Abstract
We examined the predictive value of pretransplant positron emission tomography/computed tomography and marrow involvement evaluation on outcomes of 66 patients with mantle cell lymphoma treated with hematopoietic cell transplantation (HCT). Residual disease detected by either method prior to autograft was associated with increased relapse rates at 2 years and worse 5-year disease-free survival. Allograft recipients had favorable long-term outcomes despite the presence of residual disease pre-HCT.
Background
The prognostic roles of 18F-fludeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) imaging and marrow involvement evaluation on outcomes following autologous and allogeneic hematopoietic cell transplantation (HCT) for mantle cell lymphoma (MCL) are uncertain and require more data.
Patients and Methods
We categorized 66 patients with MCL who received HCT (38 autologous and 28 allogeneic) on the basis of pre-HCT residual disease (RD) status as assessed by marrow MCL morphology and flow/molecular analysis and PET/CT imaging to RD positive (RD+) (either or both measures positive) and RD− (both negative). We analyzed the predictive value of these RD detection methods on transplant outcomes.
Results
The 2-year relapse rate after autograft was significantly higher in pre-HCT RD+ patients (46% [95% CI 16–77%]) than in patients who were RD− (19% [95% CI 0–42%]; P = .02), leading to worse 5-year disease-free survival (DFS) in RD+ patients (46% [95% CI 14%–73%] vs. 68% [95% CI 33–87%], P = .04). In multivariate analysis, RD+ status was associated with a reduction in DFS (hazard ratio, 5.6; P = .02). Most allogeneic HCT recipients had advanced disease and most were RD+ (12 PET/CT+; 5 marrow-positive). The 5-year DFS and relapse rates after allogeneic HCT were 34% and 25% for all patients and 40% and 33% for RD+ recipients, suggesting that active disease at the time of allograft does not preclude long-term remissions in advanced MCL.
Conclusion
Both autologous and allogeneic HCT lead to promising long-term survival. RD detected prior to autograft was associated with increased relapse and worse 5 year DFS. Allograft recipients had favorable long-term outcomes even in presence of pre-HCT detectable disease.
Keywords: Allogeneic transplantation, Autologous transplantation, Minimal residual disease, MIPI score, PET scan
Introduction
Mantle cell lymphoma (MCL) comprises 5%–10% of all of non-Hodgkin lymphomas (NHL). It predominately affects older male patients and commonly presents in advanced stage with extranodal involvement and marrow infiltration.1 The median survival in patients with MCL ranges from 3 to 7 years.2 Although the disease is incurable with chemotherapy alone, complete responses can be achieved in 90% of patients with rituximab and intensive chemotherapies containing high-dose cytarabine.3 These remissions are often consolidated with hematopoietic cell transplantation (HCT); however, there is wide variation in reported outcomes.4–9
Recent advances in the understanding of the clinical, molecular, and genetic characteristics of MCL have identified prognostic factors useful to develop risk-adapted therapies. The Mantle Cell International Prognostic Index (MIPI) comprises 4 nonmodifiable factors (age, white cell count at diagnosis, lactate dehydrogenase level, and performance status) and was developed to predict overall survival (OS) from diagnosis.10 The discriminatory power of MIPI has been validated in the era of rituximab and intensive immunochemotherapy. Previous studies have shown that a high-risk MIPI score indicates worse disease-free survival (DFS) after autologous HCT or rituximab plus hyper-CVAD/MA (cyclophosphamide, vincristine, Adriamycin [doxorubicin], dexamethasone/methotrexate, cytarabine) chemotherapy.11,12 In addition to the MIPI, the presence of residual MCL in marrow as assessed by polymerase chain reaction (PCR) for the immunoglobulin heavy chain (IgH) has been correlated with an increased risk of relapse and progression after autologous HCT.13 A uniqueMCL flow cytometric phenotype defined by surface expression of CD20, CD5, surface light chain, and FMC7 or CD79b and the absence of CD23 has also been validated for pretransplant bone marrow evaluation.14
Recently, positron emission tomography/computed tomography (PET/CT) imaging has become an important advance in noninvasive lymphoma assessment. PET/CT has been applied for assessing disease burden and response to therapy in Hodgkin lymphoma and diffuse large B-cell lymphoma; however, its utility as a stratification tool in MCL is debated.15–19 Particularly, there is little data on prognostic utility of pre-HCT PET/CT imaging on transplant outcomes in MCL. For the past decade we have used PET/CT imaging and PCR/flow cytometric bone marrow assessment prior to HCT to restage MCL patients enrolled on transplant protocols at the University of Minnesota. In this single-institution analysis, we investigated the impact of residual disease (RD) as detected by either pretransplant PET/CT scan or MCL marrow assessment on transplant outcomes in 66 patients with MCL who received consolidation autologous or allogeneic HCT.
