Abstract
BACKGROUND
Disorders of bone metabolism, most notably osteoporosis, are highly prevalent and predispose to fractures, causing high patient morbidity and mortality. Diagnosis and monitoring of bone metabolic defects can present a major challenge as these disorders are largely asymptomatic and radiographic measures of bone mass respond slowly to changes in bone physiology.
CONTENT
Bone turnover markers (BTMs) are a series of protein or protein derivative biomarkers released during bone remodeling by osteoblasts or osteoclasts. BTMs can offer prognostic information on fracture risk that supplements radiographic measures of bone mass, but testing using BTMs has to take into account the large number of preanalytic factors and comorbid clinical conditions influencing BTM levels. BTMs respond rapidly to changes in bone physiology, therefore, they have utility in determining patient response to and compliance with therapies for osteoporosis.
SUMMARY
BTMs are a useful adjunct for the diagnosis and therapeutic monitoring of bone metabolic disorders, but their use has to be tempered by the known limitations in their clinical utility and preanalytic variables complicating interpretation.
Osteoporosis is the most common disorder of bone metabolism, with approximately 1 in 2 women and 1 in 5 men expected to experience an osteoporotic fracture during their lifetime. Osteoporotic fractures incur a high degree of morbidity and mortality, with a woman’s lifetime risk of dying from a hip fracture roughly equivalent to her risk of dying from breast cancer. Owing to these clinical consequences, diagnosis, treatment, and monitoring of treatment for osteoporosis are of critical importance. A major challenge in this regard is that osteoporosis is asymptomatic until presenting with a fracture; thus clinical diagnosis and subsequent treatment rely on radiologic and laboratory testing in patients at risk based on clinical history and demographics.
Ultimately, the pathogenesis of osteoporosis emerges from an imbalance in the ability of osteoclasts to resorb bone vs the ability of osteoblasts to form bone, with the etiology of this imbalance depending on the clinical risk factors for osteoporosis present in a given patient. These risk factors can include hormonal changes after menopause, advanced age, treatment with glucocorticoids, other endocrinopathies such as hyperthyroidism or hyperparathyroidism, or chronic systemic inflammation. While radiographic techniques remain the mainstay for the diagnosis of osteoporosis, radiographic measures of bone mass have been demonstrated to only account for a portion of the total fracture risk (1, 2 ). Additionally, radiographically detectable changes in bone mass can lag months to over a year behind specific insults or therapies influencing bone mass; thus there is a desire for readouts that respond more rapidly to changes in bone physiology (3 ).
As osteoporosis emerges directly from alterations in the number or activities of osteoblasts and osteoclasts, it follows that biomarkers of the activity of these cells reflect current levels of bone turnover. They may also provide additional information beyond radiographic assessments of total bone mass, as low total bone mass may occur with either high or low levels of bone turnover, and bone in low-turnover forms of osteoporosis may not have the same biophysical properties and fracture risk as the same amount of bone in a high-turnover form of osteoporosis. Notably, this reasoning applies equally to all other disorders of low bone mass or local bone erosion, including skeletal metastases of solid tumors or selected hematologic malignancies such as multiple myeloma. Thus bone turnover markers (BTMs)3 can also provide information on disease activity in oncologic and rheumatologic conditions affecting bone.
Introduction to Specific BTMs
BTMs can be categorized first as reflecting either bone resorption or formation, and then further categorized into matrix products that are liberated during bone resorption or formation or cellular products that are directly secreted into the circulation at levels commensurate with the number or activity of osteoclasts or osteoblasts (Fig. 1). Here, we introduce several of the most commonly used BTMs.
Fig. 1. Summary of commonly measured BTMs.
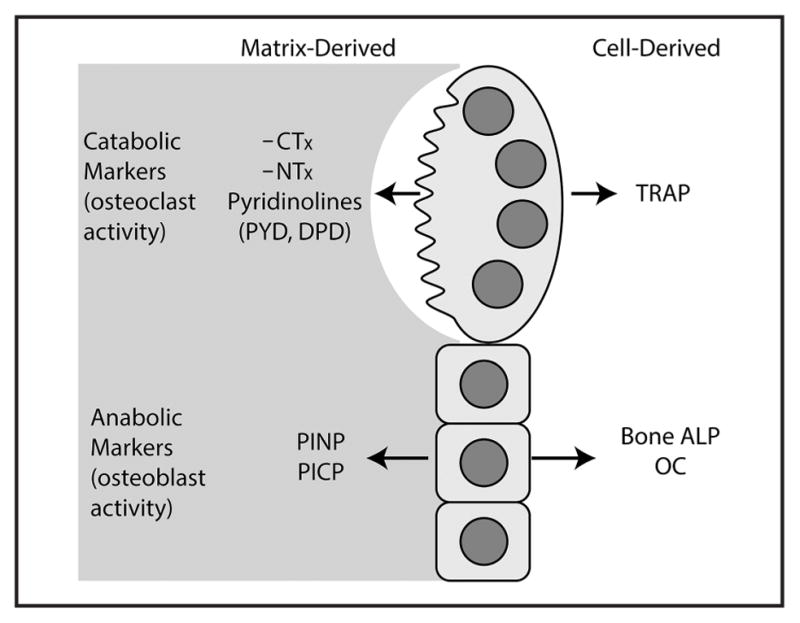
The commonly measured BTMs are listed according to whether they are released by the catabolic activity of osteoclasts or the anabolic activity of osteoblasts.
C- and N-Terminal Telopeptides of Type I Collagen
Type I collagen is the most abundant protein component of bone, and C- and N-terminal telopeptides of type I collagen (CTX and NTX) are both fragments of type I collagen from the telopeptide region, a nontriple-helical portion near the ends of mature collagen (Fig. 2). The telopeptides are cleaved during osteoclastic resorption of bone, resulting in their liberation into the circulation at a rate proportional to bone resorption activity. CTX is the specific product of cathepsin K-mediated bone resorption, as direct digestion of bone with cathepsin K but not alternative catabolic enzymes, such as matrix metalloproteinases, causes CTX release (4 ). In contrast, another BTM, the C-terminal cross-linked telopeptide of type I collagen (ICTP) is released by matrix metalloproteinase or trypsin digestion of bone. ICTP has been suggested to respond more to pathways of bone resorption activated by skeletal metastases of solid tumors than those activated in postmenopausal osteoporosis. However, clinical application of ICTP is limited by it being available only as a manual RIA, thus it will not be further considered here.
Fig. 2. Structure of type I procollagen.
