Abstract
Syk and ZAP-70 nonreceptor protein tyrosine kinases (PTKs) are essential elements in several cascades coupling immune receptors to intracellular responses. The critical role of these kinases in promoting the propagation of intracellular signaling requires a tight regulation of their activity, thus the existence of a negative feedback loop regulating their expression can be hypothesized. Herein, we have investigated whether ubiquitin-dependent proteolysis could be a mechanism responsible for controlling the fate of Syk and ZAP-70 after their immunoreceptor-induced activation. We found that both Syk and ZAP-70 become ubiquitinated in response to aggregation of the low affinity Fc receptor for IgG (CD16) on human natural killer cells. We confirmed the identity of the major in vivo ubiquitinated kinase species by performing an in vitro ubiquitination assay. In addition, we found that after CD16 stimulation, ubiquitinated forms of Syk and ZAP-70 associate with the receptor complex. After CD16 engagement, we also observed a decrease in the stability of Syk and ZAP-70 PTKs that is counteracted by pretreatment with either proteasome or lysosomal inhibitors. Moreover, in the presence of the proteasome inhibitor, epoxomicin, we observed an accumulation of ubiquitinated forms of both kinases. Our findings provide evidence of ligand-induced ubiquitination of nonreceptor PTKs belonging to the Syk family and propose the ubiquitin-dependent proteasome-mediated degradation pathway as a mechanism for attenuating the propagation of intracellular signaling initiated by immune receptor engagement.
The activation of protein tyrosine kinases (PTKs) is an early essential event in the transduction of signals from multisubunit immunoreceptors, including the B and T cell receptors for antigen and the widely distributed receptors for the Fc portion of immunoglobulins (1–4). Even though these receptors do not possess intrinsic PTK activity, their clustering activates several classes of cytoplasmic PTKs, namely members of the Src, Csk, Tec, and Syk families (1–8). Biochemical and genetic studies have demonstrated that PTKs of the Src family are responsible for the phosphorylation of immunoreceptor-associated invariant subunits, which contain conserved immunoreceptor tyrosine-based activation motifs (ITAMs) within their cytoplasmic domains (9, 10). The phosphorylated ITAMs create binding sites for a second class of cytosolic PTKs belonging to the Syk family (3, 11). This PTK family comprises only two known members, Syk and ZAP-70 (7, 8). Syk is present in all hematopoietic cells, whereas ZAP-70 expression is restricted to T lymphocytes and natural killer (NK) cells. The structure of Syk and ZAP-70 is highly conserved and is characterized by the presence of tandem Src homology 2 domains, a linker region containing several tyrosine residues acting as docking sites for downstream effectors such as phospholipase C-γ, Vav, and Cbl (12–14), and a catalytic domain.
Recruitment of Syk and ZAP-70 PTKs to the immunoreceptor complex requires binding of both Src homology 2 domains to the phosphorylated ITAMs. This recruitment leads to tyrosine phosphorylation and conformational changes of Syk and ZAP-70, events required for the increase of enzymatic activity, which is essential for the propagation of signaling cascade (15–18).
The potency of Syk and ZAP-70 activity as a biochemical switch used by immunoreceptors for the control of many cellular functions requires a tight regulation. Although tyrosine phosphatases could revert the phosphorylation status of Syk and ZAP-70, thus turning off their activity, additional mechanisms of negative control may be hypothesized.
Ubiquitination is a posttranslational modification responsible for selective protein degradation (19–21). It has been proposed that the extent of ubiquitination can control the fate of proteins. The formation of at least a four-subunit-long multiubiquitin chain represents a recognition signal for proteasome degradation (20, 22); on the other hand, cell surface receptors modified by single or a few ubiquitin (Ub) molecules appear to be subjected to internalization and lysosomal degradation (23, 24).
The present study is addressed to investigate whether Syk family PTKs undergo ligand-induced ubiquitination and the potential role of this modification in controlling the intracellular level of these enzymes.
NK cells are especially suitable for this purpose because they express both Syk and ZAP-70 PTKs, which are rapidly activated by ligation of a number of activating receptors, including the low affinity Fc receptor for IgG (CD16), whose ligand binding α chain is coupled to the ITAM-containing ζ and γ subunits (25–27).
Materials and Methods
Chemical Reagents and Antibodies.
