Summary
It has been reported that functionally distinct cancer stem cells (CSCs) exist in human bladder cancer (BCa). Here, we found that Sox2, a transcription factor that is well characterized as a marker for stem cells, is upregulated in both mouse and human BCa. Sox2 expression is absent in normal urothelial cells, but it begins to be expressed in pre-neoplastic bladder tumors and continues to be expressed in invasive mouse BCa. Using s as a reporter of Sox2 transcriptional expression, we demonstrated that Sox2-expressing cells mark a subpopulation of tumor cells that fuel the growth of established BCa. SOX2-positive cells also expressed other previously reported BCa CSC markers, including Keratin14 (KRT14) and CD44v6. Ablation of Sox2-expressing cells within primary invasive BCa led to enhanced tumor regression, supporting the essential role of SOX2-positive cells in regulating BCa maintenance and progression. Our data show that Sox2 is a marker of bladder CSCs and indicate it as a potential clinical target for BCa therapy.
Keywords: bladder cancer, Sox2, cancer stem cell
Highlights
-
•
Sox2 expression marks bladder cancer stem cells in vivo
-
•
SOX2-positive cells are a subpopulation of KRT14-expressing cancer stem cells
-
•
SOX2-positive cells are a subpopulation of CD44v6-expressing cancer stem cells
-
•
Elimination of Sox2-expressing cells led to reduction of bladder cancer progression
In this article, Yang and colleagues assess the renewal and differentiation potential of Sox2-expressing cells in bladder cancer, by in vitro clonogenic assays, in vivo transplantation, and lineage-tracing experiments. Their data indicate that Sox2 expression marks the bladder cancer stem cells, which are necessary for tumor growth and maintenance.
Introduction
Bladder cancer (BCa) is the sixth most common malignancy in males worldwide, affecting ∼429,800 people annually and is responsible for ∼165,100 deaths (Torre et al., 2015). Treatment with chemotherapeutics or surgical resection for BCa usually fails due to the existence of cancer stem cells (CSCs). CSCs play a pivotal role in tumor recurrence and metastasis and make complete elimination of the tumor difficult. The ability of these bladder CSCs to drive cancer initiation and progression make them ideal targets for anticancer therapies. Improvement in clinical treatment strategies requires further understanding of the CSC population and their molecular biology.
Research on the phenotypic and functional properties of urothelial CSCs has revealed that they are not characterized by a one-marker-fits-all approach; instead, various markers, including the aldehyde dehydrogenase 1 family, member A1 (ALDH1A1), cytokeratin 14 (CK14), and CD44v6, are used to isolate CSCs from patient specimens and to successfully establish cancer cell lines (Chan et al., 2010, Ho et al., 2012a). These CSCs appear to differ considerably and may contribute to the heterogeneity of BCa (Hatina and Schulz, 2012).
The transcription factor SOX2 is a member of the SRY-related HMG-box (SOX) family. It plays an essential role in cell fate determination, thereby regulating developmental processes (Sarkar and Hochedlinger, 2013). Aberrant expression of SOX2 has been reported in many types of cancers and is correlated with the presence of CSCs (Ferone et al., 2016, Leis et al., 2012, Lundberg et al., 2016). As for BCa, SOX2 expression is associated with tumor progression and prognosis (Kitamura et al., 2013, Ruan et al., 2013). Nevertheless, SOX2-expressing cells have yet to be identified in the normal human urothelium, and definitive proof that SOX2-positive cells correspond to urothelial malignancies remains elusive.
Here, we provide unequivocal evidence that the expression of Sox2 is rare in the normal bladder epithelia but remarkably elevated in BCa of both mouse and human patient origin. SOX2+ cells isolated from BCa tissue had a much greater ability to reform secondary tumors in vivo and spheres in vitro compared with SOX2 cells. Lineage-tracing experiments showed that SOX2+ cells give rise to a spectrum of bladder tumors. SOX2+ cells are highly coincident with KRT14- and CD44v6-positive urothelial CSCs. Furthermore, ablation of SOX2+ cells in BCa by administration of tamoxifen to Sox2CreER:Rosa-DTA mice led to a strong regression of invasive BCa. These findings suggest that SOX2 marks a population of tumor cells necessary for tumor growth and maintenance in vivo and will inspire future studies regarding their role in bladder tumorigenesis and their use in medicine applications.