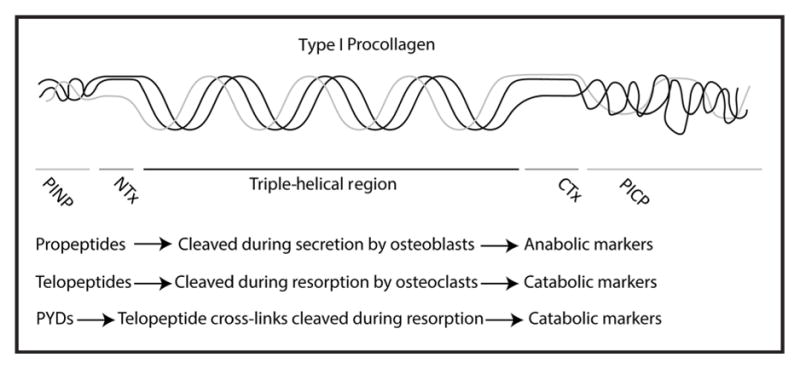
A cartoon of triple-helical type I procollagen is displayed indicating the regions that correspond to the nontriple-helical telopeptides and propeptides flanking the core triple-helical region.
The NTX assay recognizes a peptide from the N-terminal telopeptide of the α2-collagen chain, though α1-collagen peptides are included in the cross-linked complex. The 2 most widely used automated CTX assays recognize an octapeptide epitope of the α1-chain telopeptide, which contains a lysine residue that forms cross-links. Notably, the CTX octapeptide also contains an aspartyl that gradually undergoes isomerization and racemization, converting from the newly synthesized form (βCTX) to an isomerized form (βCTX) over time. Due to the presence of the crosslink-forming lysine in the CTX peptide, these are present both as monomers (βCTX and βCTX) and dimers (β- βCTX, β- βCTX, and β- βCTX). The relative abundance of βCTX to βCTX has been proposed to provide information on the duration of time between collagen deposition and resorption, though further validation is needed to determine the potential clinical utility of this ratio (5 ). CTX assays can display different degrees of selectivity for βCTX vs βCTX and the various species of CTX dimers, with most of the assays under the CrossLaps branding recognizing predominantly β- βCTX, and the Alpha CTX assays recognizing β- βCTX.
In general, CTX and NTX assays show broadly similar clinical utility and test characteristics, with head-to-head comparisons of CTX and NTX showing relatively modest differences in performance (6 ). Both CTX and NTX are small enough to be renally cleared, and accordingly, serum/plasma and urine are both suitable sample types. In clinical practice, CTX is most commonly analyzed in serum, whereas NTX is often run on urine. Though serum NTX assays are available at many reference laboratories, urine is the preferred sample as serum NTX shows less robust changes to antiresorptive treatment (7 ).
Pyridinoline and Deoxy-Pyridinoline
Type I collagen in bone displays an extensive network of covalent cross-links that contribute to the overall biomechanical properties of bone. Pyridinoline (PYD) and deoxy-pyridinoline (DPD) are specific cross-links formed within structural collagens between lysine or hydroxylysine residues in the telopeptide region with specific sites within the collagen triple helix, with PYD having 2 forms, hydroxylysyl PYD formed from 3 hydroxylysine residues and lysyl PYD which is derived from 1 lysine and 2 hydroxylysine residues. Hydroxylysine is formed by the action of lysyl hydroxylase [procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 (PLOD1)],4 which is defective in type VI Ehlers–Danlos syndrome. Thus, the decreased amount of hydroxylysine seen in type VI Ehlers–Danlos syndrome results in a reduction in the ratio of hydroxylysyl PYD to lysyl PYD, and this can be used as supporting evidence for a diagnosis of type VI Ehlers–Danlos syndrome (8 ). Whereas PYD is found in several tissues including bone and cartilage, DPD is predominantly found in bone, and is therefore considered to be a more specific marker of bone turnover. PYD and DPD are liberated upon resorption of bone and subsequently cleared renally (9 ).
In the urine, approximately 30%–50% of PYDs are present as free species, and the rest are bound to peptides or larger protein complexes (10 ). The free species are hypothesized to be formed from metabolism of peptide-bound PYDs after release from bone (11 ). PYDs can be quantified using detection of their native fluorescence on HPLC or by ELISA, with the distinction between free and total depending on whether or not an acid hydrolysis is performed to allow analysis of the peptide-bound fraction (12 ).
N- and C-Terminal Propeptides of Type 1 Collagen
Osteoblasts secrete type 1 collagen as an intact molecule containing the N- and C-terminal propeptides, which are subsequently cleaved in the extracellular space. Thus, N-and C-terminal propeptides of type 1 collagen (PINP and PICP) levels are markers of type 1 collagen secretion by osteoblasts. Both PINP and PICP appear to have similar characteristics as analytes, but PINP has been more extensively studied and will, therefore, be the focus of consideration here. PINP is present initially as a trimer of the propeptides from the 3 protein chains in type 1 collagen and is subsequently converted to monomeric forms in circulation. Accordingly, assays may measure both monomeric and trimeric PINPs (total PINP) or just trimeric PINP (intact PINP). Trimeric PINP is cleared via hepatic uptake via scavenger receptors, whereas monomeric PINP is predominantly cleared renally. Accordingly, whereas total and intact PINP show good correlation in healthy controls, total PINP concentrations are greatly increased in patients on hemodialysis owing to impaired clearance of monomeric PINP (13 ).
Bone-Specific Alkaline Phosphatase
While total alkaline phosphatase (ALP) levels measured by enzymatic activity can show an association with bone remodeling activity, particularly in cases of extreme high turnover disorders, such as Paget disease of bone, the utility of total ALP in this context is blunted by total ALP activity representing the products of 4 ALP genes [alkaline phosphatase, intestinal (ALPI), alkaline phosphatase, liver/bone/kidney (ALPL), alkaline phosphatase, placental (ALPP), alkaline phosphatase, placental like 2 (ALPPL2)] encoding numerous enzyme isoforms, with each isoform responding to a different set of tissue-specific disease processes. Of these, only the bone-specific isoform of the ALPL gene reflects bone anabolic activity, as it is directly released into the circulation in a manner proportional with the number and differentiation state of osteoblasts (14 ). While historically there were a number of methods to determine isoform-specific ALP levels or activity, immunoassays that specifically recognize bone-specific ALP (bone ALP) are currently preferred for monitoring bone metabolic activity. Importantly, the current immunoassays available for bone ALP do not show perfect specificity and still display some degree of cross-reactivity toward liver ALP. Thus, increased bone ALP levels must be interpreted with caution in patients with liver disease.
Bone ALP expression is acquired early in the differentiation of osteoblasts from mesenchymal progenitors, and bone ALP itself plays a key role in degrading the natural inhibitor of mineralization pyrophosphate, as evidenced by the potentially severe rickets-like skeletal disease in patients with hypophosphatasia due to mutations in ALPL.