All chemicals and drugs, unless otherwise mentioned, were obtained from Sigma. The following mouse mAbs were used for immunofluorescence and cytofluorimetric analysis: anti-CD3 (Leu4), anti-CD16 (Leu11c), and anti-CD56 (Leu19), all of which were purchased from Becton Dickinson. Anti-CD16 (B73.1) and anti-CD56 (B159.5.2) mAbs were kindly provided by G. Trinchieri (Schering Plough, Dardilly, France) and B. Perussia (Jefferson Cancer Institute, Philadelphia), respectively. Goat anti-mouse IgG F(ab′)2 fragment (GAM) was purchased from Cappel (Cooper Biomedical). Anti-human ZAP-70 and anti-phosphotyrosine (anti-PY) 4G10 mAbs were purchased from Upstate Biotechnology; anti-Syk (IgG2a, sc-1240), anti-actin (IgG1, sc-8432), anti-ζ (IgG1, sc-1239), and anti-Ub (polyclonal IgG, sc-9133) were purchased from Santa Cruz Biotechnology; anti-Ub mAb FK2 (PW8810) and the proteasome inhibitor epoxomicin were purchased from Affinity Research Products (Mamhead, Exeter, U.K.). Rabbit reticulocyte lysate (L415/1–3) was purchased from Promega.
Preparation of Human NK Cells.
Human NK cell cultures were obtained by coculturing nylon nonadherent peripheral blood mononuclear cells (4 × 105 cells/ml) with irradiated (3,000 rads) RPMI 8866 cells (1 × 105 cells/ml) for 10 days at 37°C in a humidified 5% CO2 atmosphere as described (28, 29). On day 10, the cell population was routinely 80–95% CD56+, CD16+, and CD3− as assessed by immunofluorescence and cytofluorimetric analysis. The experiments were performed on NK cell populations that were >90% pure.
Cell Stimulation and Lysate Preparation.
Highly purified cultured human NK cells were incubated with anti-CD16 mAb (B73.1, 1 μg/106 cells) for 30 min on ice. After washing off unbound antibody, cells were resuspended at 108/ml in prewarmed RPMI 1640 medium, and GAM (1.5 μg/106 cells) was added for the indicated length of time at 37°C. Cells (5 × 107/ml) were then lysed in a buffer containing 1% Triton X-100/50 mM Tris⋅HCl, pH 8/150 mM NaCl/2.5 mM EGTA, pH 8/2.5 mM EDTA, pH 8/1.5 mM MgCl2/1 mM PMSF/1 mM Na3VO4/50 mM NaF, pH 8/5 mM N-ethyl-maleimide and 1 μg/ml each of aprotinin, leupeptin, and pepstatin, as described (29). Lysates were cleared of debris by centrifugation at 15,000 × g for 15 min, and the protein concentration was determined by using the Bradford protein assay (Bio-Rad). For experiments requiring proteasome and lysosomal inhibitors, cells were pretreated with 10 μM epoxomicin for 4 h or 20 mM NH4Cl for 8 h, respectively. For experiments requiring inhibition of protein synthesis, cells were pretreated overnight with 10 μg/ml cycloheximide and then with 10 μM epoxomicin for the last 4 h or 20 mM NH4Cl for the last 8 h. Cell viability was >90% before stimulation.
Immunoprecipitation, Electrophoresis, and Immunoblotting.
Immunoprecipitation was performed as described (29). Immunoprecipitates were washed five times with lysis buffer, and bound proteins were eluted with SDS-sample buffer, resolved by SDS/PAGE on standard or minigels (NOVEX, San Diego) and transferred electrophoretically to nitrocellulose filters.
After blocking nonspecific reactivity, filters were probed with specific antibodies diluted in TBS-T (20 mM Tris⋅HCl, pH 7.8/150 mM NaCl/0.05% Tween 20). After extensive washing, immunoreactivity was detected by using an enhanced chemiluminescence detection kit (Amersham Pharmacia).
In Vitro Ubiquitination Assay.
Anti-ZAP-70 and anti-Syk immunoprecipitates, used as source of substrate for in vitro ubiquitination, were washed five times with lysis buffer, once with ubiquitination buffer (50 mM Tris⋅HCl, pH 7.5/0.5 mM MgCl2/0.1 mM ATP/0.1 mM DTT/1 mM creatine phosphate), and incubated in 40 μl of the same buffer supplemented with 70% (vol/vol) rabbit reticulocyte lysates, 10 units of creatine phosphokinase, and 10 μg of Ub for 2 h at 30°C. After in vitro ubiquitination, all samples were washed three times with lysis buffer, eluted with SDS-sample buffer, resolved by SDS/PAGE, and transferred electrophoretically to nitrocellulose filters.
