Abstract
Following stroke, patients are commonly left with debilitating motor and speech impairments. This article reviews the state of the art in neurological repair for stroke and proposes a new model for the future. We suggest that stroke treatment—from the time of the ictus itself to living with the consequences—must be fundamentally neurological, from limiting the extent of injury at the outset, to repairing the consequent damage. Our model links brain and behaviour by targeting brain circuits, and we illustrate the model though action observation treatment, which aims to enhance brain network connectivity. The model is based on the assumptions that the mechanisms of neural repair inherently involve cellular and circuit plasticity, that brain plasticity is a synaptic phenomenon that is largely stimulus-dependent, and that brain repair required both physical and behavioural interventions that are tailored to reorganize specific brain circuits. We review current approaches to brain repair after stroke and present our new model, and discuss the biological foundations, rationales, and data to support our novel approach to upper-extremity and language rehabilitation. We believe that by enhancing plasticity at the level of brain network interactions, this neurological model for brain repair could ultimately lead to a cure for stroke.
Introduction
Following a large stroke that produces considerable impairments, patients can recover a degree of function, and many can walk, drive, communicate, and interact sufficiently for good quality of life.1,2 The vast majority of patients, however, can no longer work,3,4 and many cannot maintain independence.5,6 Furthermore, a substantial number of stroke survivors do not retain enough function to maintain social connectedness, leading to isolation and loneliness. Finally, depression and anxiety are common after stroke,7 which exacerbates an already difficult situation.
The ultimate goal of stroke treatment—after treatment of the initial insult to limit its extent and severity— should be the repair and reorganization of the injured brain to bring about a cure. Although such a statement might seem obvious, this perspective differs fundamentally from current poststroke therapeutic strategies. The emphasis of both motor therapy and speech and language therapy after stroke is on education, and therapists work intensively with patients to ameliorate motor or language impairments and to improve function. The most effective types of such ‘re-education’ involve focused instruction and practice that are based on theories about how therapeutic behaviours affect specific impairments. The more common approach, however, is to use ad hoc, individually tailored instruction and practice. Whereas researchers tend to favour the former approach, practitioners tend to use the latter.
Current practice in rehabilitation focuses on ways to circumvent deficits (compensation) rather than to cure them (remediation), as this strategy is the most efficient way to achieve good functional outcome.8,9 In compensatory recovery, behaviour is changed to meet environmental needs, and neurological restoration is bypassed. Existing tools for outcome assessment generally do not distinguish compensation from remediation9,10 and, as compensation is a more rapid and cost-effective approach, it tends to be the preferred option for insurers, therapists and—in many cases—patients. Moreover, medical reimbursement is geared towards the most rapid achievement of functional goals, assessed via the functional independence measure (FIM).11 Patients and families are typically given low expectations of rehabilitation, as such relearning can be quite meagre and can take tremendous effort over a long time.
In this context, a number of theoretical approaches to re-education have been proposed for treatment of both motor impairments and speech and language deficits. Some of these strategies have emphasized parallels between stroke recovery and learning,12–16 such as occurs in child development,17–20 whereas others have built complex multicomponent functional models to enable targeting of fractionated portions of impaired movements21–24 or linguistic skill.25 In addition, some models have aimed at development of compensatory behaviours that bypass known functional deficits.19,21,22,26,27
The above examples of theory-driven approaches to re-education emphasize how both the theory and the therapy address the repair process at a behavioural level. For active clinicians, practical common sense plays a more prominent part than theory, frequently leading therapists to work on rote learning of impaired skills and elimination of perceived obstacles, such as the effects of altered upper motor neuron influences on the arm or mouth. Despite the clear rationality of these educational endeavours, no strong medical evidence exists to support a given intervention over another for any specific patient or set of deficits. Granted, different types of behavioural interventions can lead to different short-term gains, but whether the type of therapy affects long-term outcomes remains unclear.28–30 Furthermore, treatment by a highly trained expert might not have advantages over treatment by a trained volunteer or by a robotic device.31 The result is essentially a stalemate in long-term stroke outcomes for over half a century.
In this article, we argue for a paradigm shift in poststroke therapy towards physiological repair of the underlying damage. We discuss findings from basic neuroscience, especially those concerning organization of the motor system, that can serve as a theoretical framework for treatment of poststroke aphasia and upper-limb motor dysfunction. We review studies that have tested this model in translational research to treat poststroke deficits as well as impairments in other motor syndromes. Promising biological interventions in development are beyond the scope of this article, and have been reviewed elsewhere.32–34 Two approaches in particular— constraint-induced therapy for upper-limb function35–37 and language,38,39 and therapy using mirrors40–42—have been motivated by biological rationales and lead to consistent changes in the brain.
A new model
In light of increasing understanding of the physiological underpinnings of poststroke deficits, and limited efficacy of current approaches to rehabilitation, the time is right to start on a completely different therapeutic track, to rethink basic assumptions of poststroke therapy, and to effect a paradigm shift43 in the way we view stroke and its consequences. Towards this goal, we advocate a biomedical model in which both motor therapy and speech therapy are understood in terms of physical repair (remediation) of the neural circuits that underlie the impaired functions. This perspective assumes that damage to the brain produces the impairment, and that repair or reorganization of the affected neural circuits can lead to a cure for the disease. Remediation and repair can be achieved via two basic routes: direct restoration, in which the original circuits are reinstated; and/or indirect restoration, in which related neural circuits are recruited to perform the original functions.44 For example, therapy for gait, hand motor function, speech and language, memory, attention and affect could be devised for direct or indirect rebuilding of damaged neural circuits that mediate those functions.
