipoma-related surgery before initiating everolimus, and the median GFR at baseline was 86 (range, 23–178) mL/min/1.73 m2. Fifty patients (44.6%) had ≥1 target SEGA lesion.
Table 1. Patient baseline characteristics.
| Characteristic | Everolimus N = 112 |
|---|---|
| Age, median (range), years | 32.2 (18.1–61.6) |
| Age category, years, n (%) | |
| <30 | 49 (43.8) |
| ≥30 | 63 (56.3) |
| Sex, n (%) | |
| Male | 39 (34.8) |
| Female | 73 (65.2) |
| Race, n (%) | |
| White | 99 (88.4) |
| Asian | 11 (9.8) |
| Othera | 2 (1.8) |
| Diagnosis of TSC,b n (%) | 107 (95.5) |
| Diagnosis of sporadic LAM, n (%) | 5 (4.5) |
| Diagnosis of LAM, n (%) | 24 (21.4) |
| Presence of ≥1 SEGA lesion, n (%) | 50 (44.6) |
| Presence of ≥1 skin lesion, n (%) | 107 (95.5) |
| Facial angiofibromas or forehead plaque | 105 (93.8) |
| Ungual or periungual fibroma | 68 (60.7) |
| Hypomelanotic macules (three or more) | 69 (61.6) |
| Shagreen patch | 54 (48.2) |
| 'Confetti' skin lesions | 30 (26.8) |
| Prior renal angiomyolipoma—related surgery, n (%) | 42 (37.5) |
| Prior nephrectomy, n (%) | 21 (18.8) |
| Bilateral renal angiomyolipoma lesions, n (%) | 88 (78.6) |
| Longest diameter of largest renal angiomyolipoma lesion, n (%) | |
| ≥8 cm | 33 (29.5) |
| ≥4 cm and <8 cm | 64 (57.1) |
| ≥3 cm and <4 cm | 7 (6.3) |
| <3 cm | 6 (5.4) |
| Unknown | 0 |
| Not applicable | 2 (1.8) |
| Target renal angiomyolipoma lesions ≥1 cm, n (%) | |
| 0 | 2 (1.8) |
| 1–5 | 43 (38.4) |
| 6–10 | 67 (59.8) |
| Sum of volumes of target renal angiomyolipoma lesions | |
| Number of patients with ≥1 target renal angiomyolipoma | 110 |
| Median (range), cm3 | 92.1 (2.8–1611.5) |
Abbreviations: LAM = lymphangioleiomyomatosis; SEGA = subependymal giant cell astrocytoma; TSC = tuberous sclerosis complex.
aDesignates patients who were of mixed race.
bAll patients with diagnosed TSC had ≥2 major features of TSC. In patients with both LAM and renal angiomyolipoma, another feature must have been identified to assign TSC diagnosis.
Everolimus exposure
The median time on study was 47.2 (range, 0.9–65.3) months, and the median duration of everolimus exposure was 46.9 (range, 0.5–63.9) months. The median dose intensity was 8.7 (range, 1.9–19.3) mg/day, and the relative dose intensity (ratio of administered dose to planned dose) was 0.86. Most (82.1%) patients were exposed to everolimus for ≥2.8 years, and 12.5% had exposure for ≥4.5 years. Ten (8.9%) patients discontinued the study because of AEs. Approximately 80% of patients required ≥1 dose interruption and/or reduction; AEs were the most common reason (66.1% for dose interruptions, 59.8% for dose reductions). At 4 years into the study, approximately 60% of patients were taking 10 mg/day of everolimus (as per protocol), approximately 30% of patients were taking between 5 and <10 mg/day of everolimus (mostly 5 mg/day) and 10% to 15% of patients were taking between 0 and <5 mg/day (mostly 5 mg/every other day) out of the 58 patients who had at least 4 years of exposure to everolimus in the study.