Results
Syk and ZAP-70 Tyrosine Kinases Become Ubiquitinated After CD16 Engagement on Human NK Cells.
We first investigated whether Syk and ZAP-70 tyrosine kinases were subjected to ubiquitination upon CD16 engagement.
Cultured NK cells, unstimulated or stimulated with anti-CD16 mAb for the indicated length of time, were lysed and immunoprecipitated with anti-Syk or anti-ZAP mAb (Fig. 1 A and B, respectively). Immunoprecipitates were resolved by 8% SDS/PAGE, transferred to nitrocellulose, and immunoblotted with the same mAbs used for immunoprecipitation, anti-PY, or anti-Ub mAb.
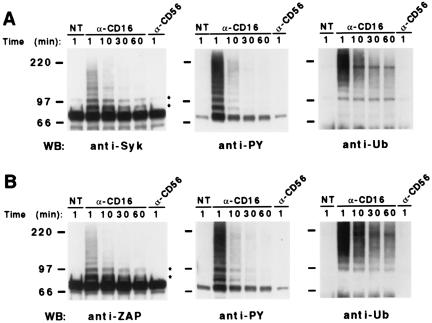
Figure 1.
CD16 engagement induces ubiquitination of Syk and ZAP-70 tyrosine kinases on human NK cells. Cultured NK cells (4 × 107/sample) were left untreated (NT) or incubated with anti-CD16 (B73.1) or anti-CD56 mAb for 30 min at 4°C, stimulated with GAM at 37°C for the indicated length of time, and lysed. All samples were immunoprecipitated with anti-Syk (A) or anti-ZAP (B) mAb, split in two, resolved by 8% SDS/PAGE, and transferred to nitrocellulose. One membrane was immunoblotted with anti-Syk or anti-ZAP (Left) and the second one was sequentially immunoblotted, after stripping, with anti-Ub (Right) and anti-PY (Center) mAbs. These results represent one of three independent experiments. The positions of molecular weight markers and IgH are indicated. The two major modified kinase species are indicated by *.
Besides the main form migrating around 70 kDa, additional bands of higher molecular mass reacting with anti-Syk and anti-ZAP mAbs are rapidly induced after receptor engagement (1 min) and are still evident at 1 h of stimulation (Fig. 1 Left). Most of these induced forms (indicated by asterisks) show a regular increase of the molecular weight and are tyrosine phosphorylated as demonstrated by anti-PY immunoblot (Fig. 1 Center). Immunoblotting with anti-Ub mAb (Fig. 1 Right) reveals that receptor triggering induces the appearance of Syk and ZAP-70 molecular species modified by a few Ub molecules, together with a smear in the high molecular weight region, characteristic of multiubiquitination (29). Similar results were obtained by using a polyclonal anti-Ub Ab (data not shown). Control samples treated with anti-CD56 mAb were comparable with untreated samples. The same immunoprecipitates blotted with anti-Lck mAb do not show any reactivity in the 70-kDa Mr range or higher (data not shown).
To confirm the identity of the ubiquitinated kinase forms observed in vivo, we performed an in vitro ubiquitination assay by using Syk and ZAP-70 as substrate immunoprecipitated from unstimulated NK cells (Fig. 2 A and B, respectively). Protein G-Sepharose beads conjugated with anti-Syk or anti-ZAP mAb alone were used as negative control (Fig. 2, lanes 1–3). All samples were incubated with ubiquitination reaction buffer in the presence (Fig. 2, lanes 2, 3, 5, and 6) or absence (Fig. 2, lanes 1 and 4) of rabbit reticulocyte lysate added (Fig. 2, lanes 3 and 6) or not (Fig. 2, lanes 2 and 5) with Ub and subjected to in vitro ubiquitination. The samples were then resolved by SDS/PAGE and blotted with anti-Syk (Fig. 2A Top) or anti-ZAP (Fig. 2B Top) mAb. Several modified species of both kinases were induced in vitro. The same membranes stripped and reblotted with anti-Ub mAb confirm the presence of ubiquitinated kinase species (Fig. 2 A and B, Middle). The most prominent of these ubiquitinated forms (indicated by asterisks) correspond to the main forms of Syk and ZAP-70 induced by CD16 engagement, clearly indicating that the increase in the molecular mass of both kinases is because of their ubiquitination. Similar results also were obtained by using purified fractions I and II of reticulocyte lysates containing the enzymes responsible for Ub conjugation (data not shown). A basal level of Syk and ZAP-70 tyrosine phosphorylation, which is not affected by in vitro ubiquitination, is revealed by an anti-PY blot (Fig. 2 A and B, Bottom). The result suggests that, under the experimental conditions used in vitro, this basal level of Syk and ZAP-70 phosphorylation is sufficient to promote kinase ubiquitination. When the same assay was performed by using as substrates Syk and ZAP-70 immunoprecipitated from stimulated NK cells, besides the presence of ubiquitinated kinase forms induced in vivo, a more extensive in vitro ubiquitination was obtained (data not shown). This result supports a role for tyrosine phosphorylation in controlling the extent of kinase ubiquitination.