If the goal of motor or language therapy is to stimulate cerebral plasticity as a means of improving function, precise definition of ‘plasticity’ is essential to enable measurement of this parameter. In this Review, we use the notion developed by Donald Hebb,45 as articulated by Johansson,46 that neuronal cortical connections can be remodelled by experience,45,47 owing to changes in chemistry and anatomy48–50 Plasticity in adult animals is defined at the cellular and molecular level by increases in dendritic branching, the number of synapses per neuron, and expression of genes encoding trophic factors; and at the systems level by changes in cortical representation areas, and in cortical maps that develop in response to sensory input, experience and brain lesions.46
Rebuilding brain circuits to recover speech and motor functions does not depend solely on endogenous biological factors, but also on exogenous input, as brain plasticity is inherently stimulus-dependent.51,52 Developmentally, neural networks are shaped by intensive experience inherent in years of practice, and some evidence suggests that brain remodelling after injury might require similar levels of experience.53–57 For example, in all studies of pharmacological intervention for stroke rehabilitation, drug efficacy is dependent on accompanying behavioural practice.58 This finding is consistent with the well-established notion in synaptic physiology that plasticity depends on stimulus-driven patterns of neural activity59 Furthermore, when computational neural network models are experimentally ‘damaged’, functional restoration is not possible solely through replacement of lost ‘tissue’, but requires additional training.60,61 Moreover, the nature of the triggering stimulus probably affects the degree of neural circuit modification.
Despite the dependence of drug effectiveness on concomitant behavioural interventions, little work has been undertaken to address the interaction between brain physiology and clinical phenotype. The very few approaches from the pre-imaging era that were based on biological rationales have not been validated, or at least not completely, with modern biological tools. For example, the biological rationale of Melodic Intonation Therapy,62,63 the only aphasia therapy approved by the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology,64 was not supported by subsequent imaging studies.65
Physiology of therapy
Since the now classic studies of Lawrence and Kuypers in macaques,66,67 a large body of literature has focused on the effects of lesions in the corticospinal tract (CST) on motor deficits and extent of recovery. Reports tend to agree on the existence of a correlation between ipsilateral or contralesional CST damage and degree of motor deficits,68–71 whereas the association between CST integrity and degree of motor recovery is less clear.72
A complicating issue is the nature of the CST itself, which is not one tract but multiple tracts. In addition to efferent fibres originating in cortical area M1 (which are sometimes equated with the CST), dorsal and ventral premotor regions, the supplementary motor area, the motor cingulate cortex and parietal regions (superior parietal cortex and S1) communicate directly with the spinal cord,73,74 terminating onto both spinal motor neurons and interneurons.75,76 Direct corticofugal connections are thought to underpin the ability to perform skilled hand movements, whereas corticofugal fibres from association motor regions target inter-neurons,77–79 providing modulatory effects on motor neuronal activity.80
The consequences of the organization of the CST fibres are twofold. The first consequence is the magnitude of the effects of different pathways on the physiology of spinal motor neurons. For example, activation of a single corticomotor neuron in the primary motor cortex can produce direct responses in single motor units or multi-units on electromyography.81 Lesions in these direct M1 efferents in stroke, therefore, produce permanent impairment of individualized finger movements, but not other movements that can recover.66,67,82–84 The second consequence of CST organization relates to motor repair. The organization of different cortical regions and their spinal projections, as determined by imaging studies, have suggested potential mechanisms of recovery through recruitment of association motor regions.85–88 A more direct physiological assessment of this hypothesis through use of transcranial magnetic stimulation (TMS) in patients with stroke has confirmed these imaging findings.89
The mirror neuron system
We posit that successful treatment of aphasia or upper-limb motor dysfunction after stroke requires neural repair and reorganization that theoretically includes neural rewiring through stimulus-dependent plasticity. This approach requires a carefully tailored stimulus or behavioural intervention that can modulate relevant circuits to produce desired neural connectivity. The behavioural intervention must be developed according to findings from basic neuroscience that link brain circuitry with behaviour.
Among the most remarkable neurophysiological findings of the past several decades is the discovery of mirror neurons in various regions of the macaque cerebral cortex. Mirror neurons are notable because they discharge both during the execution of goal-directed actions performed with different biological effectors (for example, the mouth or hand), and during observation of another individual performing the same or a similar action .90,91 Increasing evidence suggests that a similar mirror neuron system is present in the human brain, and that it could have a role in action recognition, coding of motor intentions, and motor learning.92–95 The anatomy and physiology of the mirror neuron circuit, and its possible role in several cognitive functions96–98 and rehabilitation99,100 have been previously reviewed.
Motor imitation
Motor imitation is sometimes incorrectly regarded as a relatively unimportant cognitive task. However, it is particularly developed in humans, and intrinsically linked to language and culture.101–103 Imitation of actions involves motor observation, motor imagery, and action execution. Imitation of speech requires these steps as well as other highly complex processes at additional levels (for example, phoneme identification and word recognition).
Involvement of the mirror neuron system in motor imitation, especially imitation of hand actions, has been suggested by brain imaging studies104–106 and basic neurophysiology investigations that highlighted the importance of a network between premotor frontal regions and association regions in the parietal and temporal lobes.98,107
Behaviourally, observation of the lips, tongue and mouth of a speaker improves speech perception, particularly under noisy conditions108 or when the auditory signal is degraded.109,110 This effect arises from shared neural substrates for action observation and action execution,111–113 particularly of the mouth and lips during speech.114–118 The neural mechanism that links the behaviour with the brain network is observation–execution matching,119,120 whereby a perceiver matches observed actions to a repertoire of previously executed actions via a circuit that includes posterior inferior frontal and ventral premotor cortices, the inferior parietal lobule, and the posterior superior temporal sulcus.116,121,122
To investigate the putative human mirror neuron network for observation and production (execution) of speech, we analysed functional MRI brain imaging data for effective connectivity among active brain regions in healthy adults.123 Participants performed a simple task that involved observation and imitation of an audiovisual clip of an individual saying a simple consonant– vowel syllable. We focused on six brain regions: ventral premotor cortex and inferior frontal gyrus (combined region); inferior parietal lobule (including intraparietal sulcus); primary motor and sensory cortices; dorsal premotor cortex; posterior superior temporal gyrus and sulcus; and anterior superior temporal gyrus and sulcus.