Patients and Methods
Study Design
We analyzed outcomes of all consecutive patients ≥ 18 years of age with a diagnosis of MCL who underwent autologous or allogeneic HCT at the University of Minnesota from 1999 to 2010. A diagnosis of MCL was confirmed according to the World Health Organization criteria. Patient data prospectively collected in the University of Minnesota Transplant Database were supplemented with data from individual medical records, imaging files, and pathology reports. Treatment responses were evaluated according to criteria described by Cheson et al.20 The MIPI score was calculated as previously described.10 Outcomes included DFS, OS, treatment-related mortality (TRM), and relapse rates. Pretransplant disease burden was determined by PET/CT scan or bone marrow MCL assessment. Transplantation protocols were approved by the institutional review board (IRB), and informed consent for clinical data collection was obtained prior to treatment. We also obtained IRB approval to retrospectively review imaging studies and pathologic materials of transplanted patients.
RD Definitions
Patients were defined as RD negative (RD−) if both the PET/CT scan and marrow were negative for disease prior to HCT. Patients were defined as RD positive (RD+) if the results of either 1 (PET/CT or marrow) or both methods were positive prior to HCT.
RD Measurement by PET/CT Scan
Patients underwent imaging by PET/CT ≤ 4weeks before HCT, and all PET/CT scans were reviewed by a nuclear medicine radiologist (JF) who was blinded to clinical outcomes. We used a Siemens Biograph 16 PET scanner with HD detector system for all patients. Criteria proposed by the Imaging Subcommittee for International Harmonization Project for lymphoma were used.15 PET/CT scans were positive if focal or diffuse 18F-fludeoxyglucose (FDG) uptake above the surrounding background in a location incompatible with normal anatomy and physiology was identified. The standardized uptake value (SUV), representing the ratio of the tumoral tracer concentration to the average tracer concentration in the entire body, was used. PET/CT-negative patients had no evidence of metabolically active MCL.
RD Measurement by Bone Marrow Examination
A hematopathologist (MAL) performed a central review of data from bone marrow trephine biopsies and aspirates collected 10 to 30 days prior to HCT. As this retrospective study spanned 11 years, the methods used to evaluate RD varied. Morphology was interpreted from core biopsies. If lymphoid aggregates were absent on multiple hematoxylin-eosin—stained levels, the core biopsies were interpreted as negative. Any lymphoid aggregates with atypical morphologic features were immunostained and deemed positive if stains were diagnostic of MCL independent of percentage involvement. A molecular assay to detect common breakpoints comprising t(11;14) translocations was employed during the early years, as previously described.21 A subset of cases had aspirate material evaluated for at (11;14) by fluorescence in situ hybridization (FISH) or conventional G-banding karyotypic analysis. We performed 4- or 8-color flow cytometry using antibodies to the following antigens: CD5, CD10, CD14, CD19, CD20, CD23, CD45, CD79b, polyclonal kappa, and lambda light chains. Analysis was performed on BD FACSCalibur or BD FACSCanto II, and flow cytometry data were analyzed by FACSDiva, FCS Express, or Kaluza. For marrow analysis, a minimum of 100,000 events was collected to identify B-cell clones aberrantly coexpressing CD5 and light chain restriction. Small CD5+ B-cell populations with monotypic light chains were tested for CD79b expression and absence of CD23 to confirm the MCL immunophenotype. In a few cases, clonality studies by PCR amplification of VDJ rearrangements were performed as previously described.21,22 RD positivity was defined as any evidence of disease by morphology, molecular assays, or flow cytometry; for a patient to be RD−, all test results had to be negative.
Conditioning Regimens
Myeloablative conditioning (MAC) for allogeneic HCT recipients consisted of cyclophosphamide (CY) 60 mg/m2/day intravenously (I.V.) for 2 days combined with fractioned total body irradiation (TBI) 13.20 Gy over 4 days or busulfan (BU) 1 mg/kg/day by mouth every 6 hours for 4 days. Umbilical cord blood (UCB) graft recipients received I.V. fludarabine (FLU) 25 mg/m2/day for 3 days in addition to TBI and CY. The reduced-intensity conditioning (RIC) allogeneic HCT protocol enrolled patients > 55 years of age who were receiving related donor HCT, patients > 45 years of age who were receiving unrelated UCB, and patients with comorbidities or previous HCT. The regimen consisted of 2.00 Gy of TBI plus FLU 40 mg/m2/day I.V. for 5 days (days 6 through −2) with either CY 50 mg/kg/day I.V. on day −6 or BU 1 mg/kg/day by mouth every 6 hours on days −8 and −7.9 The autologous HCT regimen consisted of CY 60 mg/kg for 2 days and TBI (1.65 Gy twice a day × 4 days; total 13.20 Gy) or CY 1.5 g/m2 daily for 4 days, BCNU (carmustine) 300 mg/m2 for 1 day, and etoposide (VP16) 150 mg/m2 I.V. × 6 doses.