Figure 2.
In vitro ubiquitination of Syk and ZAP-70 kinases. Syk (A) and ZAP (B) immunocomplexes from unstimulated NK cells (lanes 4–6) or protein G-Sepharose beads conjugated to anti-Syk (A) or anti-ZAP (B) mAb (lanes 1–3) were incubated with the ubiquitination reaction buffer at 30°C for 2 h in the presence or absence of rabbit reticulocyte lysates and added Ub, as indicated. Samples were washed with lysis buffer, resolved by 8% SDS/PAGE, and first immunoblotted with anti-Syk (A, Top) or anti-ZAP (B, Top) mAb, and after stripping sequentially reprobed with anti-Ub (Middle) and anti-PY (Bottom) mAbs. The positions of molecular weight markers, IgH, and ubiquitinated kinase species are indicated. These results represent one of three independent experiments.
An accumulation of Syk and ZAP-70 molecular species modified by a few Ub molecules was observed when in vitro ubiquitination was performed in the presence of Ub mutated in the lysine 48 (K48R), the amino acid residue involved in the formation of multiubiquitinated chains (data not shown).
Ubiquitinated Forms of Syk and ZAP-70 Coprecipitate with Tyrosine-Phosphorylated ζ Chain After CD16 Engagement.
CD16 engagement on human NK cells can rapidly induce the recruitment of both Syk and ZAP-70 PTKs to receptor complex, leading to their phosphorylation and activation (25–27, 30). To investigate whether Syk and ZAP-70 ubiquitinated forms coprecipitate with the CD16-associated ζ chain, lysates from NK cells unstimulated or stimulated with anti-CD16 mAb for the indicated length of time were immunoprecipitated with anti-ζ mAb. Immunoprecipitates were resolved by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-Syk, anti-ZAP, or anti-Ub mAb (Fig. 3A). Both kinases coprecipitated with the ζ chain also in the untreated sample. Receptor stimulation led to a further recruitment of the kinases, as demonstrated by an increased amount of the 70-kDa Syk and ZAP-70 species. In addition, several ubiquitinated forms of both kinases coprecipitated with the ζ chain and were more evident after 1 min of stimulation; this result correlates with the kinetics reported in Fig. 1.
Figure 3.
Ubiquitinated forms of Syk and ZAP-70 associate with CD16 complex after receptor engagement. Lysates of NK cells (2 × 108/sample) unstimulated (NT) or stimulated for the indicated length of time (as in Fig. 1) were immunoprecipitated with anti-ζ mAb, loaded in duplicate on 8% SDS/PAGE (A) and 4–20% gradient SDS/PAGE (B), and transferred to nitrocellulose. (A) The membrane was sequentially immunoblotted, after stripping, with anti-Ub (Right), anti-Syk (Left), and anti-ZAP (Center) mAbs. (B) The membrane was sequentially immunoblotted with anti-PY (Upper) and anti-ζ (Lower) mAbs. These results represent one of three independent experiments. The positions of molecular weight markers are indicated.
In Fig. 3B, the anti-PY blot of ζ immunoprecipitates (Upper) shows a basal level of phosphorylated ζ that could explain the association between ζ and the kinases observed in the untreated sample. After CD16 engagement, the increase of ζ tyrosine phosphorylation correlated with a further recruitment of both kinases. An equal amount of ζ chain was immunoprecipitated in each lane, as demonstrated by anti-ζ blot (Fig. 3B, Lower).
These data strongly suggest that after CD16 engagement, both ubiquitinated and nonubiquitinated Syk and ZAP-70 PTKs associate with the receptor complex through the tyrosine-phosphorylated ζ subunit.
Expression of Syk and ZAP-70 Decreases After CD16 Engagement.