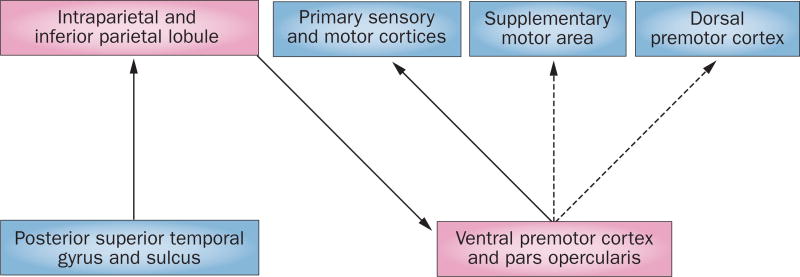
We used our results to generate a model (Figure 1) in which connections from posterior superior temporal to inferior parietal cortex, from inferior parietal to ventral premotor cortex, and from ventral pre motor to primary sensory–motor cortex were among the strongest in both hemispheres during execution and observation. In addition to the results illustrated here in the left hemisphere, there were interesting and subtle differences in connections within the right hemisphere and between hemispheres.123
Figure 1.
Possible mirror neuron network for syllable observation and imitation. A model of shared brain networks activated by observation and execution of speech, as determined by functional MRI in humans.123 Solid lines show connections that are common to observation and imitation, whereas dashed lines show connections that are more important in imitation than in observation. Pink rectangles represent core nodes in the putative ‘mirror neuron’ network. These networks suggest that a flow of information during imitation—starting at the posterior superior temporal cortex and ending in the motor cortex—enhances input to the motor cortex in the service of speech execution.
Action observation treatment
Basic neuroscience research on mirror neurons and their connections has suggested a new approach to poststroke treatment that is based on principles of motor physiology. Although this new direction does not yet constitute a fully developed rehabilitation model, it could provide the means by which such a goal can be achieved.
A rehabilitative approach that is based on findings in physiology enables direct assessment of changes through application of imaging and/or electrophysiology, and direct measures of CST excitability via TMS. Development of functional biomarkers could enable customization of therapy on the basis of an individual’s neurophysiological measures. By monitoring the long-term neuroanatomical and neurophysiological consequences of therapy, functions with the largest burden of impairment can be specifically targeted at the individual level. We suggest that systematic observation of meaningful actions followed by their execution (action observation treatment [AOT]) could be a viable rehabilitative strategy for patients with motor impairment and aphasia after stroke.
AOT for motor dysfunction
In AOT, patients with motor impairment carefully and systematically observe a series of videos that display everyday actions such as drinking coffee, reading the newspaper, or walking. Actions are chosen on the basis of their ecological value. Every action is divided into three or four motor segments with increasing degrees of difficulty. For example, in hand therapy, the action of having a cup of coffee can be decomposed into motion segments of reaching for the cup, turning the spoon, and bringing the coffee to the mouth. Each motion segment is presented for 3 min in the observation phase. At the end of the segment, patients perform the observed action (execution phase). The total time for the session— including instructions—takes about 90 min, and sessions are repeated daily for 4 weeks (Figure 2).
Figure 2.
Visual stimuli for action observation treatment. During action observation treatment, patients watch video sequences containing daily life hand and arm actions124 (top panels) or leg and foot actions132 (bottom panels) for 6 min, and then perform the action for 6 min, using the same movement and objects shown in the video clip. On each day of treatment, a ‘unit’ of three limb movements of increasing complexity is presented. In each video, the presented action is shown from three perspectives. A complete session consists of three or four such videos. Patients typically undergo 20 rehabilitation sessions over 20 consecutive weekdays. During both observation and execution, patients are instructed to focus on the goal of the action rather than on the movement per se.
Results in patients
AOT has been used for rehabilitation of patients with chronic ischaemic stroke (>6 months after the acute event), cerebral palsy, or Parkinson disease (PD), and in patients with non-neurological disorders, such as those undergoing orthopaedic surgery of the hip or knee.
In a pivotal randomized controlled study in patients with chronic ischaemic stroke in the territory of the middle cerebral artery,124 AOT was applied to treat upper-limb motor functions. Patients in the control group observed video clips that were related to historical, scientific or geographical issues and had no specific motor content. In this study, the Stroke Impact Scale, the Wolf Motor Function Test, and the Frenchay Arm Test were the functional scales used to quantify changes in motor abilities. The results showed significant improvement of motor functions over a 4-week treatment compared with the stable pretreatment baseline and the control group. Similar results have since been reported by another group.125 The improvement lasted for at least 8 weeks after the end of the intervention. Functional MRI during object manipulation before and after therapy showed a significant increase in activity in the bilateral ventral premotor cortex, bilateral superior temporal gyrus, the supplementary motor area and the contralateral supramarginal gyrus. On the basis of these data, we concluded that action observation promotes recovery of motor functions after stroke by reactivation of motor areas that contain the putative human correlate of the macaque mirror neuron system.
In a randomized controlled study,126 we investigated the efficacy of AOT in complementing pharmacology for PD. For this trial, participants in the active group observed videos depicting everyday life actions, including postural actions and walking, whereas those in the control group observed movies devoid of specific motor content. The active group improved more from baseline relative to the control group as measured on two functional scales: the Unified Parkinson Disease Rating Scale and the FIM.
AOT has also been used to reduce freezing of gait in patients with PD.127 Given that the mirror neuron system is heavily interconnected with the basal ganglia and has a role in motor planning and motor learning,128 it is possible that AOT promotes reorganization and maintenance of cortical loops and cortical connections with the striatum and thalamus.129 This notion is also supported by the fact that action observation in PD is accompanied by increases in beta oscillatory activity of the subthalamic nucleus in association with the alpha and beta desynchronization on EEG that is seen over the motor cortex.130
In an additional randomized controlled study, AOT was used for treatment of upper-limb motor dysfunction in children with cerebral palsy aged 6–11 years.131 One group of children observed daily actions appropriate for their age, whereas another group observed documentaries with no specific motor content. Functional evaluation using the Melbourne Assessment Scale of upper-limb motor functions showed that children undergoing AOT performed significantly better than controls after treatment. These results potentially provide insight into the ontogenesis of the mirror neuron system: the apparent targeting of central motor representations of actions in these children by AOT suggests that the mirror neuron system is fully functional at this age. Furthermore, these findings raise the question of whether this treatment affected an already developed motor representation in these children, or rather contributed to development of new motor representations of the presented actions.