Renal angiomyolipoma response
Of the 112 patients with ≥1 target renal angiomyolipoma at baseline, a confirmed response was achieved in 65 patients (58.0%; 95% CI, 48.3%–67.3%) at any time. The median time to angiomyolipoma response was 2.89 months (95% CI, 2.79 to 3.19). A best overall result of stable disease (between <50% reduction in lesion volume from baseline and <25% increase in volume over nadir) was reported in 34 patients (30.4%; 95% CI, 22.0%–39.8%); one patient (0.9%; 95% CI, 0.0%–4.9%), experienced disease progression within 18 weeks of the start of everolimus as a best response. Twelve (10.7%; 95% CI, 5.7%–18.0%) patients were not evaluable for response, primarily because of missing baseline kidney volume measurements.
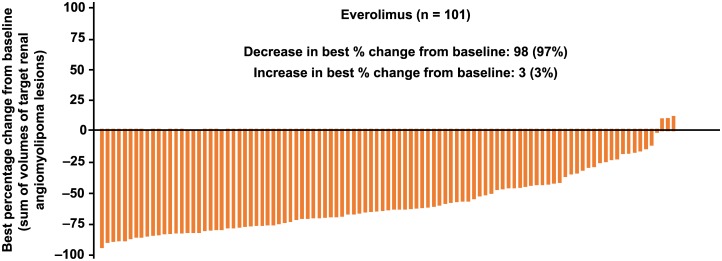
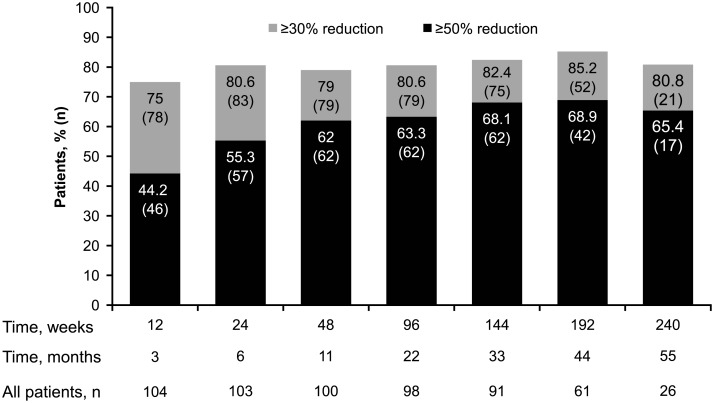
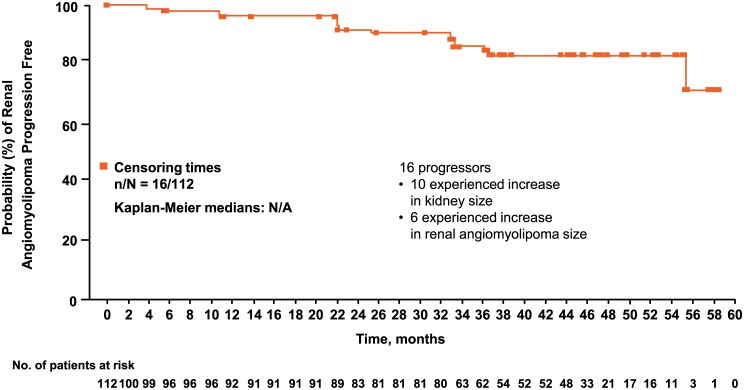
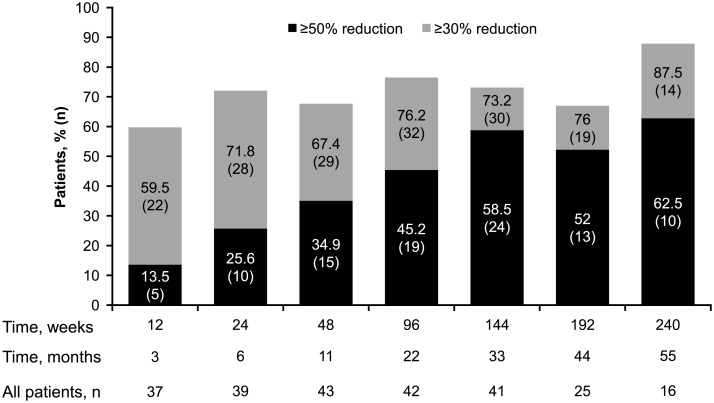
At least one reduction from baseline in the sum of volumes of target angiomyolipoma lesions was observed in 98 (97%) of 101 evaluable patients at some point in the study (Fig 2). Renal angiomyolipoma volume reductions of ≥30% were maintained for ≥75% of patients at all time points. The proportion of patients achieving ≥50% reductions increased over time (Fig 3). The longitudinal analysis confirmed that longer treatment duration was associated with lower post-baseline sum volumes of angiomyolipoma lesions, especially within the first 3 months after treatment was initiated. During the initial 3-month period, a 46% (95% CI, 37%–54%) mean reduction in the post-baseline sum volumes of angiomyolipoma lesions was estimated. Beyond this period, three additional months of treatment resulted in a slight reduction of less than 2% (95% CI, <1%–2%) in the post-baseline sum volumes of angiomyolipoma lesions. Overall, renal angiomyolipoma progression was observed in 16 (14.3%; 95% CI, 8.4%–22.2%) patients (Fig 4). Reasons for progression were increased angiomyolipoma size (6 patients) and kidney enlargement (10 patients); 9 continued the treatment despite disease progression because of a perceived clinical benefit. Progressive disease was preceded by dose reduction or interruption in 13 of the 16 patients (81.3%), similar to the reduction/interruption rate in the overall study population (80.4%, 90/112). Only 2 of the 65 patients who achieved a confirmed angiomyolipoma response had disease progression based on their lowest angiomyolipoma volume during the course of the trial. The duration from the first response to angiomyolipoma progression or the last available radiologic assessment ranged from 3.0 to 55.5 months.
Fig 2. Best percentage reduction in the sum volume of target renal angiomyolipomas each individual patient reported at any time point in the study in 101 evaluable patients.a.