Results
Sox2 Expression Is Elevated in BCa Tissue
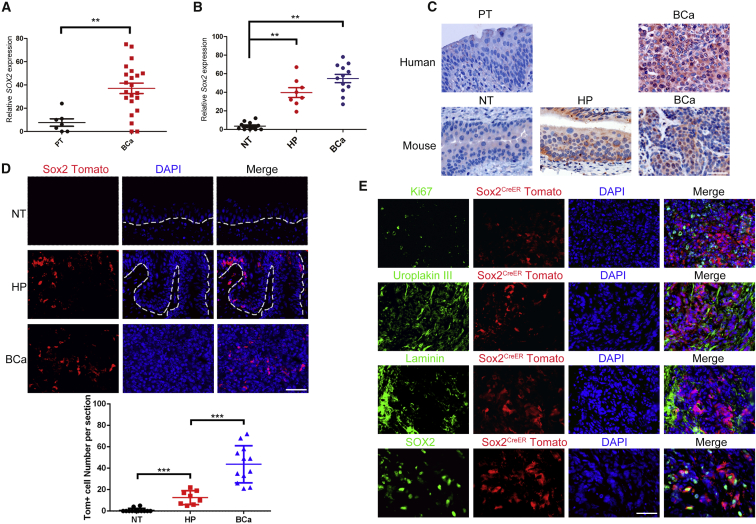
To gain insight into the role of Sox2 in BCa progression in vivo, we examined its expression in samples from a cohort of 22 BCa patients by immunohistochemistry (n = 22) and found Sox2 expression was significantly higher than in the para-tumor samples (n = 7; Figures 1A and 1C). We further crossed Sox2CreER mice with R26tdTomato mice and made use of an N-butyl-N-4-hydroxybutyl nitrosamine (BBN)-induced bladder carcinogenesis mouse model that shares molecular similarities with the human disease (Williams et al., 2008). Specifically, urothelial hyperplasia in mice (n = 8) was induced by administration of BBN for 14 weeks, whereas invasive BCa in mice (n = 12) was induced by administration of BBN for 26 weeks. We found that the expression of Sox2 in mice with urothelial hyperplasia and mice with invasive BCa was elevated compared with normal bladder tissue. In fact, Sox2 expression was hardly detected in the normal bladder tissue in mice but began to be broadly observed in hyperplasia tissue and BCa samples, ranging from several scattered cells to aggregated clusters (Figures 1B and 1C). When tamoxifen was applied to these tumor-bearing Sox2CreER:R26tdTomato mice as well as the non-treatment normal control mice, we found Sox2-tdTomato expression was absent in bladder sections from normal mice. In contrast, we began to observe Sox2-tdTomato in hyperplasia tissue and this was readily observed in BCa samples 3 days after tamoxifen injection (Figure 1D). Immunofluorescent staining with specific antibodies for SOX2 showed a bona fide Sox2 expression indicated by Tomato fluorescence. Moreover, Ki67 (a proliferation indicator) and Uroplakin III (a differentiation indicator) staining showed that SOX2+ cells (Tomato+) do not overlap with Ki67- and Uroplakin III-expressing cells, suggesting that SOX2+ cells in BCa may be quiescent and stem cell-like (Figure 1E).
Figure 1.
Sox2 Expression in Normal Bladder Tissue and BCa Samples
(A and B) Quantitative measurement of SOX2 in (A) human and (B) mice bladder tumor tissues (BCa, n = 22 for human samples, and n = 12 for mice samples), hyperplasia tissue (HP, n = 8), and normal tissues (NT, n = 12)/para-tumor tissue (PT, n = 7). The intensities of immunostaining were quantitatively measured using Image-Pro Plus 6.0 image analysis software. ∗∗p < 0.01, Student’s t test and one-way ANOVA.
(C) Representative image of immunohistochemical staining by SOX2 antibody in both human and mouse BCa samples and hyperplasia tissue (HP) compared with human para-tumor tissue (PT) and mouse normal bladder tissues (NT).
(D) Permanent labeling of SOX2+ cells by activation of a tdTomato transgene in samples from SOX2CreER;R26tdTomato mouse with tamoxifen injection at different stages of tumor progression (same sample numbers used as in B). ∗∗∗p < 0.001 by one-way ANOVA; dashed lines represent the basement membrane.
(E) Representative sections from the mouse BCa samples were stained by the indicated antibodies.
Scale bars, 50 μm.