To investigate whether receptor engagement affects Syk and ZAP-70 stability, we analyzed the steady-state levels of both PTKs by Western blot of whole-cell lysates. NK cells were pretreated with cycloheximide to block protein synthesis, and kinase expression levels were analyzed at different times of stimulation (Fig. 4). Both PTKs were stable in the absence of CD16 stimulation, their half-life being ≈10 h. After stimulation, the expression of Syk and ZAP-70 remained substantially stable during the first 6 h, began to decrease (40–50%) after 8 h, and was reduced more than 70% after 12 h.
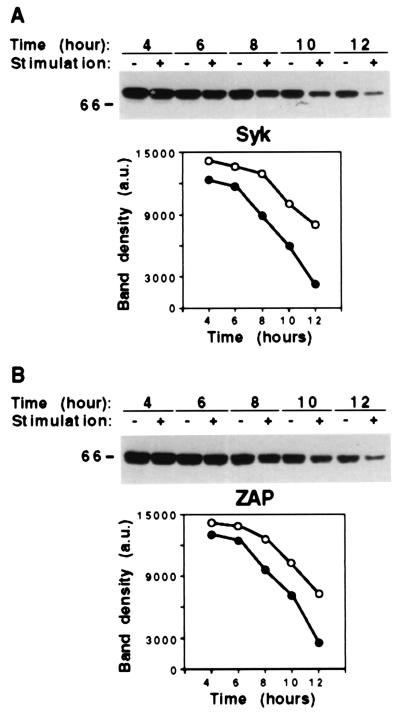
Figure 4.
CD16-induced decrease of Syk and ZAP-70 kinase stability. Cultured NK cells were pretreated overnight with cycloheximide at the final concentration of 10 μg/ml. Cells were then incubated with anti-CD16 mAb, stimulated with GAM, and lysed after the indicated times. Total cell lysates (20 μg) from each sample were separated by 8% SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-Syk (A) or anti-ZAP (B) mAb. These results represent one of three independent experiments. The positions of molecular weight markers are indicated. Densitometric analysis of the bands from unstimulated (●) or stimulated (○) samples are reported in the graphs.
These results indicate that CD16 engagement promotes a more rapid degradation of Syk and ZAP-70.
CD16-Induced Syk and ZAP-70 Degradation Is Sensitive to Proteasome and Lysosomal Inhibitors, but Ubiquitinated Kinase Forms Accumulate Only in the Presence of Proteasome Inhibitors.
To determine the cell compartment (proteasome vs. lysosome) involved in the ligand-induced Syk and ZAP-70 degradation, we examined the effect of epoxomicin, a selective and irreversible inhibitor of the proteasome proteolytic activities (31), and of ammonium chloride (NH4Cl), which inhibits lysosome function (Fig. 5). Cells were pretreated or not with epoxomicin (Fig. 5A) or NH4Cl (Fig. 5B), stimulated with anti-CD16 mAb, and analyzed after 8 h for the expression of Syk and ZAP-70 by Western blot of whole-cell lysates. Both proteasome and lysosomal inhibitors almost completely prevented the receptor-triggering mediated PTK degradation. The blots were reprobed for actin to verify an equal loading of proteins. Similar results were obtained by using another specific proteasome inhibitor, MG132, and monensin, which is known to inhibit the lysosome function (data not shown).
Figure 5.
CD16-induced Syk and ZAP-70 degradation is sensitive to proteasome and lysosomal inhibitors. (Left) NK cells pretreated overnight with cycloheximide were incubated for the last 4 h in the presence of vehicle alone (dimethyl sulfoxide) or 10 μM epoxomicin (B), or for the last 8 h in the presence of medium alone or 20 mM NH4Cl (C). Unstimulated (−) or anti-CD16-stimulated (+) cells were lysed after 8 h, and 20 μg of whole-cell lysate from each sample was separated by 8% SDS/PAGE, transferred to nitrocellulose, and sequentially immunoblotted, after stripping, with anti-Syk, anti-ZAP, and anti-actin mAbs, as indicated. (Right) Quantitation was performed by densitometry. The results are expressed as relative protein level of anti-CD16-stimulated samples with respect to that of unstimulated cells. The error bars in the graphs represent the standard deviations of three independent experiments.
The ability of CD16 engagement to accelerate the degradation of the main kinase forms is evident only after 8 h in contrast to the rapid CD16-induced Syk and ZAP-70 ubiquitination. In an effort to find a more direct link between ubiquitination and degradation, we decided to correlate the two events following the fate of CD16-induced ubiquitinated kinase forms stimulating the cells for a shorter length of time and in the absence of cycloheximide. Cells were pretreated or not with epoxomicin for 4 h (Fig. 6A) or NH4Cl for 8 h (Fig. 6B), stimulated with anti-CD16 mAb for the indicated length of time, and analyzed for the presence of ubiquitinated kinase forms by Western blot of whole-cell lysates. Using anti-Ub mAb, we found accumulation of high molecular weight protein–Ub conjugates in proteasome-inhibited NK cells. The same blot reprobed with anti-Syk and anti-ZAP mAbs shows a specific accumulation of both ubiquitinated kinase forms.