Interestingly, in patients with non-neurological disorders, AOT might also improve motor recovery. In a randomized controlled trial in postsurgical orthopaedic patients, all of whom received conventional physiotherapy, those who observed video clips showing daily actions and subsequently imitated them scored better on functional scales than did patients who observed video clips with no motor content and then executed the same actions as patients in the AOT group.132 These findings are particularly interesting because they suggest that a treatment that affects brain representations of the lower limb can affect performance even when motor impairment has a non-neurological cause.
Underlying physiology
The clinical phenotype of patients with stroke after AOT is encouraging, and preliminary studies in humans suggest cortical reorganization results from this intervention.124 Direct measures of repair, however, must be identified before the precise physiological effects of this therapy can be determined.133–135 In this context, numerous reports are available on lesion analysis regarding the association between specific brain lesions and concomitant motor deficits and recovery.72,136
On the basis of motor physiology and knowledge of the many cortical sources of spinal projections, as well as mechanisms of recovery involving association motor regions, we can discuss the possible mechanisms of the effect of AOT on physiological recovery. In a small study involving four patients after stroke, TMS of the ipsilesional dorsal premotor cortex (PMd) increased the amplitude and reduced the latency of motor evoked potentials in the affected hand, representing a facilitatory effect on hand motor function.89 The investigators concluded that plastic changes in PMd after stroke might enable reorganization of motor circuits, perhaps with establishment of direct connections of the PMd with the spinal cord. Changes in the PMd may be reinforced via the ventral premotor cortex (PMv), which expands (in macaques) in proportion to M1 lesion size.137 Extending these notions to AOT, in which both PMd and PMv are clearly involved, a potential mechanism for repair could involve reorganization of the corticofugal system, whereby the function of the PMd shifts at the level of the spinal cord from a modulatory role to a role similar to that of the primary motor cortex. At the same time, the PMv would reinforce not only motor output via the PMd but also any residual outflow from M1.138
AOT for aphasia
We believe that the action observation–execution matching system could be of considerable benefit in aphasia therapy after stroke, particularly for speech production. The role of this system in predicting the consequences of motor activity,139,140 and in comprehension of sentences that describe actions,141 gives this approach great potential in aiding language recovery more generally.
Results in patients
AOT for aphasia is currently at an earlier stage on the translational path than motor rehabilitation, and randomized controlled trials are lacking for this indication. Preliminary data142,143 show that observation and execution of action might favour retrieval of action-related words in aphasic patients with a selective deficit for verb retrieval, which supports the notion that the motor system interacts with the language system.
We have recently developed a therapeutic approach, called IMITATE, which is based on matching observation and execution in speech, and is currently being tested in a clinical trial in patients with aphasia following stroke.144,145 IMITATE therapy involves silent observation of audiovisually presented words and phrases that are spoken aloud by six talkers, followed by a period during which the participant orally repeats the stimuli. The clinical trial is a randomized single-blind controlled trial (the researcher, but not the participant, knows whether the participant is receiving IMITATE or a control therapy). Treatment is provided intensively (90 min per day) for 6 weeks, with weekly incremental increases in difficulty from monosyllabic words to disyllabic words, disyllabic sentences, and finally longer utterances, combined with progressively increasing rate of speech. Functional MRI scans are obtained before, during and after therapy.
Recent work in neural network computer models suggests that gradual incremental learning has theoretical advantages.146 We are currently analysing data from 19 patients with aphasia following left middle cerebral artery stroke, and have preliminary results showing significant improvement on an overall language score from pretreatment to post-treatment in the IMITATE group, but not in the control group.147
Underlying physiology
In an effort to understand the physiological mechanism of AOT for aphasia therapy, we have recently completed a polysomnography study that assessed brain plasticity related to a single (extended) session of IMITATE therapy. 148,149 Increasing evidence in healthy humans and animals suggests that slow-wave activity (SWA) during sleep plays an important part in regulating synaptic plasticity and reorganization.150–152 The theory posits that strengthening of synaptic efficacy in a specific cortical area during the day should be followed by increases in SWA in that cortical area compared with the rest of the cortex during sleep.150 This effect relies on the notion that stronger synapses lead to stronger cortico-cortical connections and, in turn, in increased synchronization among populations of neurons.153 Increased synchronization is then reflected in slow waves of larger amplitude on the EEG.154
We found that a single exposure to IMITATE resulted in increases in local SWA on EEG during subsequent sleep over the predicted target regions of AOT, particularly over the right parietal cortex (unaffected by the lesion). Furthermore, changes in SWA over the left precentral areas predicted behavioural changes, supporting the role of perilesional areas in predicting positive functional responses.155 These data suggest the value of AOT in affecting specific neural systems that are related to observation and imitation of speech as described above,156 and are consistent with existing models of language recovery that implicate both ipsilesional and contralateral circuits.157,158 The specific contributions of the two hemispheres to recovery following unilateral stroke differs depending on the size, type and location of the infarct,159,160 and differs in very early (neonatal and early childhood) stroke compared with adult stroke.161 One emerging notion is that functional connectivity between the two superior temporal gyri is a marker of receptive language outcome after aphasic stroke, both in adults162 and neonates.163
Conclusions
In this Review, we have outlined a new model for neural repair and rehabilitation in which we suggest that stroke treatment—from the ambulance to the return home— must be fundamentally neurological. This approach is quite different from current practice standards for post-stroke therapeutics, in which physical therapy and speech and language therapy emphasize education, compensation for deficits that are expected to exist permanently, or making limited gains in function through training of unspecified brain circuits.