Graft-Versus-Host Disease Prophylaxis
All allogeneic HCT patients received I.V. or oral cyclosporine from day −3 for a minimum of 100 days to a therapeutic target trough of 200 to 400 ng/mL. Patients who received MAC received additional graft-versus-host disease (GVHD) prophylaxis with methotrexate (15 mg/m2 I.V. day+1 followed by 10 mg/m2 I.V. day +3, +6 and +11). RIC recipients and recipients of UCB received mycophenolate mofetil (1–1.5 g I.V. or orally twice daily for the first 30 days).
Statistical Analysis
Analyses were performed separately for autologous and allogeneic transplants. The Kaplan-Meier method was used to estimate OS and DFS, while cumulative incidence was used to estimate TRM and risk of relapse.23,24 The test of equality of survival data between different groups was determined by the log-rank test. Cox multiple regression models were conducted for OS and DFS. Competing risk regression was employed for nonrelapse mortality (NRM) and risk of relapse. RD was the primary factor being considered for each endpoint. In univariate analysis, we tested the patient, disease, and transplant characteristics described in Table 1. The backward method was used to determine the final model, with a P value of < .05 considered significant in all statistical tests. Statistical analysis was performed with Statistical Analysis System statistical software version 9.2 (SAS Institute). Groups with P values ≤ .05 were considered statistically different.
Table 1.
Patient, Disease, and Transplant Characteristics
| Allogeneic HCT | Autologous HCT | |
|---|---|---|
| Total no. of patients (n = 66) | 28 | 38 |
| Median age, years (range) | 51.5 (39–66) | 63 (34–73) |
| Gender | ||
| Male | 23 (82%) | 30 (79%) |
| Stage at diagnosis, n (%) | ||
| III | 4 (14%) | 1 (3%) |
| IV | 20 (72%) | 36 (95%) |
| Bone marrow involvement at diagnosis, n (%) | 21 (75%) | 32 (84%) |
| Extranodal involvement, n (%) | 10 (45%) | 16 (43%) |
| Blastoid variant, n (%) | 2 (7%) | 5 (15%) |
| MIPI score, n (%) | ||
| Low | 8 (28%) | 12 (32%) |
| Intermediate | 10 (45%) | 15 (41%) |
| High | 6 (23%) | 10 (27%) |
| Missing | 4 (14%) | 1 (3%) |
| Number of regimens pre-HCT, n (%) | ||
| 1 | 11 (39%) | 27 (71%) |
| 2 | 7 (25%) | 6 (16%) |
| ≥3 | 10 (36%) | 5 (13%) |
| Rituximab during induction, n (%) | 24 (86%) | 34 (89%) |
| Initial chemotherapy, n (%) | ||
| CHOP | 20 (71%) | 25 (66%) |
| HyperCVAD/MAC | 5 (18%) | 10 (26%) |
| Other | 3 (11%) | 3 (8%) |
| Response to initial chemotherapy, n (%) | ||
| CR or PR | 21 (75%) | 31 (86%) |
| SD | 0 | 1 (3%) |
| Progression | 7 (25%) | 4 (11%) |
| Elevated LDH pre-HCT, n (%) | 6 (23%) | 4 (11%) |
| Elevated B2 microglobulin pre-HCT, n (%) | 2 (7%) | 12 (33%) |
| Time from diagnosis to HCT, mo (range) | 17.73 (4.8–121.13) | 7.6 (3.7–75.1) |
| Prior autologous HCT, n (%) | 5 (18%) | 0 |
| Disease status at HCT, n (%) | ||
| CR1/PR1 | 2/4 (7%/14%) | 23/6 (61%/16%) |
| ≥CR2/PR2 | 3/6 (11%/21%) | 2/2 (5%/5%) |
| PIF-sensitive | 7 (25%) | 5 (13%) |
| Resistant | 6 (23%) | 0 |
| Chemosensitivity, n (%) | 22 (77%) | 38 (100%) |
| Conditioning, n (%) | ||
| Myeloablative | ||
| CY/TBI | 4 (14%) | 36 (94%) |
| CY/etoposide/Carmustine | 2 (6%) | |
| Flu/CY/TBI | 8 (82%) | |
| CY/Bu | 1 (4%) | |
| Reduced intensity | 15 (54%) | 0 |
| Source of stem cells, n (%) | ||
| Matched related donor | 17 (61%) | 0 |
| Umbilical cord blood | 10 (36%) | 0 |