a11 patients were considered “non-evaluable” due to missing overall angiomyolipoma response status at each radiological assessment. Among the 12 patients with a best overall response with the status “not evaluable”, only one patient reported at least one radiological assessment with a non-missing overall angiomyolipoma response status.
Fig 3. Renal angiomyolipoma response rate with everolimus over time.
Fig 4. Time to renal angiomyolipoma progression.
Skin lesion response
Of the 107 patients with ≥1 skin lesion at baseline, investigators noted response in 73 patients (68.2%; 95% CI, 58.5%–76.9%; complete clinical response [n = 1, 0.9%; 95% CI, 0.0%–5.1%] and partial response of ≥50% to <100% [n = 72, 67.3%; 95% CI, 57.5%–76.0%]); 29 (27.1%; 95% CI, 19%–36.6%) patients had stable disease. The proportion of responders increased steadily over time (from 23% [24/103] at week 12 to 70% [39/56] at week 204). The median time to skin lesion response was 8.41 months (95% CI, 5.59–11.53). None of the 73 responders experienced progression of skin lesions.
Subependymal giant cell astrocytoma lesion response
Among 50 patients with ≥1 baseline SEGA lesion measuring ≥1.0 cm, the response rate was 48% (24/50; 95% CI, 33.7%–62.6%), and 42% of patients (21/50; 95% CI, 28.2%-56.8%) achieved stable disease (five were not evaluable). Patients experienced gradual decreases in median SEGA volumes over time (week 12, –35.2%; week 144, –53.3%; and week 240, –54.4%). More than half the patients had ≥30% reduction in SEGA volume at all time points, and the proportion of patients with ≥50% reduction in SEGA volume increased over time (Fig 5). The median time to SEGA response was 8.31 months (95% CI, 5.55–11.30 months). None of the 24 SEGA responders experienced SEGA progression during the study.
Fig 5. Reduction in SEGA volume with everolimus over time in patients with renal angiomyolipomas.
Changes in pulmonary function
Among 29 patients with LAM who were treated with everolimus, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and DLCO decreased slightly over time. The median percentage change in FEV1 from baseline was –3.45% at week 24, –5.88% at week 96, and –9.00% at week 192. The median percentage change in FVC was 0% at week 24, –1.25% at week 96, and –4.29% at week 192. The median percentage change in DLCO was –2.69% at week 24, –10.19% at week 96, and –10.62% at week 192. In addition, there was an increase in total lung capacity (median percentage change in TLC: week 24, 0%; week 96, 0.89%; week 192, 7.13%) and residual volume (median percentage change in RV: week 24, 0%; week 96, 3.94%; week 192, 14.29%) over time. Percentage changes and absolute values over time are provided in Table 2.