Our neurological model is based on three critical assumptions: that the mechanisms of neural repair inherently involve cellular and circuit plasticity; that brain plasticity is fundamentally a synaptic phenomenon that is largely stimulus-dependent; and that brain repair must incorporate biological interventions— ultimately to replace or augment some lost brain tissue—via behavioural interventions that are carefully tailored for re organization of specific brain circuits. Notably, recovery can be viewed as part of a continuum in which certain types of large brain lesions require augmentation of the anatomical substrate in addition to alteration of network connectivity in the existing (and new) neural substrate.
We have proposed a novel approach to rehabilitation of motor impairment and speech that aims at remediation of functions and promotion of plasticity in networks that were previously associated with prelesion behaviours. AOT is a good example of an approach that harnesses the putative mirror neuron system, which is involved in both execution and understanding of everyday life actions. Development of AOT not only depends on knowledge of the underlying biology, but therapeutic efficacy relates closely to the nature of the biological changes that it produces.
What are the features of action–observation— followed by imitation of the action—that could promote plasticity in the appropriate networks for motor skill and speech? First, this network can be triggered by multiple sensory inputs (visual, auditory and proprioceptive), and/or through additive effects if such inputs are weakened by disease. Furthermore, the widely distributed nature of the network suggests many anatomical and physiological routes to network activation. Second, activation of the network during motor observation increases the excitability of cortical motor outputs via the corticospinal path associated with execution of those movements, even in the absence of overt movements. Third, the network is strongly associated with goal-oriented, ecologically valid actions that were previously present in the repertoire of the patient. Consequently, observation followed by imitation of an observed action avoids the fragmentation of the actions into smaller components, as is typically done in current rehabilitative practice, instead emphasizing execution of the action as a whole. Fourth, its effects can be applied to numerous neurological and non-neurological conditions.
Future clinical trials should assess how this treatment affects neuronal circuits that are involved in motor control and speech, and how this approach could be integrated with other biological approaches to neurorehabilitation.
Key points.
The ultimate goal of stroke treatment—after the initial insult has been appropriately limited in extent and severity—should be to repair the injured brain to effect a cure
Current practice focuses on compensation, which is cheaper and quicker than brain repair and remediation, but involves low outcome expectations
Rebuilding brain circuits to recover motor functions and speech depends on endogenous biological factors and exogenous input, as brain plasticity is inherently stimulus-dependent
Several stroke treatment programmes based on physiological rationales aimed at repairing brain circuits are currently undergoing testing
Action observation treatment for hand motor dysfunction is based on macaque research in action understanding, and has shown some preliminary success for treatment of stroke and other neurological injuries
Action observation treatment for speech and language dysfunction seems to affect brain plasticity and have some benefit
Review criteria.
The articles in this Review were found through a search of PubMed, focusing on search terms “brain repair”, “stroke” , “treatment”, “plasticity”, “hand movement”, and “language”. We focused on articles that studied animal or human physiology for the construction and application of treatments for post-stroke therapy.
Acknowledgments
This research was supported by the National Institute of Deafness and other Communication Disorders (NIDCD) under grants R01-DC003378 and R01-DC007488 (to S. L. Small), and by the National Institute of Neurological Disorders and Stroke under grant R01-NS054942 (to A. Solodkin). Additional support was provided by the James S. McDonnell Foundation under the Brain Network Recovery Group and Virtual Brain Project grants to the Rotman Research Institute. This support is gratefully acknowledged.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed to researching data for the article, discussion of the content, writing of the article and to review and/or editing of the manuscript before submission.
Contributor Information
Steven L. Small, Department of Neurology, University of California, Irvine, 200 Manchester Avenue, Suite 206, Orange, CA 92697, USA
Giovanni Buccino, Dipartimento di Scienze Mediche e Chirurgiche, Università Magna Graecia di Catanzaro, Campus Universitario “Salvatore Venuta”, Viale Europa—Localitá Germaneto, 88100 Catanzaro, Italy.
Ana Solodkin, Department of Neurobiology and Anatomy, University of California, Irvine, Hewitt Hall, Room 1505, Irvine, CA 92697, USA.