| Matched unrelated donor | 1 (4%) | 0 |
| Autologous | 0 (0%) | 38 (100%) |
| Bone marrow involved prior HCTa, n (%) | ||
| Positive | 5 (18%) | 2 (5%) |
| Negative | 21 (75%) | 34 (90%) |
| Missing | 2 (7%) | 2 (5%) |
| PET/CT assessment prior to HCT, n (%) | ||
| Positive | 12 (43%) | 11 (29%) |
| Negative | 3 (11%) | 23 (61%) |
| PET missing | 13 (46%) | 4 (10%) |
| Residual disease prior to HCTb, n (%) | ||
| Positive | 15 (54%) | 13 (35%) |
| Negative | 2 (7%) | 21 (55%) |
| Missing | 11 (39%) | 4 (10%) |
| Median follow-up, years (range) | 6.7 (1–10.3) | 3.5 (1.1–10.2) |
Abbreviations: Bu = busulfan; CHOP = cyclophosphamide, vincristine, Adriamycin, prednisone; CR = complete remission; CY = cyclophosphamide; Flu = fludarabine; HCT = hematopoietic cell transplantation; HyperCVAD = hyperfractionated cyclophosphamide, vincristine, Adriamycin, dexamethasone; LDH = lactate dehydrogenase; MAC = myeloablative conditioning; MIPI = mantle cell international prognostic index; PET/CT = positron emission tomography/computed tomography; PIF = primary induction failure; PR = partial remission; SD = stable disease; TBI = total body irradiation.
Assessed by morphology, flow cytometry, and molecular assays.
RD positivity is defined as pre-HCT positive PET/CT and/or bone marrow involvement.
Results
Patient Characteristics
Patient, disease, and graft characteristics are reported in Table 1. Two-thirds of autologous HCT recipients were > 60 years of age, and most had stage III–IV disease and marrow involvement at diagnosis. Most patients were in first remission (first complete remission (CR1) 61%, first partial remission (PR1) 16%) and all were chemosensitive. The median age of allogeneic recipients was 51 years, most patients had advanced MCL, 5 allogeneic recipients received prior autologous HCT, half received ≥ 2 chemotherapy regimens, only 2 were in CR1, and 23% were chemoresistant. Allogeneic recipients received similar proportions of RIC (54%, n = 15) and MAC (46%, n = 13) but a greater proportion of related donor grafts (61%, n = 17) than UCB (36%, n = 10). In both groups, about half had extranodal MCL at diagnosis and most patients received a rituximab (R) and CHOP (cyclophosphamide, Adriamycin, vincristine, prednisone) induction regimen. The time from transplant to diagnosis in allogeneic and autologous groups was 17 months and 7 months, respectively. The median follow-up for autologous and allogeneic groups was 3.5 years and 6.7 years, respectively.
RD in HCT Recipients
We categorized patients into RD+ or RD− groups on the basis of pre-HCT PET/CT scans and pre-HCT bone marrow evaluation. Most autologous recipients had negative PET/CT (n = 23) and marrow (n = 34) results. Pre-HCT MCL was detected in the bone marrow of 2 patients (5%; 1 by morphology and 1 by flow cytometry; both had negative PET/CT results) and on PET/CT scan in 11 patients (29%). As a result, more than half of auto-HCT recipients were RD− (55%) and 35% were RD+ (Table 1). In allogeneic recipients, MCL was detected prior to HCT in the bone marrow of 5 patients (3 by morphology and 2 by flow cytometry or molecular assay) and on PET/CT scan in 12 out of 15 patients in whom PET/CT was performed. Thus, 54% of allogeneic HCT recipients were RD+ and 7% were RD− (Table 1). Only CT but no PET data were available for 10% of auto-HCT recipients and 39% of allogeneic HCT recipients (missing RD).