Table 2. Pulmonary function tests in patients with LAM over time.
| Everolimus n = 29 |
|||
|---|---|---|---|
| Week 24 n = 23 |
Week 96 n = 22 |
Week 192 n = 18 |
|
| FEV1 | |||
| Baseline, median (min, max), L | 2.8 (1.2, 3.6) | 2.55 (1.2, 3.5) | 2.8 (1.2, 3.6) |
| Assessment, median (min, max), L | 2.6 (1.3, 3.6) | 2.55 (1.0, 3.5) | 2.45 (0.9, 3.4) |
| Median percentage change from baseline | –3.45 | –5.88 | –9 |
| FVC | |||
| Baseline, median (min, max), L | 3.6 (1.8, 5.2) | 3.6 (1.8, 5.2) | 3.65 (2.1, 5.2) |
| Assessment, median (min, max), L | 3.6 (2.0, 5.7) | 3.6 (1.7, 5.4) | 3.6 (2.1, 5.7) |
| Median percentage change from baseline | 0 | –1.25 | –4.29 |
| DLCO | |||
| Baseline, median (min, max), mmol/min/Kpa | 6.01 (2.7, 9.6) | 5.76 (2.7, 9.6) | 6.01 (2.7, 9.6) |
| Assessment, median (min, max), mmol/min/KPa | 5.74 (1.3, 8.2) | 5.23 (2.2, 9.4) | 5.95 (2.4, 7.0) |
| Median percentage change from baseline | –2.69 | –10.19 | –10.62 |
Abbreviations: DLCO = diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Safety outcomes
Most of the reported AEs were of grade 1 or 2 in severity. Individual grade 3 AEs occurred at a frequency ≤4.5%, and the most frequently reported that occurred in ≥2% were decreased blood phosphorus (laboratory abnormality; 4.5%), hypophosphatemia (per investigator; 2.7%), amenorrhea (3.6%), and epilepsy (2.7%). Individual grade 4 AEs were infrequent; the most common was blood uric acid increased (2.7%). The most common AEs suspected to be related to everolimus (≥20% of the patients) were stomatitis (42%), hypercholesterolemia (30.4%), acne (25.9%), and aphthous stomatitis and nasopharyngitis (21.4% each). Emergence of individual AEs was generally highest in the first year and decreased each subsequent year (Table 3). Serious AEs were observed in 37.5% of the patients, most frequently epilepsy (5.4%) and pneumonia (2.7%). Several AEs that have been previously noted with everolimus were monitored closely for their clinical impact. Infections were noted in 91.1%. Most infections involved the upper respiratory tract and were mild to moderate in severity. In addition to administration of appropriate non-drug therapy and concomitant medication, infections lead to everolimus dose interruptions or adjustments in 41 patients (36.6%). Stomatitis/related events were reported in 73.2% of patients. Hemorrhage-related events (prolonged activated partial thromboplastin time, decreased hemoglobin, menorrhagia, epistaxis, vaginal hemorrhage, international normalized ratio increased, and metrorrhagia) were reported in 49.1% of patients. Female fertility—related events (including secondary amenorrhea) occurred in 47.9% (34 of 71 females between 10 and 55 years of age) of patients, and 31% (22/71) experienced ≥1 episode of amenorrhea. Three patients reported grade 3 amenorrhea suspected to be related to everolimus. Two patients reported amenorrhea events requiring dose adjustment; none led to study discontinuation. Noninfectious pneumonitis occurred in two patients (both grade 2).