References
- 1.Gosman-Hedstrom G, Claesson L, Blomstrand C. Consequences of severity at stroke onset for health-related quality of life (HRQL) and informal care: a 1-year follow-up in elderly stroke survivors. Arch. Gerontol. Geriatr. 2008;47:79–91. doi: 10.1016/j.archger.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch. Phys. Med. Rehabil. 2002;83:1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 3.Kuoppala J, Lamminpaa A. Rehabilitation and work ability: a systematic literature review. J. Rehabil. Med. 2008;40:796–804. doi: 10.2340/16501977-0270. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak MA, Kittner SJ. Return to work after ischemic stroke: a methodological review. Neuroepidemiology. 2002;21:159–166. doi: 10.1159/000059516. [DOI] [PubMed] [Google Scholar]
- 5.Pan JH, Song XY, Lee SY, Kwok T. Longitudinal analysis of quality of life for stroke survivors using latent curve models. Stroke. 2008;39:2795–2802. doi: 10.1161/STROKEAHA.108.515460. [DOI] [PubMed] [Google Scholar]
- 6.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Ageing. 2007;36:316–322. doi: 10.1093/ageing/afm014. [DOI] [PubMed] [Google Scholar]
- 7.Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry. 2002;52:253–264. doi: 10.1016/s0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- 8.Granger CV, Hamilton BB, Sherwin FS. Guide for the Use of Uniform Data Set for Medical Rehabilitation. Uniform Data Set for Medical Rehabilitation Project Office; 1986. [Google Scholar]
- 9.Aten JL, Caligiuri MP, Holland AL. The efficacy of functional communication therapy for chronic aphasic patients. J. Speech Hear. Disord. 1982;47:93–96. doi: 10.1044/jshd.4701.93. [DOI] [PubMed] [Google Scholar]
- 10.Frattali CM, Thompson CM, Holland AL, Wohl CB, Ferketic MM. The FACS of life ASHA facs—a functional outcome measure for adults. ASHA. 1995;37:40–46. [PubMed] [Google Scholar]
- 11.Dodds T, Martin D, Stolov W, Deyo R. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- 12.Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Brain Res. Rev. 2001;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 13.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- 14.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 15.Johansen-Berg H, Scholz J, Stagg CJ. Relevance of structural brain connectivity to learning and recovery from stroke. Front. Syst. Neurosci. 2010;4:146. doi: 10.3389/fnsys.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dipietro L, et al. Learning, not adaptation, characterizes stroke motor recovery: evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans. Neural Syst. Rehabil. Eng. 2012;20:48–57. doi: 10.1109/TNSRE.2011.2175008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huseyinsinoglu BE, Ozdincler AR, Krespi Y. Bobath Concept versus constraint-induced movement therapy to improve arm functional recovery in stroke patients: a randomized controlled trial. Clin. Rehabil. 2012;26:705–715. doi: 10.1177/0269215511431903. [DOI] [PubMed] [Google Scholar]
- 18.Luke C, Dodd KJ, Brock K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin. Rehabil. 2004;18:888–898. doi: 10.1191/0269215504cr793oa. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang RY, Chen HI, Chen CY, Yang YR. Efficacy of Bobath versus orthopaedic approach on impairment and function at different motor recovery stages after stroke: a randomized controlled study. Clin. Rehabil. 2005;19:155–164. doi: 10.1191/0269215505cr850oa. [DOI] [PubMed] [Google Scholar]
- 21.Gharbawie OA, Whishaw IQ. Parallel stages of learning and recovery of skilled reaching after motor cortex stroke: “oppositions” organize normal and compensatory movements. Behav. Brain. Res. 2006;175:249–262. doi: 10.1016/j.bbr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Roby-Brami A, et al. Motor compensation and recovery for reaching in stroke patients. Acta Neurol. Scand. 2003;107:369–381. doi: 10.1034/j.1600-0404.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian SK, Lourenco CB, Chilingaryan G, Sveistrup H, Levin MF. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil. Neural Repair. 2013;27:13–23. doi: 10.1177/1545968312449695. [DOI] [PubMed] [Google Scholar]
- 24.Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clin. Neurophysiol. 2007;118:164–176. doi: 10.1016/j.clinph.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson CK, Shapiro LP. Treating agrammatic aphasia within a linguistic framework: treatment of underlying forms. Aphasiology. 2005;19:1021–1036. doi: 10.1080/02687030544000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil. Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 27.Lum PS, et al. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top. Stroke Rehabil. 2009;16:237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 28.Anderson E, Anderson TP, Kottke FJ. Stroke rehabilitation: maintenance of achieved gains. Arch. Phys. Med. Rehabil. 1977;58:345–352. [PubMed] [Google Scholar]
- 29.Davidoff GN, Keren O, Ring H, Solzi P. Acute stroke patients: long-term effects of rehabilitation and maintenance of gains. Arch. Phys. Med. Rehabil. 1991;72:869–873. doi: 10.1016/0003-9993(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 30.Roth EJ, Lovell L. Community skill performance and its association with the ability to perform everyday tasks by stroke survivors one year following rehabilitation discharge. Top. Stroke Rehabil. 2007;14:48–56. doi: 10.1310/tsr1401-48. [DOI] [PubMed] [Google Scholar]
- 31.Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J. Rehabil. Res. Dev. 2012;49:479–496. doi: 10.1682/jrrd.2010.10.0210. [DOI] [PubMed] [Google Scholar]
- 32.Aichner F, Adelwohrer C, Haring HP. Rehabilitation approaches to stroke. J. Neural Transm. Suppl. 2002;2002:59–73. doi: 10.1007/978-3-7091-6137-1_4. [DOI] [PubMed] [Google Scholar]
- 33.Floel A, Cohen LG. Translational studies in neurorehabilitation: from bench to bedside. Cogn. Behav. Neurol. 2006;19:1–10. doi: 10.1097/00146965-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Small SL, Llano DA. Biological approaches to aphasia treatment. Curr. Neurol. Neurosci. Rep. 2009;9:443–450. doi: 10.1007/s11910-009-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liepert J, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci. Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 36.Taub E, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 37.Wolf SL, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berthier ML, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann. Neurol. 2009;65:577–585. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]
- 39.Pulvermuller F, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 40.Altschuler EL, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–2036. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 41.Michielsen ME, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil. Neural Repair. 2011;25:223–233. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- 42.Wu CY, Huang PC, Chen YT, Lin KC, Yang HW. Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2013;94:1023–1030. doi: 10.1016/j.apmr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn TS. The Structure of Scientific Revolutions. University of Chicago Press; 1962. [Google Scholar]
- 44.Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens. Mot. Res. 1998;15:173–189. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- 45.Hebb DO. The Organization of behavior. Wiley; 1949. [DOI] [PubMed] [Google Scholar]
- 46.Johansson BB. Brain plasticity stroke rehabilitation. The Willis lecture. Stroke. 2000;31:223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 47.Hebb DO. The effects of early experience on problem solving at maturity. Am. Psychol. 1947;2:306. [Google Scholar]