Sox2 Expression Marks a Tumor-Propagating Population of BCa
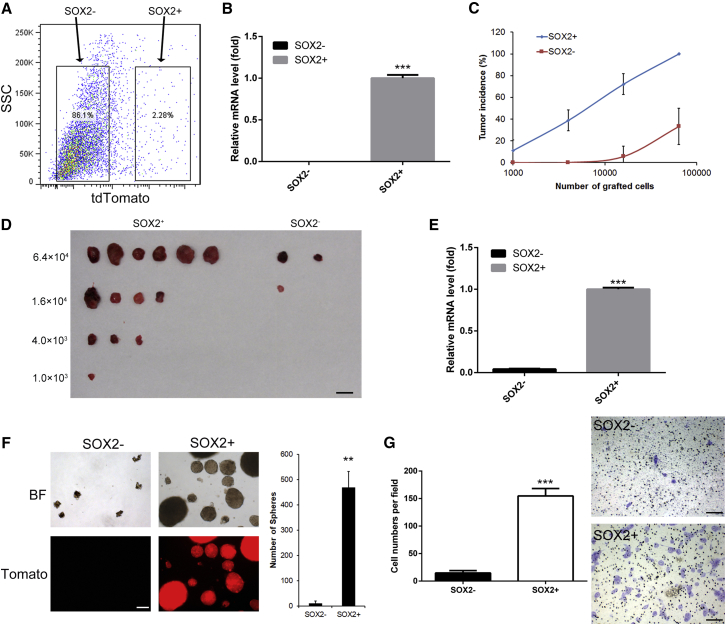
The current gold standard assay to evaluate CSC potential is to transplant highly purified and properly identified cancer cell populations in a limiting dilution fashion into immune-deficient mice to assess their ability to form secondary tumors (Beck and Blanpain, 2013). To investigate the tumorigenicity of SOX2+ cells in BCa, Sox2+ (Tomato+) and SOX2- (Tomato−) cells were isolated from mice with invasive BCa samples by flow cytometry (Figure 2A). The percentage of viable cells was examined using trypan blue staining for each group to exclude the effect of possible differences in cell viability after cell sorting on the following assays (Figure S1). Sox2 mRNA level in sorted tomato cells was hardly detected by qPCR (Figure 2B). SOX2+ and SOX2− cells were then injected subcutaneously into immune-deficient mice, and tumor formation was measured over time. SOX2+ cells exhibited a significantly higher tumor-propagating potential than the negative cells comprising the tumor bulk (Figures 2C and 2D). Not surprisingly, the Sox2 mRNA level was much lower in SOX2− xenograft samples than in SOX2+ xenografts (Figure 2E). In addition, these different populations were also seeded on low-attachment plates in clonogenic densities. After a 2-week culture period, we found that the SOX2+ cells produced many more spheres with ideal spherical shape and sharp edges than the SOX2− cells (Figure 2F, p < 0.01). Fluorescence microscopy also confirmed the sphere-forming capacity of SOX2+ cells. Moreover, we also assessed the invasiveness of the two cell types using a transwell chamber assay. The SOX2+ cells had significantly higher invasion potential than SOX2− cells (Figure 2G, p < 0.001). These results illustrate the clonogenic potential of SOX2+ cells from BCa in vivo and in vitro.
Figure 2.
SOX2 Marks BCa-Tumor-Propagating Cells
(A) Representative gating scheme with typical tomato+ and tomato− frequencies for FACS of a BCa tissue from Sox2CreER;R26tdTomato mice.
(B) mRNA levels of Sox2 were examined by qPCR in sorted Tom+/− cells, respectively.
(C and D) Percentage of tumor-free mice 5 weeks after subcutaneous injection of different dilutions of SOX2-positive or -negative cells into immunodeficient mice in triplicate experiments. In each replicate, 6 mice were used per dilution per condition (SOX2+, SOX2-). Image of a tumor from one dilution assay is shown in (D). Scale bars, 10 mm.
(E) mRNA levels of Sox2 were examined by qPCR from the tumor samples generated by SOX2+/− cells, respectively.
(F) Tomato-negative (SOX2−) and Tomato-positive (SOX2+) cells were FACS sorted and cultured in stem cell medium. Cultures at 10 days are shown, and the sphere numbers are plotted. BF, bright field. Scale bars, 250 μm.
(G) Matrigel invasion assays were performed with SOX2+/− cells (36 hr) to examine the effects of Sox2 expression on tumor cell invasion. Scale bars, 50 μm.
∗∗p < 0.01, ∗∗∗p < 0.001, t test. All error bars represent the SD of three independent experiments.