Figure 6.
The proteasome inhibitor, epoxomicin, induces accumulation of ubiquitinated Syk and ZAP-70 kinase forms. NK cells were pretreated for 4 h in the presence of vehicle alone (dimethyl sulfoxide) or 10 μM epoxomicin (A) or 8 h in the presence of medium alone or 20 mM NH4Cl (B). Unstimulated (−) or anti-CD16-stimulated cells for the indicated length of time were lysed, and 20 μg of whole-cell lysate from each sample was separated by 8% SDS/PAGE, transferred to nitrocellulose, and sequentially immunoblotted, after stripping, with anti-Ub, anti-Syk, anti-ZAP, and anti-actin mAbs, as indicated. These results represent one of four independent experiments. The positions of molecular weight markers are indicated.
In the presence of NH4Cl, neither anti-Ub or anti-kinase blots revealed accumulation of ubiquitinated-kinase forms (Fig. 6B), although we have observed inhibition of the delayed ligand-mediated PTK degradation (see Fig. 5B). Similar results were obtained by using other lysosomal inhibitors (data not shown).
Taken together, our results indicate that upon CD16 engagement on human NK cells, both the proteasome and the lysosomal compartments contribute to the degradation of Syk and ZAP-70 kinases and that only the Ub/proteasome pathway appears to be responsible for the degradation of CD16-induced ubiquitinated kinase forms.
Discussion
In the present study, we demonstrate that both Syk and ZAP-70 tyrosine kinases undergo ubiquitination after CD16 engagement on human NK cells, thus providing evidence of ligand-induced ubiquitination of nonreceptor PTKs belonging to the Syk family. Ubiquitinated forms of these PTKs are also tyrosine phosphorylated (Fig. 1), suggesting that ubiquitination affects the activated forms of PTKs. This hypothesis is further supported by our preliminary evidence indicating a requirement of PTK activity for the induction of Syk and ZAP-70 ubiquitination (unpublished observations).
The involvement of Syk and ZAP-70 tyrosine phosphorylation in the control of their ubiquitination is also suggested by the results of the in vitro ubiquitination assay. A basal level of Syk and ZAP-70 tyrosine phosphorylation is sufficient to promote kinase ubiquitination that further increases with the extent of Syk and ZAP-70 tyrosine phosphorylation induced by receptor engagement.
These findings suggest that CD16 engagement could either promote ubiquitination of basal tyrosine-phosphorylated kinase forms or induce quantitative and/or qualitative changes of Syk and ZAP-70 phosphorylation required for kinase ubiquitination. It is likely that in vivo the recruitment of adaptor proteins and/or enzymes necessary to promote kinase ubiquitination requires the presence of new phosphorylation sites.
In accordance with our results, recent evidence has demonstrated that only the active forms of Blk and Src kinases are specifically targeted for a Ub-dependent degradation (32, 33). Ubiquitinated forms of Syk and ZAP-70 are also found associated with the phosphorylated ζ chain upon CD16 engagement.
Taken together, our results suggest that Syk and ZAP-70 ubiquitination may occur in proximity of the plasma membrane, after PTK recruitment and activation. A recent finding by Winberg et al. (34) demonstrates that after Epstein–Barr virus infection of a B cell line, the viral latent membrane protein 2A promotes ubiquitination of Lyn and Syk tyrosine kinases. On both their and our systems, kinase ubiquitination could thus represent a modification required to inactivate and/or target the kinases for degradation, thus attenuating the propagation of intracellular signaling. The result presented in Fig. 1 leaves open two possibilities. The rapid induction of kinase ubiquitination suggests that this modification may negatively affect the enzymatic activity and/or interfere with the recruitment of other signaling molecules; on the other hand, the persistence of ubiquitinated kinase forms suggests a role for this modification in Syk and ZAP-70 degradation. Although we are actually testing the first possibility, herein we provide evidence suggesting a direct correlation between CD16-induced kinase ubiquitination and degradation.