Osteocalcin
Osteocalcin (OC) is a 49-amino acid, calcium-binding peptide secreted by mature osteoblasts that is the most abundant noncollagen protein found in bone. OC has recently reemerged as a molecule of intense interest in basic and translational skeletal biology owing to the discovery that it functions as a bone-derived hormone influencing male fertility, glucose homeostasis, behavior due to direct effects in the central nervous system, and muscle function in mice (15–17 ). Most OC secreted by osteoblasts is incorporated into the organic matrix that will later ossify into bone, however, a small fraction is secreted into the circulation. For this reason, OC is widely considered a bone formation marker, and, indeed, OC concentrations correlate with direct measurements of bone formation by histomorphometry (18 ). However, the fraction of OC incorporated into the organic bone matrix can be liberated during osteoclastic bone resorption. Thus, bone resorption can potentially directly impact serum OC concentrations, though the practical significance of this effect is unclear.
OC is also known to contain 3 glutamic acids (amino acids 13, 17, and 20) that undergo vitamin K-dependent γ-carboxylation, which increases the affinity of OC for hydroxyapatite (19 ). Consistent with this vitamin K-dependent posttranslational processing, treatment with vitamin K antagonists, such as warfarin, increases the relative amount of uncarboxylated or under-carboxylated OC and can lower total serum OC concentrations (20 ). A fraction of OC subsequently undergoes decarboxylation, and only uncarboxylated OC is able to regulate glucose homeostasis (21 ). While there has been substantial interest in the clinical applications of selective measurement of uncarboxylated OC, this has yet to be implemented as a clinical assay outside of a research setting.
Notably, samples for OC measurement have special collection and transportation requirements, due to the instability of OC. It is recommended that samples be kept near 4 °C and processed within 4 h of collection. Sample hemolysis is consistently observed to reduce OC levels, likely by enhancing OC degradation. As this instability of OC is largely a property of the labile 6-amino acid C-terminal sequence, an assay of a more stable fragment lacking this sequence, the N-Mid-OC fragment (amino acids 1–43), has found clinical utility alongside measurement of intact OC (22 ).
Preanalytic Factors in the Measurement of BTMs
Insofar as BTMs provide an integrated systemic measurement of bone turnover, preanalytic factors influencing bone turnover around the time of collection will impact their concentrations. These preanalytic factors can be divided into controllable factors, such as seasonal or circadian variation, and uncontrollable factors including demographic variables such as age and sex. Among the controllable factors, bone resorption displays significant circadian variation, and serum CTX, NTX, and OC concentrations peak in early morning between midnight and 8 AM with a nadir in the afternoon (23 ). This circadian effect can be generally observed with most BTMs, though CTX displays the highest amplitude in circadian variation (24 ). Additionally, seasonal variation is observed in bone turnover, with a peak in bone remodeling occurring during winter months, though the degree of coupling varies with premenopausal women showing the greatest seasonal variation (25 ). Bone resorption levels drop post-prandially, which is a significant contributor to the rise in CTX occurring during the early morning, which is typically the longest period of fasting each day. This post-prandial drop in bone remodeling has been shown to result from the effects of gastrointestinal hormones, such as glucagon-like peptide-2, to reduce bone resorption (26 ). Thus, measurement of serum CTX on a fasting morning draw is recommended to increase consistency of measurement. Bone formation markers appear to be less impacted by these factors (27 ). Exercise has been observed to cause acute changes in the concentrations of BTM markers, thus it is recommended that exercise be avoided for 48 h before sample draw (28 ). Current smoking habit and low BMI are also associated with higher basal BTM concentrations (29 ). Bone turnover also varies with the menstrual cycle, rising during the mid to late follicular phase and falling during the midluteal phase (30 ). Thus, in premenopausal women, ideal practice is to sample during the follicular phase of the menstrual cycle to obtain a consistent baseline in BTM concentrations.
In addition to these factors influencing bone turnover, patient factors influencing BTM clearance will also influence measurement. Most notably, CTX, NTX, monomeric PINP and OC display renal clearance and will generally be increased in renal insufficiency (31 ). Tartrate-resistant acid phosphatase (TRAP), an enzyme secreted from osteoclasts that correlates with resorption activity, is one of the few catabolic BTMs that is not renally cleared, thus it may have utility in the setting of renal failure. However, this utility of TRAP assays is limited by a generally inferior ability to predict risk of fracture in comparison to CTX or NTX (32 ). Assays that specifically measure the osteoclast-derived TRAP isoform, TRAP5b, as opposed to all serum TRAP activity may improve these limitations (33 ). For anabolic BTMs, bone ALP is not renally cleared and may have utility in the setting of renal insufficiency (34 ). Most BTMs are present in type I collagen in nonskeletal tissues, therefore disease processes involving matrix remodeling in other tissues, such as systemic sclerosis, congestive heart failure, or dilated cardiomyopathy have been shown to increase BTM concentrations (35–37 ).
Demographic factors are an uncontrollable source of preanalytic variation in BTMs. Men in their 20s and 30s generally display higher basal concentrations of BTMs than women (38, 39 ). However, after age 50, this reverses, as women display a more rapid increase in baseline resorption levels with aging, most likely associated with the menopausal transition (40 ). As BTM concentrations reflect both the total area of bone surface available for remodeling and the relative remodeling activity at each of those sites, the greater relative skeletal mass in young men may account for their increased baseline concentrations of BTM. Greatly increased levels of both formation and resorption markers are seen in growing children, showing a correlation with the rate of change in height, peaking during puberty (41 ). This pubertal peak is more pronounced for CTX or NTX than DPD, where increased concentrations are observed without a discrete pubertal peak (42 ). BTMs tend to remain at relatively low basal levels during adulthood until an increase is observed in postmenopausal women. These demographic effects on BTM levels highlight the challenge in generating reference intervals across categories of age and sex, especially as many of the standard reference intervals for BTMs were established in young women. Populations where bone metabolism is less widely studied, including men and children have less available data to use in establishing reference intervals (43 ).
When determining bone mass by the most common radiographic technique, dual-energy x-ray absorptiometry (DXA), it is common to primarily compare patient values not to an age- and sex-matched reference interval (termed Z-scores), but instead to a sex-matched reference interval from healthy, young adults (T-scores). This is justified on the grounds that it is the absolute value of bone mass that determines fracture risk, not whether the patient’s bone mass is within reference limits for age. Similar considerations may also extend to BTM markers, as it has been proposed that reference intervals in women be drawn from the relative nadir in BTMs during the ages of 35–45 (29 ).
Applications of BTMs
The majority of osteoporotic fractures occur not in individuals with osteoporosis but rather in individuals with osteopenic bone mineral density (BMD). Though osteopenic patients (BMD T-score −1 to −2.5 by DXA scanning) are at lower individual risk for fracture than osteoporotic patients (T-score <–2.5), the larger total number of osteopenic patients means that most fractures will occur in this subset at the population level (44 ). Thus, as most fractures occur in a large group of patients with relatively low fracture incidence, additional tools to predict fracture risk are desperately needed to identify the individuals who would optimally benefit from osteoporosis pharmacotherapy. One approach to this problem is to apply advanced radiographic techniques such as the DXA-derived trabecular bone score and high-resolution peripheral quantitative computed tomography to provide additional risk stratification data (45, 46 ). Measurements of BTMs have been studied as another potential approach to this problem. Here, we will summarize the utility of BTMs in the clinical management of osteoporosis, focusing primarily on postmenopausal osteoporosis.