Genetic Labeling and Lineage Tracing of SOX2+ Cells in BCa
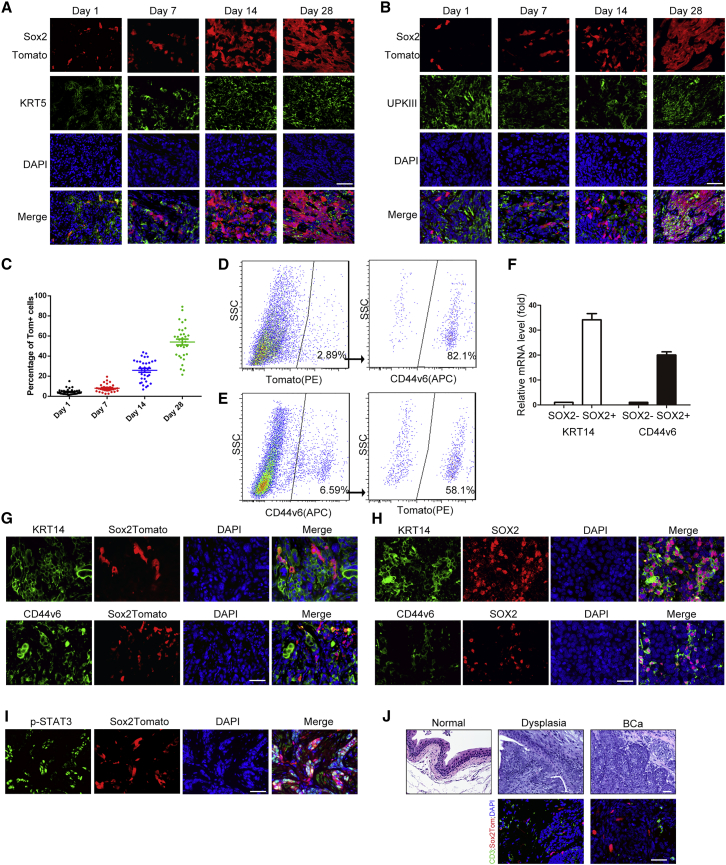
As shown in Figure 1E, we have generated Sox2CreER:R26tdTomato mice, and CreER expression is indicated by co-expression of Sox2 and Tomato in BCa samples. In the absence of tamoxifen administration, no labeled cells were observed in any BCa samples from these mice, demonstrating the absence of leakiness in these mice (data not shown). To investigate the role of SOX2+ cells in tumor maintenance and progression, 12 male Sox2CreER:R26tdTomato mice were exposed to BBN for 24 weeks, followed by a 4-mg dose of tamoxifen that was administered for 3 consecutive days. The mice were then killed on successive days following the tamoxifen regimen (3 mice per time point). Twenty-four hours after the final tamoxifen injection, Tomato-labeled SOX2+ cells were evident in the BCa tissue, and the frequency of positively labeled cells increased progressively (Figures 3A–3C). Twenty-eight days after the last tamoxifen injection, more than 50% of tumor cells were positive for Tomato, indicating that they were derived from the SOX2+ cells initially marked at the time of the injections (Figures 3A–3C). Immunofluorescent staining confirmed that some of these Tomato+ cells still expressed Sox2 (Figure S2), which further demonstrated the self-renewal capacity of this population in vivo. To determine the relationship with other known CSC markers, we co-stained with another in vivo CSC marker of BCa, KRT5 (Van Batavia et al., 2014). At the early stages, co-staining indicated that the SOX2+ cells seemed to arise from these KRT5+ stem cells. The SOX2+ cells then began to proliferate and differentiate until, after 28 days, there were large SOX2+ colonies, of which only some contained KRT5+ cells (Figure 3A). In contrast, the SOX2+ cells were initially negative for Uroplakin III 24 hr after the final tamoxifen injection yet, after 28 days, many of the SOX2+ cells were also positive for Uroplakin III (Figure 3B). These results support the notion that Sox2+ lineages in the BCa are capable of self-renewal and differentiation.
Figure 3.
SOX2+ Cells Are the Cells of Origin of BCa
(A and B) Representative images of tdTomato-labeled SOX2+, and Laminin (A) or Uroplakin III (B) stained cells at 1, 7, 14, and 21 days of tracing.
(C) Percentage of the Tom+ cells in the tumor area at each time point after tamoxifen injection, 12 fields for each mouse (3 mice per time point) were selected for calculation.
(D and E) Representative gating scheme with typical Tomato+ (PE) and CD44v6+ (APC) frequencies for FACS of BCa tissue from Sox2CreER;R26tdTomato mice.
(F–H) Relative mRNA level of Krt14 and CD44v6 of SOX2+/− cells isolated from BCa tissue from Sox2CreER;R26tdTomato mice. Error bars represent the SD of three independent experiments. Immunofluorescent staining on invasive BCa sections from mouse (G) and human patient samples (H), showing the SOX2+ cell population and extensive co-localization with KRT14 or CD44v6.