Table 3. Adverse events by preferred term regardless of relationship to study drug and by year of emergence (>15% of patients).
| Adverse events, n (%) | ≤12 months N = 112 |
13–24 months n = 101 |
25–36 months n = 100 |
37–48 months n = 91 |
49–60 months n = 52 |
|---|---|---|---|---|---|
| Stomatitis | 46 (41.1) | 9 (8.9) | 5 (5.0) | 5 (5.5) | 2 (3.8) |
| Nasopharyngitis | 36 (32.1) | 21 (20.8) | 20 (20.0) | 20 (22.0) | 6 (11.5) |
| Acne | 28 (25.0) | 8 (7.9) | 6 (6.0) | 2 (2.2) | 0 |
| Headache | 26 (23.2) | 11 (10.9) | 6 (6.0) | 4 (4.4) | 1 (1.9) |
| Hypercholesterolemia | 25 (22.3) | 13 (12.9) | 11 (11.0) | 7 (7.7) | 1 (1.9) |
| Aphthous stomatitis | 21 (18.8) | 15 (14.9) | 9 (9.0) | 5 (5.5) | 2 (3.8) |
| Fatigue | 19 (17.0) | 2 (2.0) | 4 (4.0) | 4 (4.4) | 2 (3.8) |
| Cough | 18 (16.1) | 4 (4.0) | 4 (4.0) | 3 (3.3) | 0 |
| Diarrhoea | 17 (15.2) | 7 (6.9) | 7 (7.0) | 4 (4.4) | 1 (1.9) |
| Mouth ulceration | 17 (15.2) | 6 (5.9) | 5 (5.0) | 2 (2.2) | 0 |
| Nausea | 17 (15.2) | 5 (5.0) | 2 (2.0) | 3 (3.3) | 0 |
Note that an adverse event is counted only in the time period in which it started.
None of the treated patients reported angiomyolipoma-related bleeding during the study. The most common AEs requiring additional therapy included nasopharyngitis and stomatitis (33% each). One death (status epilepticus) was reported and was not suspected by the investigator to be treatment related.
Renal function
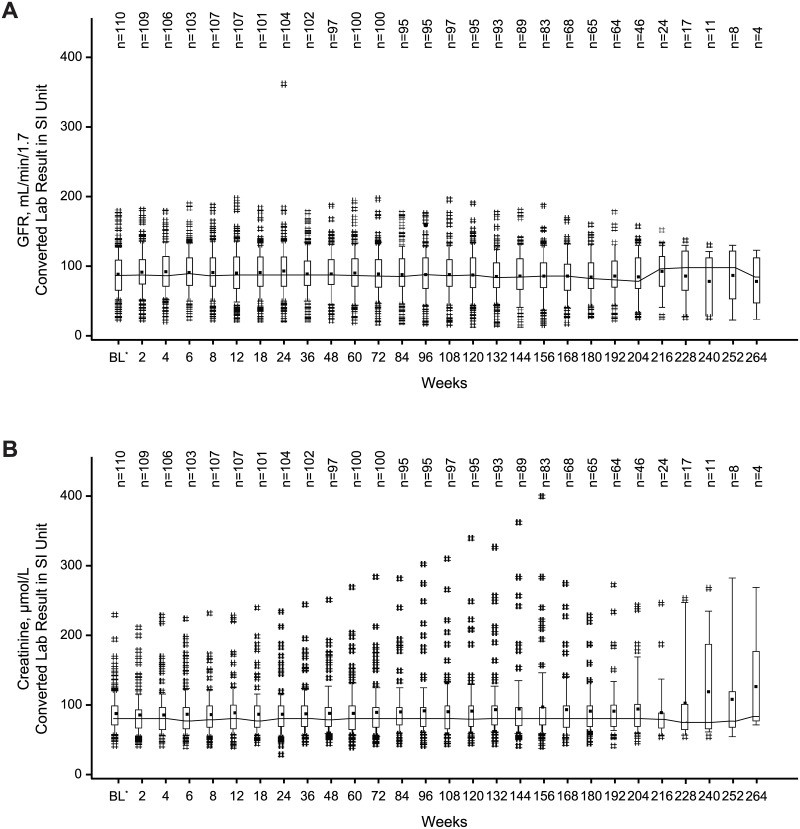
Most patients had GFR ≥30 mL/min/1.73 m2 or normal serum creatinine values while receiving treatment with everolimus (92.9% and 83.9%, respectively). Median GFR and serum creatinine values remained stable during the everolimus treatment (Fig 6). Severe renal impairment (postbaseline GFR <30 mL/min/1.73 m2) was observed in eight (7.1%) patients. All eight patients had GFR <60 mL/min/1.73 m2 at baseline, including three patients with GFR <30 mL/min/1.73 m2 before everolimus initiation. In addition, 8 of 23 patients with GFR <60 mL/min/1.73 m2 at baseline attained a GFR below 30 mL/min/1.73 m2 while on everolimus, whereas in 15 patients GFR remained stable or increased. Grade 1 or 2 elevations in serum creatinine were observed in 15.2% of patients, and one patient (0.9%) had a grade 3 elevation in serum creatinine. Among the 18 patients with grade ≥1 elevations in serum creatinine, half reported normal creatinine level at baseline and half reported grade 1 or 2 serum creatinine increase before starting everolimus. Renal events occurred in 5% (4/79) of everolimus- and 15% (6/39) of placebo-treated patients during the double-blind period (median treatment duration, 8.8 and 7.8 months, respectively) [16,17] and in 20.5% of the 112 everolimus-treated patients for this final analysis (treatment duration, 46.9 months). Significant worsening of existing proteinuria (continuous urine dipstick ≥2 stages higher than baseline) occurred in two patients.