Outcomes After Autologous Transplantation
At 5 years, the DFS and OS for all patients undergoing an autologous transplant were 59% (95% CI, 37%–76%) and 62% (95% CI, 39%–79%), respectively, with a cumulative incidence of relapse at 5 years of 29% (95% CI, 11%–48%). At 2 years, all relapses occurred in RD+ patients (31%; 95% CI 6%–55%) compared with no relapse in the RD− group (P = .02). All relapses (n = 4) in the RD group occurred after 2 years, yet DFS at 5 years in RD+ patients remained significantly lower than that of the RD− group (46% vs. 68%; P = .04) (Fig. 1A). Patients with a positive PET/CT scan (and negative marrow result; n = 21) had an increased 2-year relapse rate compared with that of those with a negative PET/CT scan (HR 0.02; P< .01).
Figure 1.
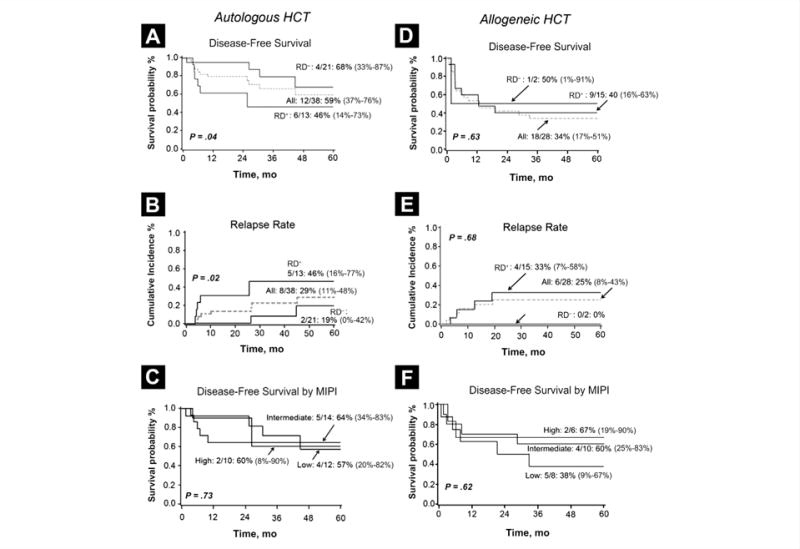
Outcomes Following Autologous Hematopoietic Cell Transplantation. (A) Disease-Free Survival by Residual Disease Status; (B) Cumulative Incidence of Relapse at 5 Years by Residual Disease. (C) Disease-Free Survival by Low, Intermediate, and High MIPI; Outcomes Following Allogeneic HCT. (D) 5-Year Disease-Free Survival by Residual Disease. (E) Cumulative Incidence of Relapse at 5 Years by Residual Disease. (F) Disease-Free Survival by Low, Intermediate, and High MIPI
Abbreviations: HCT = hematopoietic cell transplantation; MIPI = Mantle Cell Lymphoma International Prognostic Index; RD = residual disease.
Given the late relapses in patients with a negative PET/CT scan, the 5-year DFS rates of PET/CT+ and PET/CT− groups were similar (66% [95% CI, 34%–86%] vs. 55% [95% CI, 16%–82%]; P = .21). Of 2 patients with marrow disease detected by flow cytometry, 1 relapsed and 1 died of NRM.
In univariate analysis of all autologous transplant recipients, DFS was significantly lower in patients older than 60 years (43%; 95% CI, 18%–66%) compared with that in patients younger than 60 years (92%; 95% CI, 57–99%; P = .046), in patients with extranodal MCL at diagnosis (25%; 95% CI, 2%–63%) vs. patients without extranodal disease (77%; 95% CI, 48%–91%; P = .02), and in patients with a ≥ 8-month period from diagnosis to HCT (32%; 95% CI, 7%–62%) than in patients with < 8 months from diagnosis to HCT (79%; 95% CI, 51%–92%; P = .03). Patients treated with rituximab had improved DFS compared with that of patients who received no rituximab (66% [95% CI, 41%–83%] vs. 0%; P < .01). Remission status (CR1/PR1 vs. other), type of induction chemotherapy (CHOP vs. cytarabine-containing), or MIPI score had no influence on relapse, OS, or DFS rate (Fig. 1C). The 1-year NRM after autologous HCT was 5% (95% CI, 0%–12%).
In the multivariate analysis adjusted for age, extranodal MCL, MIPI, and time from diagnosis to HCT, RD+ status was associated with increased risk for poor OS (hazard ratio [HR], 3.9; P = .06) (Table 2)and DFS(HR, 5.6; P = .02). The only other variable associated with worse DFS in auto-HCT recipients was age > 60 years (HR, 10; P = .04) (Table 2).
Table 2.