Use of BTMs to Predict Bone Loss
As total bone mass reflects the balance of activity between osteoclasts and osteoblasts, there is intense interest in measuring the activities of these cell lineages in vivo. Bone resorption increases rapidly with the menopausal transition (47 ). Since bone resorption and formation are typically coupled, increased indices of osteoblast activity are usually noted along with increased bone resorption by osteoclasts. Several studies have investigated the relationship between BTMs, menopausal status, and subsequent bone loss. Overall, while a clear relationship has been documented between perimenopausal BTM levels and subsequent bone loss, the association between BTM levels and subsequent bone loss in elderly women is less obvious (48 ). Therefore, the positive predictive value of altered BTM levels for accelerated bone loss in elderly white women is modest (2 ).
Currently, most women are not systematically screened for osteoporosis at the time of the menopausal transition. Therefore, incorporating routine monitoring of BTMs into clinical practice to identify the “rapid losers,” who might go on to develop osteoporosis many years later, presents substantial challenges. In the absence of prospective randomized clinical trials designed to assess the efficacy and cost-effectiveness of such a screening program, use of BTMs is not currently recommended as a public health measure to identify patients at increased risk of rapid bone loss (49 ).
One approach to increase the utility of BTMs in predicting bone loss is based on the observation that, ultimately, bone mass reflects the amount bone formed minus the amount of bone resorbed. Thus, ideally, both bone formation and bone resorption BTMs would be combined into a single integrated measure reflecting the net bone loss, though equating BTM measurements with changes in the volume of bone tissue is a challenge. The bone balance index is a creative solution to this problem, and is based using regression to determine the relative amounts of OC vs urine NTX seen in a patient cohort with stable bone mass (50 ). Then, patients are assessed relative to this regression standard to determine if their amount of NTX relative to OC is above or below the amount expected to correspond to stable bone mass. Initial validation suggests that the bone balance index may be a useful method to predict bone loss, however, further study is needed to see how widely applicable this approach proves.
Use of BTMs to Predict Fracture Risk
Given that BMD only accounts for a portion of fracture risk, many studies have been performed to determine the relationship between incident BTM concentrations and subsequent fracture risk. Notably, distinction must be drawn between the ability of BTMs to predict bone loss, as discussed above, with their ability to predict fracture risk, as patients can have markedly differing fracture risks at a given overall level of bone mass due to variation in demographic, clinical, and bone microarchitectural factors. Overall, prospective studies analyzing the relationship between bone formation markers and subsequent fracture risk have failed to show clear utility for anabolic BTMs for this application (2 ). In contrast, multiple studies have demonstrated that increased markers of bone resorption are predictive of subsequent fragility fracture (51 ). Interestingly, increased resorptive markers are linked to increased fracture risk only for a period of up to 5 years after initial assay, as this association is not detectable in longer-term follow-up studies (52 ).
Notably, comorbid clinical conditions can alter the relationship between BTMs to predict fracture risk. As one of the best-studied examples, BMD measurements underestimate fracture risk in individuals with diabetes (53 ). Therefore, how to best apply BTMs to estimate fracture risk in diabetic patients represents an area of active investigation.
Despite these findings linking increased concentrations of resorptive BTMs and fracture risk, few data exist regarding the utility of such measurements in routine clinical practice. ROC analyses have failed to demonstrate that the combination of low BMD and increased BTMs detects more women at risk for fracture than low BMD alone (54 ).
In summary, while BTMs represent powerful research tools for epidemiologists studying fracture risk across populations, current evidence is insufficient to recommend their routine use to identify individual patients who would optimally benefit from osteoporosis pharmacotherapy. However, distinction should be made for patients with “secondary” bone loss such as hyperparathyroidism, hyperthyroidism, vitamin D deficiency, and paraproteinemia, as BTMs may have utility in these higher-risk patient subsets. Additionally, as the unmet clinical need of reducing morbidity and mortality from osteoporotic fractures is driving active investigation of how to best apply BTMs for the routine monitoring of fracture risk, new applications for BTMs in this domain may emerge in the future.
Use of BTMs in Monitoring Osteoporosis
In contrast with the limitations to the use of BTMs for identifying patients at risk of rapid bone loss, use of BTMs to guide osteoporosis therapy has clearer potential utility. The pattern of change in BTMs in response to treatment is well described, and these changes have been used to predict both increases in bone density and therapeutic efficacy in reducing fracture risk.
TREATMENT EFFECT
Antiresorptives directly inhibit bone resorption by osteoclasts and accordingly result in relatively rapid decreases in bone resorption markers. The degree of inhibition of bone resorption varies with each antiresorptive based on the dose and mechanism of action. This inhibition of bone resorption secondarily causes a decrease in bone formation markers, due to physiologic mechanisms linking osteoclast and osteoblast activity. As some of this coupling effect is mediated directly by osteoclasts, antiresorptive agents which inhibit osteoclast resorptive capacity while leaving osteoclasts present will have a lesser effect on bone formation than agents that reduce osteoclast numbers. For instance, inhibitors of cathepsin K, an investigational class of antiresorptives, decrease levels of CTX (55 ). However, as inhibitors of cathepsin K do not affect osteoclast numbers, existing osteoclasts are still able to stimulate osteoblast recruitment and differentiation. Thus the decrease in bone formation markers with cathepsin K inhibitors is less than the decrease observed with bisphosphonates and denosumab, agents that can block osteoclast differentiation or kill active osteoclasts (4, 56 ).
This linkage between osteoclasts and osteoblasts can also function in the opposite direction, with agents targeting osteoblasts influencing bone resorption by osteoclasts. Recombinant human parathyroid hormone (PTH) 1–34 (teriparatide) is typically characterized as an anabolic agent, but results in increases in both bone formation and resorption markers (55, 57 ). Anti-sclerostin monoclonal antibody, an anabolic agent under investigation, also results in a dose-dependent increase in bone formation markers but, unlike teriparatide, a transient decrease in serum CTX (58 ).
Combination therapy with antiresorptive and anabolic agents has been studied in postmenopausal women. In postmenopausal women treated with denosumab and teriparatide, bone formation markers decreased with the combination of denosumab and teriparatide but less so than with denosumab alone (59, 60 ). CTX decreased similarly between denosumab monotherapy and combination therapy at month 12 and remained suppressed at month 24. This sustained inhibition of bone resorption contrasts with prior bisphosphonate-based combination trials. Combined zoledronic acid and teriparatide suppressed CTX levels only transiently, and combined alendronate and teriparatide or PTH suppressed bone resorption less than alendronate alone (55, 61, 62 ). Although direct comparisons cannot be made between trials owing to differences in trial design, this differential effect on bone resorption may explain the larger increases in BMD observed with denosumab and teriparatide combination vs bisphosphonate-based combinations.