Fig 6. Median GFR (A) and creatinine (B) over time.
Medians are connected by lines, means are displayed as dots. Boxes are drawn from P25 to P75. Whiskers extend from P10 to P90. # indicates values that lie outside [P10, P90]. *Baseline assessment is the last performed before start of everolimus. Post-baseline laboratory assessments performed at unplanned schedule or more than 28 days after discontinuation of everolimus are not presented. Only results from central laboratory are included. Abbreviations: BL = baseline; GFR = glomerular filtration rate; P = percentile.
Angiomyolipoma-related clinical events
Among the 112 patients who received everolimus, two patients underwent angiomyolipoma-related interventions. One patient receiving everolimus for 1.5 years underwent embolization after pain worsened in the right flank (progressive disease). Another patient underwent elective left partial nephrectomy following everolimus discontinuation (after 3 years of exposure) in an apparent attempt to use everolimus to reduce tumor burden prior to surgery.
Discussion
This analysis from the conclusion of treatment in EXIST-2 supports the long-term efficacy and safety of everolimus in patients with renal angiomyolipoma associated with TSC or sLAM. Renal angiomyolipoma response continued to improve from 41.8% in the core phase (median exposure, 8.8 months) to 58% in the extension phase (median exposure, 46.9 months) [16]. Clinically relevant renal angiomyolipoma reductions persisted over time as shown by the longitudinal data analysis which demonstrated an important reduction in the first 3 months of treatment and a continuous but less marked reduction later on. Low rates of renal angiomyolipoma progression were observed.
Over the 4-year duration of everolimus treatment, angiomyolipoma-related complications or procedural interventions were uncommon, and no treated patient experienced an angiomyolipoma-associated hemorrhage, whereas one-third of the patients experienced major renal interventions before entering the trial.
Everolimus was also associated with sustained reductions in SEGA volume and improvement in skin lesions. In the subset of patients with TSC-associated LAM, a lower than expected rate of decline in pulmonary function was reported. A 12-month, placebo-controlled, double-blind study in patients with LAM reported an annual decline in FEV1 of 10% in patients taking placebo (n = 43) [22], whereas the current study reported a decline of 5.9% after nearly 2 years of everolimus treatment. Together, the evidence supports a multisystem clinical impact of everolimus on various manifestations of TSC.