The analysis of the steady-state levels of Syk and ZAP-70 kinases has revealed the ability of CD16 engagement to accelerate the degradation of the main kinase forms (Fig. 4). This effect is reverted by both proteasome and lysosomal inhibitors (Fig. 5). In addition, the proteasome compartment seems to be responsible for a more rapid degradation of the ligand-induced ubiquitinated kinase forms, indicating a direct correlation between ubiquitination and proteasome degradation (Fig. 6). The evidence that ubiquitinated forms of Syk and ZAP-70 can be precipitated from the lysates after depletion of the ζ-associated PTKs (data not shown) suggests that modified kinases could be released from engaged receptor complexes and become accessible to proteasome degradation. In this regard, Geahlen and coworkers (35) have shown that activated forms of Syk are preferentially localized in the soluble fraction after BCR engagement. Even if the proteasome-dependent degradation we observed is restricted to a small fraction of kinases, it can be of functional relevance, as the degraded kinases are likely active.
The results presented in Fig. 5 indicate that the proteasome-dependent degradation we observed is not the only fate of ligand-activated kinases; they also localize in the lysosomes. Localization of activated Syk and ZAP-70 in the lysosomes is likely the result of ligand-induced CD16 receptor complex internalization. Signal transduction and endocytic/lysosomal transport are initiated simultaneously after immunoreceptor stimulation; however, the signals required for internalization of triggered receptors are not clearly defined yet (24). It has been demonstrated that Ub carries an internalization signal in its amino acid sequence that is sufficient to promote endocytosis of mono-ubiquitinated plasma membrane protein (36). We and others have demonstrated that different immunoreceptor subunits, including the CD16 ζ chain on human NK cells (29), become mono-ubiquitinated, and/or modified with short chains of Ub, after engagement of their respective receptors (37, 38). Thus, it is possible that mono-ubiquitination of Syk family PTKs and/or CD16 ζ subunit provides signals for activating the endocytic machinery, resulting in the localization of Syk and ZAP-70 together with the CD16 receptor complex in the lysosome compartment. This hypothesis is supported by recent findings demonstrating that Syk activation is necessary for the transport of internalized Fc receptors from endosome to lysosomes (39). Once the kinases reach the lysosomes, they could be degraded whether or not they are ubiquitinated.
Although we have established that the Ub/proteasome pathway is involved in limiting the expression of Syk and ZAP-70 kinases, the specific enzyme(s) responsible for their ubiquitination have yet to be determined. The enzymes that provide specificity to the Ub system are the Ub protein ligases or E3, which differ with regard to structure and/or class of signals they recognize (19–21). In particular, several RING finger proteins appear to serve as E3s, including Cbl, the product of the protooncogene c-Cbl (40). Cbl is a prominent PTK substrate detected in immune receptor-mediated signaling, and it has been shown to negatively regulate a number of PTKs, including Syk and ZAP-70 (41–45). The mechanism responsible for c-Cbl negative regulation of these kinases is not fully resolved, in part because of conflicting results. In c-Cbl-deficient cells, the activity of ZAP-70 is markedly enhanced, whereas the protein level is not substantially increased (44, 45); conversely, c-Cbl overexpression could enhance both Syk and ZAP-70 degradation (42, 43). Based on these observations, it remains to be determined whether c-Cbl can be the E3 ligase involved in Syk and ZAP-70 ubiquitination upon CD16 engagement.
Our finding suggests a role for ligand-induced Syk and ZAP-70 ubiquitination in controlling the rate of active PTK degradation, thus providing a mechanism for limiting the propagation of signal initiated by multisubunit immunoreceptors.
Acknowledgments
We are grateful to P. Birarelli, A. M. Bressan, B. Milana, A. Procaccini, and A. Sabatucci for expert technical assistance. This work was partially supported by grants from the Italian Association for Cancer Research, Istituto Superiore di Sanità Italy-USA “Therapy of Tumors” Program, and Ministero dell'Università e della Ricerca Scientifica e Tecnologica.