CLINICAL OUTCOMES
Both baseline concentrations of BTMs and the responses of BTMs early after the initiation of therapy for osteoporosis predict BMD changes. Baseline concentrations of PINP correlate positively with teriparatide-induced changes in spine and hip BMD at months 18 and 24 (63, 64 ). Early increases at months 1 and 3 in PINP were predictors of 1–2 year increases in spine BMD with teriparatide (63, 65 ). Similarly, early decreases in BTMs with bisphosphonates and denosumab correlated with long-term (2–3 years) increases in BMD (66, 67 ).
Furthermore, early changes in BTMs are associated with fracture risk reduction with some antiresorptives (67–70 ). For example, in a posthoc analysis of the Fracture Intervention Trial (FIT), greater decreases of serum PINP, bone ALP, and CTX with alendronate treatment were associated with a greater reduction in spine and hip fractures (68 ). Similarly, decreases in urine CTX and urine NTX with risedronate were associated with greater reduction in spine fractures (66, 67 ). In the Multiple Outcomes of Raloxifene (MORE) trial, changes in OC predicted spine fracture risk reduction better than changes in BMD (70, 71 ). Similar relationships were observed with PINP and zoledronic acid in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly–Pivotal Fracture Trial (HORIZON-PFT) study (72 ).
Lastly, monitoring BTMs after discontinuation of therapy would be clinically valuable if levels or changes in BTMs were established to correlate with fracture risk. However, in the Fracture Intervention Trial Long-Term Extension (FLEX) trial, levels of CTX, PINP, and bone ALP at the start of the extension did not predict bone loss over a treatment-free 5-year interval in participants who had received a mean of 5 years of alendronate during FIT (73 ). Furthermore, 1-year changes in bone ALP and urine NTX after treatment discontinuation did not predict fracture rates (74 ). Additionally, in the HORIZON extension, PINP concentrations at the entry of the extension were not a predictor of morphometric vertebral or nonvertebral fractures in participants who had received 3 years of zoledronic acid followed by 3 years of placebo (61 ). Based on these findings, monitoring BTMs after treatment is not standard practice, although this remains an area of considerable interest.
In summary, use of BTMs with osteoporosis therapy remains limited to research use and is yet to be standardized for clinical use. However, the ability to detect early changes in bone with BTMs vs with bone density scans remains extremely desirable. Clinical use of BTMs may be limited by the analytic variability of assays as previously discussed, and there is great potential to impact osteoporosis care with future improvement in BTM assays.
Use of BTMs in Renal Disease
Patients with chronic kidney disease may develop osteoporosis and/or renal bone disease. Excluding adynamic bone disease, osteomalacia, and hyperparathyroid renal bone disease is critical to make appropriate treatment decisions. Bone histomorphometry remains the gold standard to diagnose renal osteodystrophy and chronic kidney disease–mineral bone disorder but is not commonly performed owing to limited clinical availability (75 ). As already reviewed, TRAP and bone ALP are not renally cleared and may have utility in the setting of renal insufficiency. In a comprehensive review of a large sample size of individuals with bone histomorphometry, extreme high and low values of PTH correlated with bone formation rate. Additionally, the authors suggested that using bone ALP in conjunction with PTH may be helpful as low levels of bone ALP are associated with adynamic bone disease (76 ). While bone histomorphometry remains the gold standard for diagnosing bone disease in the setting of chronic kidney disease, extreme values of bone ALP may serve as a useful proxy measure.
Role of BTMs in Oncology
A number of solid tumors, most notably breast and prostate carcinoma, characteristically metastasize to bone, and primary involvement of bone are also characteristic of several hematopoietic malignancies, particular multiple myeloma. In nearly all cases, this bone involvement is characterized by alterations in bone metabolism, and these disruptions in bone metabolism commonly predispose to pathologic fractures and may contribute to sustaining the growth of these skeletal metastases. Measurement of BTMs can provide prognostic information in these settings. In patients with castration-resistant prostate cancer, lung cancer, or other solid tumors, increased levels of NTX predicted a number of negative outcomes, including skeletal-related events, disease progression and death (77 ). A detailed consideration of this topic has been the subject of recent reviews (78 ).
Early seeding of a bone metastasis leads to increased bone turnover, thus BTMs have been investigated as a potential noninvasive method to screen for subclinical bone metastases. However, a major challenge to this approach comes from the many preanalytic sources of variability in BTM concentrations combined with other endocrine and chemotherapeutic therapies having a confounding effect on bone turnover. For instance, in a cohort of 94 patients with a mixed group of solid tumors, screening of a number of BTMs showed significant increases in NTX and DPD in patients with skeletal metastases (79 ). However, the sensitivity of even the best performing BTM in this study, NTX, was too low to be of practical clinical utility. Thus, the low sensitivity of increased BTMs for detecting skeletal metastases, in part due to high preanalytic variability, likely precludes their general use as a stand-alone screening test for this indication. However, approaches to account for this variability, such as analyzing the serial change in BTM measurements over time, are currently being explored. For instance, changes in a patient’s CTX/bone ALP ratio have been proposed to predict the appearance of osteolytic lesions in multiple myeloma (80 ).
BTMs have also been investigated as prognostic markers in patients with known metastases. In patients with known bone metastases from solid tumors, increases in bone ALP or NTX predicted increased rates of skeletal-related events, such as fracture, disease progression or death (77 ). Furthermore, normalization of NTX levels after treatment was also correlated with a longer event-free and overall survival when examined across several studies of solid tumors, suggesting that BTMs may have utility in monitoring therapy in this setting (81, 82 ).
In rare cases, BTMs may themselves function as tumor markers directly secreted by primary bone tumors. Osteoid osteoma has been proposed to secrete OC (83 ). Bone ALP can be secreted from osteosarcoma, and high bone ALP has been observed in patients with osteosarcoma (84 ). However, bone ALP levels have been proposed to have greater diagnostic utility for detection of osteosarcoma in adult vs adolescent onset osteosarcoma due to the lower baseline variability of bone ALP levels in adults.
BTMs in Rheumatologic Disorders
Rheumatologic disorders such as rheumatoid arthritis can have several distinct reasons for increases in resorptive markers. First, disorders such as rheumatoid arthritis display local, particularly periarticular bone erosions. Additionally, the inflammatory milieu of the disorder promotes systemic osteopenia due to enhanced bone resorption and suppressed bone formation. This last factor is not specific to rheumatoid arthritis and is seen across a wide range of chronic inflammatory, autoimmune, or infectious disorders, especially inflammatory bowel disease.