The longer-term safety profile of everolimus was consistent with that previously reported in TSC-associated clinical settings with everolimus [23–26]. AEs occurred in most patients but were managed by dose adjustment in the majority. After the placebo arm was discontinued, it was difficult to distinguish between true everolimus side effects and AEs due to TSC (eg, infections were just as common in the placebo as in the everolimus group in the double-blind phase [16]). Some AEs, however, were predictable or known effects of mTOR inhibitors, often attributed to downregulation of cellular turnover (eg, amenorrhea, mouth ulcers). Nearly three-quarters of the patients completed this long-term study as per protocol. Stomatitis, an event known to be associated with everolimus, was the most frequent AE. While infections were common and mostly mild to moderate in severity during the study, it should be noted that severe life-threatening infections have been reported in patients treated with everolimus for TSC [27]. The frequency of emergent AEs of all types decreased over time. Renal function remained stable in most patients, and no angiomyolipoma-related bleeding events were reported. Immediate surgical intervention with embolization was performed in one patient (during the study period), and nephrectomy was performed in another (after treatment discontinuation). The observation of a single embolization while on everolimus over a median exposure of approximately 4 years indicates that treatment was successful and suggests a need for longer-term mTOR inhibition to continue to prevent or slow tumor regrowth, preserve renal function, and reduce the need for future embolization. This reduction in hemorrhage frequency could be due to restoration of a more appropriate mTORC1 axis in the TSC mutant vascular pericytes, thus preventing vascular remodeling and aneurysm formation [28]. Better angiographic data are required to assess the vascular effects on therapy [10]. Patients included in this study were at high risk for long-term renal deterioration because most of them had large, bilateral angiomyolipomas at baseline. Sustained clinical responses to everolimus in angiomyolipoma and other lesional manifestations of TSC—such as skin lesions, SEGA, and LAM—support everolimus as a viable treatment option for TSC and its potential to be a disease-modifying therapy in these patients.
Given the magnitude of effect with everolimus in the primary analysis, maintenance of an untreated arm was unethical, thus justifying the omission of the placebo arm in the extension phase. A planned noninterventional follow-up of EXIST-2 will provide longer-term data to determine angiomyolipoma characterizations for up to 1 year after the discontinuation of everolimus. Results of EXIST-2 demonstrate that everolimus is a safe and effective longer-term therapy for patients with renal angiomyolipoma associated with TSC or sLAM not requiring immediate surgical intervention. Early treatment with everolimus may prevent progressive damage to renal tissue, reduce renal-related complications and the need for renal intervention, and improve patient outcomes.
Supporting information
(DOCX)
(PDF)
(DOC)
CT = computed tomography; EIAED = enzyme-inducing antiepileptic drug; MRI = magnetic resonance imaging; sLAM = sporadic lymphangioleiomyomatosis; TSC = tuberous sclerosis complex. aEnrollment occurred between April 28, 2009, and December 30, 2010. bDose adjusted based on toxicity. cAngiomyolipoma progression by central review or occurrence of adverse event of angiomyolipoma-related bleeding grade 2 or worse.
(EPS)
Acknowledgments
The authors thank the EXIST-2 patients, study investigators, and study site personnel for their participation in this study. This study was supported by Novartis Pharmaceuticals Corporation. We thank Rama Mylapuram and Manojkumar Patel, Novartis Healthcare Pvt. Ltd. for providing editorial assistance with this manuscript. Medical editorial assistance was also provided by Traci Stuve and Robert Schoen of ApotheCom (Yardley, PA), funded by Novartis Pharmaceuticals Corporation.
Data Availability
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.
Funding Statement
The study sponsor Novartis Pharmaceuticals Corporation contributed to the study design, data analysis, and the decision to publish. Novartis authors reviewed the draft for submission. Third party support funded by Novartis was involved in the preparation of the manuscript under the direction of the authors.