Outcomes After Autologous and Allogeneic Hematopoietic Cell Transplantation: Multivariate Analysis
| Parameter | HR | 95% CI | P Value |
|---|---|---|---|
| Autologous HCT | |||
| Overall survival | |||
| RD Status (positive) | 1.82 | 0.28–11.73 | .53 |
| Age ≥60 years | 3.31 | 0.38–28.81 | .28 |
| Diagnosis to HCT >252 d | 3.27 | 0.43–24.81 | .25 |
| MIPI intermediate or high | 2.12 | 0.37–12.20 | .40 |
| Disease-free survival | |||
| RD Status (positive) | 5.60 | 1.30–24.04 | .02 |
| Age ≥60 years | 10.05 | 1.12–90.39 | .04 |
| Diagnosis to HCT >252 d | 3.51 | 0.86–14.34 | .08 |
| MIPI intermediate or high | 1.53 | 0.31–7.63 | .60 |
| Transplant-related mortality | |||
| RD Status (positive) | 1.29 | 0.19–8.76 | .79 |
| Age >60 years | 0 | – | <.01 |
| Diagnosis to HCT >252 d | 0 | – | <.01 |
| MIPI intermediate or high | 0.89 | 0.10–8.13 | .92 |
| Relapse | |||
| RD Status (negative) | 0 | – | <.01 |
| Age <60 years | 0.22 | 0.02–2.17 | .20 |
| Diagnosis to HCT >252 d | 0.86 | 0.20–3.77 | .84 |
| MIPI low | 0 | – | <.01 |
| Allogeneic HCT | |||
| Overall survival | |||
| Chemosensitivity | 1.76 | 0.32–9.68 | .52 |
| RIC | 0.79 | 0.18–3.45 | .75 |
| MIPI intermediate or high | 0.43 | 0.07–2.55 | .35 |
| Diagnosis to HCT >252 d | 0.79 | 0.16–3.80 | .77 |
| Disease-free survival | |||
| Chemosensitivity | 1.17 | 0.26–5.33 | .84 |
| RIC | 1.08 | 0.32–3.68 | .90 |
| MIPI intermediate or high | 0.53 | 0.12–2.27 | .39 |
| Diagnosis to HCT >252 d | 0.64 | 0.17–2.40 | .51 |
| Comorbidity ≥3 | 0.85 | 0.17–4.32 | .84 |
| Transplant-related mortality | |||
| RIC | 0.21 | 0.03–1.68 | .14 |
| MIPI intermediate or high | 1.81 | 0.31–10.45 | .04 |
| Diagnosis to HCT >252 d | 0.51 | 0.06–4.21 | .54 |
| Comorbidity ≥3 | 0.23 | 0.01–3.53 | .29 |
| Relapse | |||
| Chemosensitivity | 2.25 | 0.34–14.71 | .40 |
| MAC | 0 | – | <.01 |
| MIPI intermediate or high | 1.42 | 0.13–15.26 | .77 |
| Diagnosis to HCT >252 d | 2.88 | 0.28–29.18 | .37 |
| Comorbidity ≥3 | 1.44 | 0.13–16.50 | .77 |
Shown are factors considered in Cox-model multivariate analysis. The hazard ratios with 95% confidence intervals (CI) and significance probability for each factor considered are shown. The reference groups were chemorefractory disease, myeloablative conditioning, low MIPI score, diagnosis to HCT time < 252 d, and comorbidity index of 1–2.
Abbreviations: HCT = hematopoietic cell transplantation; HR = hazard ratio; MAC = myeloablative conditioning; MIPI = Mantle Cell Lymphoma International Prognostic Index; RD = residual disease; RIC = reduced-intensity conditioning.
Outcomes After Allogeneic HCT
For all allogeneic HCT recipients, the 5-year DFS and OS were 34% (95% CI, 17%–51%) and 52% (95% CI, 32%–69%), respectively. The cumulative incidence of relapse at 5 years was 25% (95% CI, 8%–43%), and NRM at 2 years was 23% (95% CI, 7%–40%). Patients who were RD+ preallograft had DFS and relapse rates of 40% and 33%, respectively (Figs. 1D and 1E). The 5-year relapse and DFS rates of patients with a positive marrow result (n = 5) were 27% and 40%, respectively, and not different from the patients with a negative pre-HCT marrow evaluation (n = 23; 25% relapse and 31% DFS). There were not enough patients with a negative PET/CT result to estimate their outcomes, and therefore, we could not evaluate the prognostic impact of PET/CT and RD on allograft outcomes.