- 48.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 49.Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 50.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J. Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr. Opin. Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitzer NC. Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci. 2012;13:94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colon-Ramos DA. Synapse formation in developing neural circuits. Curr. Top. Dev. Biol. 2009;87:53–79. doi: 10.1016/S0070-2153(09)01202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fields RD, Itoh K. Neural cell adhesion molecules in activity-dependent development and synaptic plasticity. Trends Neurosci. 1996;19:473–480. doi: 10.1016/S0166-2236(96)30013-1. [DOI] [PubMed] [Google Scholar]
- 55.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev. Disabil. Res. Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 56.Knafo S, Esteban JA. Common pathways for growth and for plasticity. Curr. Opin. Neurobiol. 2012;22:405–411. doi: 10.1016/j.conb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr. Opin. Neurobiol. 2006;16:258–264. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Small SL. In: Handbook on Adult Language Disorders: Integrating Cognitive Neuropsychology, Neurology, and Rehabilitation. Hillis A, editor. Psychology Press; 2001. pp. 397–411. [Google Scholar]
- 59.Horng SH, Sur M. Visual activity and cortical rewiring: activity-dependent plasticity of cortical networks. Prog. Brain Res. 2006;157:3–11. doi: 10.1016/s0079-6123(06)57001-3. [DOI] [PubMed] [Google Scholar]
- 60.McCloskey M, Cohen NJ. In: The Psychology of Learning and Motivation. Bower G, editor. Academic Press; 1989. pp. 109–165. [Google Scholar]
- 61.Gernsbacher MA, St John MF. In: Foreign Language Learning: Psycholinguistic Experiments On Training and Retention. Healy AF, Bourne LE Jr, editors. Laurence Erlbaum Associates; 1998. pp. 231–255. [Google Scholar]
- 62.Sparks R, Helm N, Albert M. Aphasia rehabilitation sesulting from melodic intonation therapy. Cortex. 1974;10:303–316. doi: 10.1016/s0010-9452(74)80024-9. [DOI] [PubMed] [Google Scholar]
- 63.Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch. Neurol. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- 64.[No authors listed]. Assessment: melodic intonation therapy. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1994;44:566–568. doi: 10.1212/wnl.44.3_part_1.566. [DOI] [PubMed] [Google Scholar]
- 65.Belin P, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 66.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 68.Schaechter JD, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum. Brain Mapp. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindenberg R, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ward NS, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin. Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 73.Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J. Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morecraft RJ, Louie JL, Schroeder CM, Avramov K. Segregated parallel inputs to the brachial spinal cord from the cingulate motor cortex in the monkey. Neuroreport. 1997;8:3933–3938. [PubMed] [Google Scholar]
- 75.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl Acad. Sci. USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lemon RN. Descending pathways in motor control. Annu. Rev. Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 77.Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc. Natl Acad. Sci. USA. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maier MA, et al. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb. Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- 79.Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J. Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J. Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- 81.Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog. Brain Res. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- 82.Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J. Neurosci. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J. Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- 84.Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Adv. Exp. Med. Biol. 2009;629:559–575. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weiller C, Chollet F, Friston K, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann. Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 86.Cramer SC, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 87.Gerloff C, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 88.van Meer MP, et al. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J. Neurosci. 2012;32:4495–4507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fridman EA, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 90.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 91.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 92.Jeannerod M. The representing brain: neural correlates of motor intention and imagery. Behav. Brain Sci. 1994;17:187–245. [Google Scholar]
- 93.Umilta MA, et al. I know what you are doing. A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 94.Buccino G, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 95.Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain Lang. 2004;89:370–376. doi: 10.1016/S0093-934X(03)00356-0. [DOI] [PubMed] [Google Scholar]
- 96.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 97.Cattaneo L, Rizzolatti G. The mirror neuron system. Arch. Neurol. 2009;66:557–560. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 98.Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda) 2008;23:171–179. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- 99.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn. Behav. Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Small SL, Buccino G, Solodkin A. The mirror neuron system and treatment of stroke. Dev. Psychobiol. 2012;54:293–310. doi: 10.1002/dev.20504. [DOI] [PubMed] [Google Scholar]
- 101.Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 102.Nishitani N, Schurmann M, Amunts K, Hari R. Broca’s region: from action to language. Physiology (Bethesda) 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- 103.Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn. Sci. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Iacoboni M, et al. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 105.Koski L, et al. Modulation of motor and premotor activity during imitation of target-directed actions. Cereb. Cortex. 2002;12:847–855. doi: 10.1093/cercor/12.8.847. [DOI] [PubMed] [Google Scholar]
- 106.Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc. Natl Acad. Sci. USA. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelissen K, et al. Action observation circuits in the macaque monkey cortex. J. Neurosci. 2011;31:3743–3756. doi: 10.1523/JNEUROSCI.4803-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacLeod A, Summerfield Q. Quantifying the contribution of vision to speech perception in noise. Br. J. Audiol. 1987;21:131–141. doi: 10.3109/03005368709077786. [DOI] [PubMed] [Google Scholar]
- 109.Ross LA, Saint-Amour D, Leavitt VM, Javitt DC, Foxe JJ. Do you see what I am saying? Exploring visual enhancement of speech comprehension in noisy environments. Cereb. Cortex. 2007;17:1147–1153. doi: 10.1093/cercor/bhl024. [DOI] [PubMed] [Google Scholar]
- 110.Sumby WH, Pollack I. Visual contribution of speech intelligibility in noise. J. Acoustic. Soc. Am. 1954;26:212–215. [Google Scholar]
- 111.Buccino G, et al. Neural circuits underlying imitation learning of hand actions: an event-related FMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 112.Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb. Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- 113.Tanaka S, Inui T. Cortical involvement for action imitation of hand/arm postures versus finger configurations: an fMRI study. Neuroreport. 2002;13:1599–1602. doi: 10.1097/00001756-200209160-00005. [DOI] [PubMed] [Google Scholar]