References
- 1.Osborne JP, Fryer A, Webb D: Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991; 615: 125–127. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P, Bombardieri R, Jozwiak S: Tuberous sclerosis. Lancet. 2008; 372: 657–668. doi: 10.1016/S0140-6736(08)61279-9 [DOI] [PubMed] [Google Scholar]
- 3.Crino PB, Nathanson KL, Henske EP: The tuberous sclerosis complex. N Engl J Med. 2006; 355: 1345–1356. doi: 10.1056/NEJMra055323 [DOI] [PubMed] [Google Scholar]
- 4.Budde K, Gaedeke J: Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis. 2012; 59: 276–283. doi: 10.1053/j.ajkd.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 5.Cook JA, Oliver K, Mueller RF, Sampson J. A cross sectional study of renal involvement in tuberous sclerosis. J Med Genet. 1996; 33: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewalt DH, Sheffield E, Sparagana SP, Delgado MR, Roach ES. Renal lesion growth in children with tuberous sclerosis complex. J Urol. 1998; 160: 141–145. [PubMed] [Google Scholar]
- 7.O'Callaghan FJ, Noakes MJ, Martyn CN, Osborne JP. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int. 2004; 94: 853–857. doi: 10.1111/j.1464-410X.2004.05046.x [DOI] [PubMed] [Google Scholar]
- 8.Rakowski SK, Winterkorn EB, Paul E, Steele DJ, Halpern EF, Thiele EA. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int. 2006; 70: 1777–1782. doi: 10.1038/sj.ki.5001853 [DOI] [PubMed] [Google Scholar]
- 9.Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002; 168: 1315–1325 [DOI] [PubMed] [Google Scholar]
- 10.Rabenou RA, Charles HW. Differentiation of sporadic versus tuberous sclerosis complex-associated angiomyolipoma. AJR Am J Roentgenol. 2015; 205: 292–301. doi: 10.2214/AJR.14.14255 [DOI] [PubMed] [Google Scholar]
- 11.Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002; 225: 78–82. doi: 10.1148/radiol.2251011477 [DOI] [PubMed] [Google Scholar]
- 12.Eijkemans MJ, van der Wal W, Reijnders LJ, Roes KC, van Waalwijk van Doorn-Khosrovani SB, Pelletier C, et al. Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity, and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am J Kidney Dis 2015; 66: 638–645. doi: 10.1053/j.ajkd.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 13.Sooriakumaran P, Gibbs P, Coughlin G, Attard V, Elmslie F, Kingswood C, et al. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010; 105: 101–106. doi: 10.1111/j.1464-410X.2009.08649.x [DOI] [PubMed] [Google Scholar]
- 14.Curatolo P, Moavero R. mTOR inhibitors in tuberous sclerosis complex. Curr Neuropharmacol. 2012; 10: 404–415. doi: 10.2174/157015912804143595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013; 49: 255–265. doi: 10.1016/j.pediatrneurol.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013; 381: 817–824. doi: 10.1016/S0140-6736(12)61767-X [DOI] [PubMed] [Google Scholar]
- 17.Afinitor [package insert]. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2016.
- 18.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant. 2016; 31: 111–119. doi: 10.1093/ndt/gfv249 [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Cancer therapy evaluation program: common terminology criteria for adverse events, version 3.0 (CTCAE). National Cancer Institute, Bethesda, MD: 2006. [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 21.Brookmeyer R, Crowley J. A Confidence Interval for the Median Survival Time. Biometrics. 1982; 38: 29–41. [Google Scholar]
- 22.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011; 364: 1595–1606. doi: 10.1056/NEJMoa1100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013; 381: 125–132. doi: 10.1016/S0140-6736(12)61134-9 [DOI] [PubMed] [Google Scholar]
- 24.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010; 363: 1801–1811. doi: 10.1056/NEJMoa1001671 [DOI] [PubMed] [Google Scholar]
- 25.Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant-cell astrocytoma. Neurology. 2013; 80: 574–580. doi: 10.1212/WNL.0b013e3182815428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franz DN, Agricola K, Mays M, Tudor C, Care MM, Holland-Bouley K, et al. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015; 78: 929–938. doi: 10.1002/ana.24523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trelinska J, Dachowska I, Kotulska K, Fendler W, Jozwiak S, Mlynarski W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. 2015; 26: 437–442. doi: 10.1097/CAD.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 28.Siroky BJ, Yin H, Dixon BP, Reichert RJ, Hellmann AR, Ramkumar T, et al. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol. 2014; 307: F560–F570. doi: 10.1152/ajprenal.00569.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(DOC)
CT = computed tomography; EIAED = enzyme-inducing antiepileptic drug; MRI = magnetic resonance imaging; sLAM = sporadic lymphangioleiomyomatosis; TSC = tuberous sclerosis complex. aEnrollment occurred between April 28, 2009, and December 30, 2010. bDose adjusted based on toxicity. cAngiomyolipoma progression by central review or occurrence of adverse event of angiomyolipoma-related bleeding grade 2 or worse.
(EPS)
Data Availability Statement
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.