Abbreviations
- PTK
protein tyrosine kinase
- ITAM
immunoreceptor tyrosine-based activating motif
- NK
natural killer
- GAM
goat anti-mouse IgG F(ab′)2 fragment
- PY
phosphotyrosine
- Ub
ubiquitin
References
- 1.Weiss A. Cell. 1993;73:209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch J V. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 3.Cambier J C. J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 4.Kinet J P. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 5.Bolen J B, Brugge J S. Annu Rev Immunol. 1997;15:371–404. doi: 10.1146/annurev.immunol.15.1.371. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki T, Tsukada S. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 7.Chu D H, Morita C T, Weiss A. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 8.Turner M, Schweighoffer E, Colucci F, Di Santo J P, Tybulewicz V L. Immunol Today. 2000;21:148–154. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- 9.Reth M. Nature (London) 1989;338:383–384. [Google Scholar]
- 10.Cambier J C. Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 11.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 12.Sillman A L, Monroe J G. J Biol Chem. 1995;270:11806–11811. doi: 10.1074/jbc.270.20.11806. [DOI] [PubMed] [Google Scholar]
- 13.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 14.Lupher M L, Reedquist K A, Miyake S, Langdon W Y, Band H. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- 15.Iwashima M, Irving B A, Van Oers N S C, Chan A C, Weiss A. Science. 1994;263:1136–1139. [PubMed] [Google Scholar]
- 16.Kurosaki T, Takata M, Yamamashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura T, Sakamoto H, Appella E, Siraganian R. Mol Cell Biol. 1996;16:1471–1478. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hillal O, Kurosaki T, Yamamura H, Kinet J-P, Scharenberg A M. Proc Natl Acad Sci USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershko A, Ciechanover A. Annu Rev Biochem. 1998;68:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 20.Hochstasser M. Annu Rev Genet. 1996;4:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 21.Weissman A M. Immunol Today. 1997;18:189–198. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- 22.Thrower J S, Hoffman L, Rechteiner M, Pickart C. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicke L. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 24.Strous G J, Roland G. J Cell Sci. 1999;112:1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- 25.Sthals A, Liwszyc G E, Couture C, Mustelin T, Anderson L C. Eur J Immunol. 1994;24:2491–2496. doi: 10.1002/eji.1830241035. [DOI] [PubMed] [Google Scholar]
- 26.Ting A T, Dick C J, Schoon R A, Karnitz L M, Abraham R T, Leibson P J. J Biol Chem. 1995;270:16415–16421. doi: 10.1074/jbc.270.27.16415. [DOI] [PubMed] [Google Scholar]
- 27.Leibson P J. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 28.Perussia B, Ramoni C, Anegon I, Cuturi C, Faust J, Trinchieri G. Nat Immun Cell Growth Regul. 1987;6:171–188. [PubMed] [Google Scholar]
- 29.Paolini R, Serra A, Molfetta R, Piccoli M, Frati L, Santoni A. Eur J Immunol. 1999;29:3179–3187. doi: 10.1002/(SICI)1521-4141(199910)29:10<3179::AID-IMMU3179>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Brumbaugh K M, Perez-Villar J J, Dick C J, Schoon R A, Lopez-Botet M, Leibson P J. J Immunol. 1996;157:2804–2812. [PubMed] [Google Scholar]
- 31.Meng L, Mohan R, Kwok B H B, Elodsson M, Sin N, Crews C M. Proc Natl Acad Sci USA. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oda H, Kumar S, Howley P. Proc Natl Acad Sci USA. 1999;96:9557–9562. doi: 10.1073/pnas.96.17.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris K F, Shoji I, Cooper E M, Kumar S, Oda H, Howley P. Proc Natl Acad Sci USA. 1999;96:13738–13743. doi: 10.1073/pnas.96.24.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. Mol Cell Biol. 2000;20:8526–8535. doi: 10.1128/mcb.20.22.8526-8535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters J D, Furlong M T, Asai D J, Harrison M L, Geahlen R L. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- 36.Shih S C, Sloper-Mould K E, Hicke L. EMBO J. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cenciarelli C, Hou D, Hsu K-C, Rellahan B L, Wiest D L, Smith H T, Fried V A, Weissman A M. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- 38.Paolini R, Kinet J-P. EMBO J. 1993;12:779–786. doi: 10.1002/j.1460-2075.1993.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnerot C, Briken V, Brachet V, Lankar D, Cassard S, Jabril B, Amigorena S. EMBO J. 1998;17:4606–4616. doi: 10.1093/emboj/17.16.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joazeiro C A, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y C. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 41.Ota Y, Samelson L E. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 42.Lupher M L, Jr, Rao N, Lill N L, Andoniou C E, Miyake S, Clark E A, Druker B, Band H. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 43.Rao N, Lupher M L, Ota S, Reedquist K A, Druker B J, Band H. J Immunol. 2000;164:4616–4626. doi: 10.4049/jimmunol.164.9.4616. [DOI] [PubMed] [Google Scholar]
- 44.Murphy M A, Schnall R G, Venter D J, Barnett L, Bertoncello I, Thien C B F, Langdon W Y, Bowtell D D. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naramura M, Kole H K, Hu R-J, Gu H. Proc Natl Acad Sci USA. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]