In univariate analysis, for the entire cohort of allogeneic HCT recipients, the relapse rate was significantly higher after RIC (45%; 95% CI, 18%–73%]) vs. MAC (0%; P = .03), and the 2-year NRM was lower after RIC (RIC 15% [95% CI, 0%–32%] vs. MAC 33% [95% CI 7%–59%]) resulting in similar OS at 5 years (RIC 53% [95% CI, 26%–74%] vs. MAC 51% [95% CI, 21%–75%]; P = .77). Prior autologous HCT and donor source did not influence survival (data not shown). Patients with intermediate or high vs. low MIPI score had similar relapse and survival rate, suggesting that allogeneic transplants may lead to long-term remission in some poor-risk MCL (Fig. 1F).
In multivariate regression analysis, the adjusted relapse and survival after allogeneic HCT were not influenced by clinical factors such chemosensitivity, type of conditioning, time for diagnosis to transplant, and comorbidity index (Table 2). Bone marrow involvement did not affect outcomes after allograft.
Discussion
Feasible prognostic tools for MCL are needed to identify patients at risk for rapid disease progression and stratify patients for therapeutic interventions. Here, we examined whether PET/CT scan in combination with marrow MCL assessment has a predictive value for transplant outcomes. Our results suggest promising survival with both autologous and allogeneic transplantation (OS 62% and 52% at 5 years). For patients consolidated with autologous HCT, RD+ was associated with higher risk of relapse and worse survival compared with that of RD− patients.
Patients with negative PET/CT results prior to transplant did not experience relapse in the first 2 years; however, because of late relapses (between years 2 and 5), the PET/CT scan alone was not independently associated with improved survival. Importantly, while RD+ and particularly PET/CT+ patients experienced early relapse more often, many still enjoyed prolonged survival. Our results also showed that older MCL patients were at high risk of poor outcomes mainly as a result of late relapse (beyond 2 years), but their NRM was not increased compared with that of younger patients. Therefore, patients with a positive PET/CT result and those above age 65 years should not be disqualified from autograft; rather, PET/CT result and age can be used as a stratifying tool to prevent relapse by selecting high-risk patients for alternative therapies such as maintenance rituximab or other targeted strategies peritransplant in the setting of a clinical trial.
The value of PET/CT imaging in MCL prognosis was also examined in a recent retrospective study of a cohort of 53 MCL patients treated with rituximab hyper-CVAD chemotherapy, which concluded the post-treatment, but not interim, PET/CT scan was associated with improved DFS.17
The benefit of autologous transplant after R-CHOP chemotherapy is based on evidence from randomized trial results.27 While most patients in our cohort received R-CHOP frontline induction, the recent reports from the GELA group and Nordic MCL2 trial highlighted the significant potency of cytarabine-based regimen in therapy for MCL.25,26 Although complete response (CR) was uncommon after 3 cycles of R-CHOP induction in an European MCL network trial (only 12%), 57% of patients were in CR after 3 subsequent cycles of R-DHAP (rituximab, dexamethasone, cytarabine, cis-platinum), and 5-year survival after autograft consolidation was 75%.26 While we showed similar transplant survival in patients treated with CHOP vs. cytarabine-containing induction regimen, 1 of the main questions in the field is whether transplant is needed for all patients who become PET/CT− after frontline therapy. This question should be tested in the future.
Notably, there is almost no data on the utility of pretransplant PET/CT scan in context of allogeneic HCT for MCL.28,29 Allogeneic HCT recipients are often heavily pretreated, have advanced disease, and more often have pretransplant marrow involvement and residual MCL on PET/CT imaging. Despite these differences, promising outcomes in the RD+ group suggest that allogeneic HCT may provide disease control through the combination of conditioning chemotherapy and a graft-versus-lymphoma effect. These results are consistent with favorable long-term outcomes of allograft for MCL observed by other groups.8,30 In a recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of advanced MCL, despite a refractory disease state, about 25% of MCL patients attained durable remissions after allogeneic HCT.31 Notably, because very few patients had a negative PET/CT scan prior to HCT, we cannot make any conclusions about the predictive value of PET/CT imaging for allograft outcomes.
The MIPI score has been validated in many studies as a tool for MCL risk stratification at diagnosis and remains a powerful tool for MCL prognostication.4,7,10,12 While we observed an encouraging survival of patients with high MIPI score after both autologous HCT and allogeneic HCT, given the retrospective nature of this series, it would premature to infer any additional inferences. Larger studies will be needed to study the association between MIPI and transplant outcomes.
Conclusion
The value of consolidation therapy for MCL in first remission remains a key issue for future prospective trials comparing transplant and nontransplant approaches. These trials should incorporate the risk-stratifying strategy using pre-HCT PET/CT imaging with centrally reviewed design. Our results suggest that PET/CT imaging and assessment of residual marrow MCL could be useful as a risk-stratifying tool to intensify consolidation and maintenance therapies for those patients at higher risk of relapse.