Consistent with these factors, the presence of rheumatoid arthritis is associated with an increase in nearly all resorption markers and a suppression of bone formation markers (85 ). Moreover, resorptive markers, especially CTX and PYD are associated with disease activity, correlating with the risk of radiologic progression of bone erosion (86, 87 ). Consistent with this linkage between resorptive markers and disease activity, therapy with disease modifying biologics, such as anti-tumor necrosis factor antibodies, promotes a relative normalization of the levels of bone resorption and bone formation BTMs (88 ). Notably, this normalization of bone turnover may not necessarily be tied with a clinical response to therapy (89 ).
Additionally, other rheumatologic disorders display increases in resorptive BTMs. Polymyalgia rheumatica has been associated with increases in resorptive markers (90 ). Both DPD and CTX levels are increased in psoriatic arthritis, ankylosing spondylitis, and reactive arthritis and the degree of increase tends to correlate with inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein (91 ).
BTMs in Paget Disease of Bone
Paget disease of bone is the second most common bone metabolic disorder after osteoporosis and is characterized by extremely high bone turnover leading to expansion and deformation of affected bones. In about half of affected patients, this results in symptoms, most commonly due to osteoarthritis, nerve entrapment or fracture secondary to either expansion or fragility of the affected bones. Both Paget disease and the group of rare Paget-like skeletal disorders including expansile skeletal hyperphosphatasia, familial expansile osteolysis, juvenile Paget disease, and fibrous dysplasia are characterized by a substantial increase in nearly every BTM measured (92 ). Furthermore, BTM concentrations, particularly PINP concentrations, have been shown to correlate with bone scintigraphy measures of disease activity and to respond to treatment with antiresorptives; thus BTMs have both diagnostic and disease monitoring applications for Paget disease of bone (93 ).
Conclusions
BTMs have utility in the diagnosis and management of endocrine, oncologic, and rheumatologic disorders influencing bone. However, this breadth of potential applications for BTM monitoring highlights the many physiologic factors and disease conditions influencing BTM levels that can act as potential confounders for interpreting BTMs for a given indication. These limitations have motivated increasing efforts to counter their inherent variability with methods that account for and minimize preanalytic sources of variation. Similarly, efforts are underway to bolster the ability of BTMs to predict clinical risk by incorporation into indication-specific comprehensive risk models. Both of these approaches have the promise to increase the ability of BTMs to provide clinically actionable information.
Footnotes
Disclaimer: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Nonstandard abbreviations: BTM, bone turnover marker; CTX, C-terminal telopeptide of type I collagen; NTX, N-terminal telopeptide of type I collagen; ICTP, C-terminal cross-linked telopeptide of type I collagen; PYD, pyridinoline; DPD, deoxy-pyridinoline; PINP, N-terminal propeptide of type 1 collagen; PICP, C-terminal propeptide of type 1 collagen; ALP, alkaline phosphatase; bone ALP, bone-specific alkaline phosphatase; OC, osteocalcin; TRAP, tartrate-resistant acid phosphatase; DXA, dual-energy x-ray absorptiometry; BMD, bone mineral density; PTH, parathyroid hormone; FIT, Fracture Intervention Trial; HORIZON-PFT, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly–Pivotal Fracture Trial.
Human genes: PLOD1, procollagen-lysine,2-oxoglutarate 5-dioxygenase 1; ALPI, alkaline phosphatase, intestinal; ALPL, alkaline phosphatase, liver/bone/kidney; ALPP, alkaline phosphatase, placental; ALPPL2, alkaline phosphatase, placental like 2.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: M.B. Greenblatt, Career Award for Medical Scientists from the Burroughs Wellcome Fund, the Office of the Director of the NIH under award DP5OD021351, and a Junior Investigator Award from the Musculoskeletal Transplant Foundation; J.N. Tsai, NIAMS of the NIH under award K23AR068447; M.N. Wein, NIAMS of the NIH under award K08AR067285.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26:2762–9. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 2.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grand-jean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–8. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 3.Chesnut CH, McClung MR, Ensrud KE, Bell NH, Genant HK, Harris ST, et al. Alendronate treatment of the post-menopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med. 1995;99:144–52. doi: 10.1016/s0002-9343(99)80134-x. [DOI] [PubMed] [Google Scholar]
- 4.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–67. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino H, Takahashi M, Kushida K, Ohishi T, Inoue T. The relationships between the degree of beta-isomerization of type I collagen degradation products in the urine and aging, menopause and osteoporosis with fractures. Osteoporos Int. 1999;9:405–9. doi: 10.1007/s001980050164. [DOI] [PubMed] [Google Scholar]
- 6.Fall PM, Kennedy D, Smith JA, Seibel MJ, Raisz LG. Comparison of serum and urine assays for biochemical markers of bone resorption in postmenopausal women with and without hormone replacement therapy and in men. Osteoporos Int. 2000;11:481–5. doi: 10.1007/s001980070089. [DOI] [PubMed] [Google Scholar]
- 7.Eastell R, Mallinak N, Weiss S, Ettinger M, Pettinger M, Cain D, et al. Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res. 2000;15:594–8. doi: 10.1359/jbmr.2000.15.3.594. [DOI] [PubMed] [Google Scholar]
- 8.Steinmann B, Eyre D, Shao P. Urinary pyridinoline cross-links in Ehlers-Danlos syndrome type VI. Am J Hum Genet. 1995;57:1505–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Apone S, Lee MY, Eyre DR. Osteoclasts generate cross-linked collagen N-telopeptides (NTX) but not free pyridinolines when cultured on human bone. Bone. 1997;21:129–36. doi: 10.1016/s8756-3282(97)00105-1. [DOI] [PubMed] [Google Scholar]
- 10.Kamel S, Brazier M, Neri V, Picard C, Samson L, Desmet G, et al. Multiple molecular forms of pyridinolines cross-links excreted in human urine evaluated by chromatographic and immunoassay methods. J Bone Miner Res. 1995;10:1385–92. doi: 10.1002/jbmr.5650100916. [DOI] [PubMed] [Google Scholar]
- 11.Randall AG, Kent GN, Garcia-Webb P, Bhagat CI, Pearce DJ, Gutteridge DH, et al. Comparison of biochemical markers of bone turnover in Paget disease treated with pamidronate and a proposed model for the relationships between measurements of the different forms of pyridinoline cross-links. J Bone Miner Res. 1996;11:1176–84. doi: 10.1002/jbmr.5650110817. [DOI] [PubMed] [Google Scholar]
- 12.Kamel S, Brazier M, Desmet G, Picard C, Mennecier I, Sebert J. High-performance liquid chromatographic determination of 3-hydroxypyridinium derivatives as new markers of bone resorption. J Chromatogr. 1992;574:255–60. doi: 10.1016/0378-4347(92)80037-q. [DOI] [PubMed] [Google Scholar]