- 114.Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat. Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- 115.Skipper JI, Nusbaum HC, Small SL. Listening to talking faces: motor cortical activation during speech perception. Neuroimage. 2005;25:76–89. doi: 10.1016/j.neuroimage.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 116.Skipper JI, van Wassenhove V, Nusbaum HC, Small SL. Hearing lips and seeing voices: how cortical areas supporting speech production mediate audiovisual speech perception. Cereb. Cortex. 2007;17:2387–2399. doi: 10.1093/cercor/bhl147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fadiga L, et al. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–158. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- 118.D’Ausilio A, Bufalari I, Salmas P, Fadiga L. The role of the motor system in discriminating normal and degraded speech sounds. Cortex. 2011;48:882–887. doi: 10.1016/j.cortex.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 119.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 120.Gallese V. The manifold nature of interpersonal relations: the quest for a common mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:517–528. doi: 10.1098/rstb.2002.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dick AS, Solodkin A, Small SL. Neural development of networks for audiovisual speech comprehension. Brain Lang. 2010;114:101–114. doi: 10.1016/j.bandl.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mashal N, Solodkin A, Dick AS, Chen EE, Small SL. A network model of observation and imitation of speech. Front. Psychol. 2012;3:84. doi: 10.3389/fpsyg.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ertelt D, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36(Suppl. 2):T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 125.Franceschini M, et al. Clinical relevance of action observation in upper-limb stroke rehabilitation: a possible role in recovery of functional dexterity. A randomized clinical trial. Neurorehabil. Neural Repair. 2012;26:456–462. doi: 10.1177/1545968311427406. [DOI] [PubMed] [Google Scholar]
- 126.Buccino G, et al. Action observation treatment improves autonomy in daily activities in Parkinson’s disease patients: results from a pilot study. Mov. Disord. 2011;26:1963–1964. doi: 10.1002/mds.23745. [DOI] [PubMed] [Google Scholar]
- 127.Pelosin E, et al. Action observation improves freezing of gait in patients with Parkinson’s disease. Neurorehabil. Neural Repair. 2010;24:746–752. doi: 10.1177/1545968310368685. [DOI] [PubMed] [Google Scholar]
- 128.Doyon J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 129.Obeso JA, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov. Disord. 2008;23(Suppl. 3):S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 130.Alegre M, et al. Changes in subthalamic activity during movement observation in Parkinson’s disease: is the mirror system mirrored in the basal ganglia? Clin. Neurophysiol. 2010;121:414–425. doi: 10.1016/j.clinph.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 131.Buccino G, et al. Improving upper limb motor functions through action observation treatment: a pilot study in children with cerebral palsy. Dev. Med. Child Neurol. 2012;54:822–828. doi: 10.1111/j.1469-8749.2012.04334.x. [DOI] [PubMed] [Google Scholar]
- 132.Bellelli G, Buccino G, Bernardini B, Padovani A, Trabucchi M. Action observation treatment improves recovery of postsurgical orthopedic patients: evidence for a top-down effect? Arch. Phys. Med. Rehabil. 2010;91:1489–1494. doi: 10.1016/j.apmr.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 133.Ward NS, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Starkey ML, et al. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain. 2012;135:3265–3281. doi: 10.1093/brain/aws270. [DOI] [PubMed] [Google Scholar]
- 135.Stinear CM, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 136.Weiller C. Imaging recovery from stroke. Exp. Brain Res. 1998;123:13–17. doi: 10.1007/s002210050539. [DOI] [PubMed] [Google Scholar]
- 137.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J. Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 138.Kantak SS, Stinear JW, Buch ER, Cohen LG. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil. Neural Repair. 2012;26:282–292. doi: 10.1177/1545968311420845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iacoboni M, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proc. Natl Acad. Sci. USA. 2001;98:13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iacoboni M. In: Human Brain Mapping 2003. Paus T, Bullmore E, Cohen JD, editors. Academic Press; 2003. [Google Scholar]
- 141.Tettamanti M, et al. Listening to action-related sentences activates fronto-parietal motor circuits. J. Cogn. Neurosci. 2005;17:273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- 142.Bonifazi S, et al. Action observation as a useful approach for enhancing recovery of verb production: new evidence from aphasia. Eur. J. Phys. Rehabil. Med. 2013;49:473–481. [PubMed] [Google Scholar]
- 143.Marangolo P, et al. Improving language without words: first evidence from aphasia. Neuropsychologia. 2010;48:3824–3833. doi: 10.1016/j.neuropsychologia.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 144.Lee J, Fowler R, Rodney D, Cherney L, Small SL. IMITATE: an intensive computer-based treatment for aphasia based on action observation and imitation. Aphasiology. 2010;24:449–465. doi: 10.1080/02687030802714157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Small SL. A biological basis for aphasia treatment: mirror neurons and observation- execution matching. Poznan Stud. Contemp. Linguist. 2009;45:313–326. [Google Scholar]
- 146.Elman JL. Learning and development in neural networks: the importance of starting small. Cognition. 1993;48:71–99. doi: 10.1016/0010-0277(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 147.Duncan ES, Schmah T, Small SL. 50th Annual Meeting of the Academy of Aphasia; The Academy of Aphasia; 2012. [Google Scholar]
- 148.Sarasso S, et al. Non-fluent aphasia and neural reorganization after speech therapy: insights from human sleep electrophysiology and functional magnetic resonance imaging. Arch. Ital. Biol. 2010;148:271–278. [PMC free article] [PubMed] [Google Scholar]
- 149.Sarasso S, et al. Plastic changes following imitation-based speech and language therapy for aphasia: a high density (HD) sleep EEG study. Neurorehabil. Neural Repair. doi: 10.1177/1545968313498651. http://dx.doi.org/10.1177/1545968313498651. [DOI] [PubMed]
- 150.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 151.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 152.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J. Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J. Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 154.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Meinzer M, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage. 2008;39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 156.Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain Lang. doi: 10.1016/j.bandl.2012.07.007. http://dx.doi.org/10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed]
- 157.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 158.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann. Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 159.Saur D, et al. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain. 2010;133:1252–1264. doi: 10.1093/brain/awq021. [DOI] [PubMed] [Google Scholar]
- 160.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]
- 161.Raja Beharelle A, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133:1707–1716. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–3442. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dick AS, Raja Beharelle A, Solodkin A, Small SL. Interhemispheric functional connectivity following prenatal or perinatal brain injury predicts receptive language outcome. J. Neurosci. 2013;33:5612–5625. doi: 10.1523/JNEUROSCI.2851-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]