Clinical Practice Points
Prognostic value of PET imaging prior to consolidation with autologous and allogeneic donor transplantation for mantle cell lymphoma is not known.
Our results showed that the group which had the best benefit from autologous hematopoetic cell transplant were mantle cell lymphoma patients without evidence of residual disease on either pre-transplant PET or marrow evaluation.
Most allogeneic hematopoietic cell transplant recipients had advanced disease and residual lymphoma detected by PET/CT and/or marrow, yet the long-term survival was favorable (overall survival 40%).
These results suggest that mantle cell lymphoma patients achieving PET/CT negative status prior transplant should receive autologous HCT.
While choice of consolidative therapy for patients with residual mantle cell lymphoma prior to transplant includes both autologous or allogeneic HCT; the decision should be guided by patients age, health status, co-morbidities, donor options and patients preference.
Acknowledgments
We acknowledge the support from Minnesota Medical Foundation for E.M., editorial assistance of Michael Franklin and assistance with manuscript preparation by Carol Taubert. We thank patients and their families who received their treatment at University of Minnesota Bone Marrow Transplant Program.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–8. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Kluin-Nelemans HC, Bea S, et al. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: report of the 11th annual conference of the European Mantle Cell Lymphoma Network. Leuk Lymphoma. 2013;54:699–707. doi: 10.3109/10428194.2012.733882. [DOI] [PubMed] [Google Scholar]
- 3.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemo-immunotherapy with rituximab-hyperCVAD alternating with rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–8. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 4.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–14. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 6.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–92. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 7.Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–52. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Gouill S, Kroger N, Dhedin N, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multi-center experience. Ann Oncol. 2012;23:2695–703. doi: 10.1093/annonc/mds054. [DOI] [PubMed] [Google Scholar]
- 9.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–45. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 11.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–8. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29:3023–9. doi: 10.1200/JCO.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Johnson JL, Koval G, et al. Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: final results of CALGB 59909. Haematologica. 2012;97:579–85. doi: 10.3324/haematol.2011.050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottcher S, Ritgen M, Buske S, et al. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica. 2008;93:551–9. doi: 10.3324/haematol.11267. [DOI] [PubMed] [Google Scholar]
- 15.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 16.Gill S, Wolf M, Prince HM, et al. [18F]fluorodeoxyglucose positron emission tomography scanning for staging, response assessment, and disease surveillance in patients with mantle cell lymphoma. Clin Lymphoma Myeloma. 2008;8:159–65. doi: 10.3816/CLM.2008.n.019. [DOI] [PubMed] [Google Scholar]
- 17.Hosein PJ, Pastorini VH, Paes FM, et al. Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol. 2011;86:841–5. doi: 10.1002/ajh.22126. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JB, Hall NC, Ruppert AS, et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant. 2013;48:1212–7. doi: 10.1038/bmt.2013.46. [DOI] [PubMed] [Google Scholar]
- 19.Mato AR, Svoboda J, Feldman T, et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer. 2012;118:3565–70. doi: 10.1002/cncr.26731. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 21.Coad JE, Olson DJ, Christensen DR, et al. Correlation of PCR-detected clonal gene rearrangements with bone marrow morphology in patients with B-lineage lymphomas. Am J Surg Pathol. 1997;21:1047–56. doi: 10.1097/00000478-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mitha N, McGlennen RC. Methods to detect clonal gene rearrangements in lymphomas and leukemias. Methods Mol Med. 2001;49:189–209. doi: 10.1385/1-59259-081-0:189. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 24.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158:355–62. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 26.Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 27.Pott C, Hoster E, Delfau-Larue MH, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115:3215–23. doi: 10.1182/blood-2009-06-230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodero A, Crocchiolo R, Patriarca F, et al. Pretransplantation [18-F] fluorodeoxyglucose positron emission tomography scan predicts outcome in patients with recurrent Hodgkin lymphoma or aggressive non-Hodgkin lymphoma undergoing reduced-intensity conditioning followed by allogeneic stem cell transplantation. Cancer. 2010;116:5001–11. doi: 10.1002/cncr.25357. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JR, Bomanji JB, Peggs KS, et al. Prognostic role of PET scanning before and after reduced-intensity allogeneic stem cell transplantation for lymphoma. Blood. 2010;115:2763–8. doi: 10.1182/blood-2009-11-255182. [DOI] [PubMed] [Google Scholar]
- 30.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–42. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 31.Hamadani M, Saber W, Ahn KW, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2013;19:625–31. doi: 10.1016/j.bbmt.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