- 13.Koivula M-K, Ruotsalainen V, Björkman M, Nurmenniemi S, Ikäheimo R, Savolainen K, et al. Difference between total and intact assays for N-terminal propeptide of type I procollagen reflects degradation of pN-collagen rather than denaturation of intact propeptide. Ann Clin Biochem. 2010;47:67–71. doi: 10.1258/acb.2009.009110. [DOI] [PubMed] [Google Scholar]
- 14.Kress BC, Mizrahi IA, Armour KW, Marcus R, Emkey RD, Santora AC. Use of bone alkaline phosphatase to monitor alendronate therapy in individual postmenopausal osteoporotic women. Clin Chem. 1999;45:1009–17. [PubMed] [Google Scholar]
- 15.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–41. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmas PD, Demiaux B, Malaval L, Chapuy MC, Edouard C, Meunier PJ. Serum bone gamma carboxyglutamic acid-containing protein in primary hyperparathyroidism and in malignant hypercalcemia. Comparison with bone histomorphometry. J Clin Invest. 1986;77:985–91. doi: 10.1172/JCI112400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price PA, Lothringer JW, Baukol SA, Hari Reddi A. Developmental appearance of the vitamin K-dependent protein of bone during calcification. Analysis of mineralizing tissues in human, calf, and rat. J Biol Chem. 1981;256:3781–4. [PubMed] [Google Scholar]
- 20.Knapen MHJ, Hamulyak K, Vermeer C. The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion. Ann Intern Med. 1989;111:1001–5. doi: 10.7326/0003-4819-111-12-1001. [DOI] [PubMed] [Google Scholar]
- 21.Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun. 2010;397:691–6. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenquist C, Qvist P, Bjarnason N, Christiansen C. Measurement of a more stable region of osteocalcin in serum by ELISA with two monoclonal antibodies. Clin Chem. 1995;41:1439–45. [PubMed] [Google Scholar]
- 23.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTX): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 24.Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–704. doi: 10.1007/s00198-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 25.Thiering E, Brüske I, Kratzsch J, Hofbauer LC, Berdel D, von Berg A, et al. Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci Rep. 2015;5:18138. doi: 10.1038/srep18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriksen DB, Alexandersen P, Bjarnason NH, Vilsbøll T, Hartmann B, Henriksen EEG, et al. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18:2180–9. doi: 10.1359/jbmr.2003.18.12.2180. [DOI] [PubMed] [Google Scholar]
- 27.Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–90. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- 28.Gombos Császár G, Bajsz V, Sió E, Steinhausz Tóth V, Schmidt B, Szekeres L, et al. The direct effect of specific training and walking on bone metabolic markers in young adults with peak bone mass. Acta Physiol Hung. 2014;101:205–15. doi: 10.1556/APhysiol.101.2014.001. [DOI] [PubMed] [Google Scholar]
- 29.Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R. Establishing a reference range for bone turnover markers in young, healthy women. Bone. 2008;42:623–30. doi: 10.1016/j.bone.2007.12.218. [DOI] [PubMed] [Google Scholar]
- 30.Gorai I, Chaki O, Nakayama M, Minaguchi H. Urinary biochemical markers for bone resorption during the menstrual cycle. Calcif Tissue Int. 1995;57:100–4. doi: 10.1007/BF00298428. [DOI] [PubMed] [Google Scholar]
- 31.Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. 1999;14:792–801. doi: 10.1359/jbmr.1999.14.5.792. [DOI] [PubMed] [Google Scholar]
- 32.Igarashi Y, Lee MY, Matsuzaki S. Acid phosphatases as markers of bone metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:345–58. doi: 10.1016/s1570-0232(02)00431-2. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi M, Yoh K, Miura T, Ohasi T, Rai SK, Uchida K. Development of a kinetic assay for band 5b tartrate-resistant acid phosphatase activity in serum. Clin Chem. 2000;46:469–73. [PubMed] [Google Scholar]
- 34.Miller PD. Chronic kidney disease and osteoporosis: evaluation and management. Bonekey Rep. 2014;3:542. doi: 10.1038/bonekey.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allanore Y, Borderie D, Lemaréchal H, Cherruau B, Ekindjian OG, Kahan A. Correlation of serum collagen I carboxyterminal telopeptide concentrations with cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 2003;30:68–73. [PubMed] [Google Scholar]
- 36.Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, et al. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–8. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 37.Kunishige M, Kijima Y, Sakai T, Akutagawa O, Matsuo A, Nishibe A, et al. Transient enhancement of oxidant stress and collagen turnover in patients with acute worsening of congestive heart failure. Circ J. 2007;71:1893–7. doi: 10.1253/circj.71.1893. [DOI] [PubMed] [Google Scholar]
- 38.Tsai KS, Pan WH, Hsu SH, Cheng WC, Chen CK, Chieng PU, et al. Sexual differences in bone markers and bone mineral density of normal Chinese. Calcif Tissue Int. 1996;59:454–60. doi: 10.1007/BF00369210. [DOI] [PubMed] [Google Scholar]
- 39.Midtby M, Magnus JH, Joakimsen RM. The Tromsø Study: a population-based study on the variation in bone formation markers with age, gender, anthropometry and season in both men and women. Osteoporos Int. 2001;12:835–43. doi: 10.1007/s001980170034. [DOI] [PubMed] [Google Scholar]
- 40.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–49. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 41.van Coeverden SCCM, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol. 2002;57:107–16. doi: 10.1046/j.1365-2265.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 42.Rauch F, Georg M, Stabrey A, Neu C, Blum WF, Remer T, et al. Collagen markers deoxypyridinoline and hydroxylysine glycosides: pediatric reference data and use for growth prediction in growth hormone-deficient children. Clin Chem. 2002;48:315–22. [PubMed] [Google Scholar]
- 43.Herrmann M, Seibel MJ, Seibel M. The amino- and carboxyterminal cross-linked telopeptides of collagen type I, NTX-I and CTX-I: a comparative review. Clin Chim Acta. 2008;393:57–75. doi: 10.1016/j.cca.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–9. doi: 10.1359/JBMR.050609. [DOI] [PubMed] [Google Scholar]
- 45.Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi M-L, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, et al. High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol. 2014;10:304–13. doi: 10.1038/nrrheum.2014.23. [DOI] [PubMed] [Google Scholar]
- 47.Sowers MR, Zheng H, Greendale GA, Neer RM, Cauley JA, Ellis J, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98:2854–63. doi: 10.1210/jc.2012-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer DC, Sklarin PM, Stone KL, Black DM, Nevitt MC, Ensrud KE, et al. Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res. 1999;14:1404–10. doi: 10.1359/jbmr.1999.14.8.1404. [DOI] [PubMed] [Google Scholar]
- 49.Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess. 2014;18:1–180. doi: 10.3310/hta18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shieh A, Han W, Ishii S, Greendale GA, Crandall CJ, Karlamangla AS. Quantifying the balance between total bone formation and total bone resorption: an index of net none formation. J Clin Endocrinol Metab. 2016;101:2802–9. doi: 10.1210/jc.2015-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386–93. doi: 10.1359/JBMR.0301244. [DOI] [PubMed] [Google Scholar]
- 52.Robinson-Cohen C, Katz R, Hoofnagle AN, Cauley JA, Furberg CD, Robbins JA, et al. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96:2186–93. doi: 10.1210/jc.2010-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 54.Szulc P, Bauer DC. Biochemical markers of bone turnover in osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. 4. San Diego (CA): Academic Press; 2013. pp. 1573–610. [Google Scholar]
- 55.Finkelstein JS, Leder BZ, Burnett S-AM, Wyland JJ, Lee H, de la Paz AV, et al. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab. 2006;91:2882–7. doi: 10.1210/jc.2006-0190. [DOI] [PubMed] [Google Scholar]
- 56.Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S, et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res. 2011;26:1303–12. doi: 10.1002/jbmr.341. [DOI] [PubMed] [Google Scholar]
- 57.Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, et al. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45:1053–8. doi: 10.1016/j.bone.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 58.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in post-menopausal women with low bone mineral density. N Engl J Med. 2014;370:412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 59.Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50–6. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie S-AM, Zhu Y, Foley K, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA extension study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99:1694–700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 62.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 63.Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, González de la Vera J, et al. Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int. 2011;22:1935–46. doi: 10.1007/s00198-010-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–70. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 65.Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48:798–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:530–7. doi: 10.1002/jbmr.251. [DOI] [PubMed] [Google Scholar]
- 67.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–6. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 68.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–8. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 69.Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res. 2007;22:1656–60. doi: 10.1359/jbmr.07090b. [DOI] [PubMed] [Google Scholar]
- 70.Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in post-menopausal osteoporosis. Osteoporos Int. 2001;12:922–30. doi: 10.1007/s001980170020. [DOI] [PubMed] [Google Scholar]
- 71.Sarkar S, Reginster J-Y, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res. 2004;19:394–401. doi: 10.1359/JBMR.0301243. [DOI] [PubMed] [Google Scholar]
- 72.Jacques RM, Boonen S, Cosman F, Reid IR, Bauer DC, Black DM, et al. Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:1627–34. doi: 10.1002/jbmr.1644. [DOI] [PubMed] [Google Scholar]
- 73.McNabb BL, Vittinghoff E, Schwartz AV, Eastell R, Bauer DC, Ensrud K, et al. BMD changes and predictors of increased bone loss in postmenopausal women after a 5-year course of alendronate. J Bone Miner Res. 2013;28:1319–27. doi: 10.1002/jbmr.1864. [DOI] [PubMed] [Google Scholar]
- 74.Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 2014;174:1126–34. doi: 10.1001/jamainternmed.2014.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;76(Suppl 113):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 76.Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJA. PTH–a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol. 2013;8:299–312. doi: 10.2215/CJN.09580911. [DOI] [PubMed] [Google Scholar]
- 77.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 78.Coleman R, Costa L, Saad F, Cook R, Hadji P, Terpos E, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80:411–32. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Demers LM, Costa L, Chinchilli VM, Gaydos L, Curley E, Lipton A. Biochemical markers of bone turnover in patients with metastatic bone disease. Clin Chem. 1995;41:1489–94. [PubMed] [Google Scholar]
- 80.Lund T, Abildgaard N, Andersen TL, Delaisse J-M, Plesner T. Multiple myeloma: changes in serum C-terminal telopeptide of collagen type I and bone-specific alkaline phosphatase can be used in daily practice to detect imminent osteolysis. Eur J Haematol. 2010;84:412–20. doi: 10.1111/j.1600-0609.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 82.Hirsh V, Major PP, Lipton A, Cook RJ, Langer CJ, Smith MR, et al. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol. 2008;3:228–36. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 83.Confavreux CB, Borel O, Lee F, Vaz G, Guyard M, Fadat C, et al. Osteoid osteoma is an osteocalcinoma affecting glucose metabolism. Osteoporos Int. 2012;23:1645–50. doi: 10.1007/s00198-011-1684-0. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Pei F, Tu C, Zhang H, Qiu X. Serum bone turnover markers in patients with primary bone tumors. Oncology. 2007;72:338–42. doi: 10.1159/000113063. [DOI] [PubMed] [Google Scholar]
- 85.Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999;24:381–5. doi: 10.1016/s8756-3282(98)00193-8. [DOI] [PubMed] [Google Scholar]
- 86.Garnero P, Landewé R, Boers M, Verhoeven A, Van Der Linden S, Christgau S, et al. Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis Rheum. 2002;46:2847–56. doi: 10.1002/art.10616. [DOI] [PubMed] [Google Scholar]
- 87.Krabben A, Knevel R, Huizinga TWJ, Cavet G, van der Helmvan Mil AHM. Serum pyridinoline levels and prediction of severity of joint destruction in rheumatoid arthritis. J Rheumatol. 2013;40:1303–6. doi: 10.3899/jrheum.121392. [DOI] [PubMed] [Google Scholar]
- 88.Chopin F, Garnero P, le Henanff A, Debiais F, Daragon A, Roux C, et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:353–7. doi: 10.1136/ard.2007.076604. [DOI] [PubMed] [Google Scholar]
- 89.Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther. 2007;9:R61. doi: 10.1186/ar2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnes TC, Daroszewska A, Fraser WD, Bucknall RC. Bone turnover in untreated polymyalgia rheumatica. Rheumatology. 2004;43:486–90. doi: 10.1093/rheumatology/keh072. [DOI] [PubMed] [Google Scholar]
- 91.Jadon DR, Nightingale AL, McHugh NJ, Lindsay MA, Korendowych E, Sengupta R. Serum soluble bone turnover biomarkers in psoriatic arthritis and psoriatic spondyloarthropathy. J Rheumatol. 2015;42:21–30. doi: 10.3899/jrheum.140223. [DOI] [PubMed] [Google Scholar]
- 92.Whyte MP. Paget’s disease of bone and genetic disorders of RANKL/OPG/RANK/NF-kappaB signaling. Ann NY Acad Sci. 2006;1068:143–64. doi: 10.1196/annals.1346.016. [DOI] [PubMed] [Google Scholar]
- 93.Al Nofal AA, Altayar O, BenKhadra K, Qasim Agha OQ, Asi N, Nabhan M, et al. Bone turnover markers in Paget’s disease of the bone: a systematic review and meta-analysis. Osteoporos Int. 2015;26:1875–91. doi: 10.1007/s00198-015-3095-0. [DOI] [PubMed] [Google Scholar]