(I) Immunofluorescent staining on invasive BCa sections from mouse, showing the SOX2+ cell population and extensive co-localization with phosphorylated STAT3 (p-STAT3).
(J) H&E and immunofluorescent staining of BCa sections from mouse at different stages, showing histological change and infiltrated CD3+ T cells during the tumor development.
Scale bars, 50 μm.
Previous studies have reported that KRT5+, KRT14+, and CD44v6+ cells initiate BCa (Papafotiou et al., 2016, Shin et al., 2014, Van Batavia et al., 2014), and Krt5 expression absolutely coincided with Krt14 expression in BBN-induced BCa (Papafotiou et al., 2016). To further investigate the relationship between SOX2+ cells and other reported BCa progenitors, we first analyzed CD44v6 expression in fluorescence-activated cell sorting (FACS)-sorted Sox2-Tomato+ cells from BBN-induced BCa tissue and found that more than 90% of SOX2+ cells also expressed high levels of CD44v6 (Figure 3D). The CD44v6+ population of cells was therefore isolated from BCa tissue, and we found that, of those cells, about 60% were also SOX2+ (Figure 3E). In addition, we examined the mRNA levels of CD44v6 and Krt14 in both SOX2+/− cell populations. Using flow cytometry, we found that SOX2+ cells have a significantly higher expression of both CD44v6 and Krt14 than SOX2− cells (Figure 3F). We then stained the cells with KRT14 and CD44v6 antibodies from BBN-induced BCa samples in mice. Similar to the results of KRT5 staining (Figure 3A), Tomato+ (SOX2+) cells, after 3 days of tamoxifen injection, showed a high concurrence with KRT14- and CD44v6-stained cells. Nearly 100% of SOX2+ cells were also positive for KRT14 and CD44v6, while about 40% of KRT14-stained cells and 50% of CD44v6-stained cells were SOX2 positive (Figure 3G). Double staining of the sections from patient samples with SOX2 and KRT14 or CD44v6 antibodies also showed similar results (Figure 3H). Intriguingly, when a sphere assay was performed using CD44v6+Tomato + or CD44V4+Tomato− cells respectively, both groups had the ability to form spheres in vitro, but the CD44v6+Tomato+ group formed a significantly greater number of spheres, which were also significantly larger than the CD44v6+Tomato− group (Figure S3). To explore the mechanism of how Sox2 expression regulated the stemness of BCa stem cells, we further investigated the expression of SOX2 target genes that control tumor proliferation and metabolism and are also associated with stemness in other systems. Specifically, we investigated the expression of the following SOX2 target genes: Igf2bp2, Myef2, St6gal1, Msi2, Hmga2, Tp63, Ccnd1, Ccnd2, Ccnd3, Cdc25c, Tex15, Enpp1, Rap2b, Pcdh18, Alcam, Epha7, and Mgll (Boumahdi et al., 2014, Hutz et al., 2014, Santini et al., 2014). The mRNA levels of Myef2, St6gal1, Tp63, Ccnd3, Cdc25c, Pcdh18, and Epha7, were all elevated in CD44v6+/SOX2+ cells compared with CD44v6+/SOX2− cells (Figure S4), indicating the specificity of the SOX2-regulated genes in BCa stem cells. These data indicate that SOX2+ cells may be a subpopulation of KRT14- or CD44v6-marked CSCs and possibly further enable their stem-like capabilities.
Ho et al. (2012b) have shown that Stat3 activation in CSCs leads to tumor progression and is colocalized with KRT14 + CSCs in BBN-induced mouse BCa. To investigate the possible mechanism of Sox2 activation in BCa CSCs, we also stained the cells from BBN-induced BCa in mice. We found that STAT3 signaling was activated in most of the SOX2+ (Tom+) stem cells in the tumor (84.7% ± 9.1%, Figure 3I). STAT3 signaling in CSCs can be activated by chemokines such as interferon (IFN)-γ, interleukin (IL)-6 and IL-22 which are released by infiltrated T cells, other lymphocytes, and tumor cells (Yu et al., 2007). To explore whether extracellular signaling, especially tumor-infiltrated lymphocytes, participate in the activation of Stat3 activation in SOX2+ CSCs, we then examined the presence of CD3-labeled T cells during BCa tumor development. The results showed that lymphocytes were seldom seen in normal bladder tissue, while CD3+ T cells (and probably other lymphocytes) were extensively infiltrated into the tumor microenvironment throughout tumor progression (Figure 3J).
Ablation of Sox2-Expressing Cells Abrogates BCa Progression
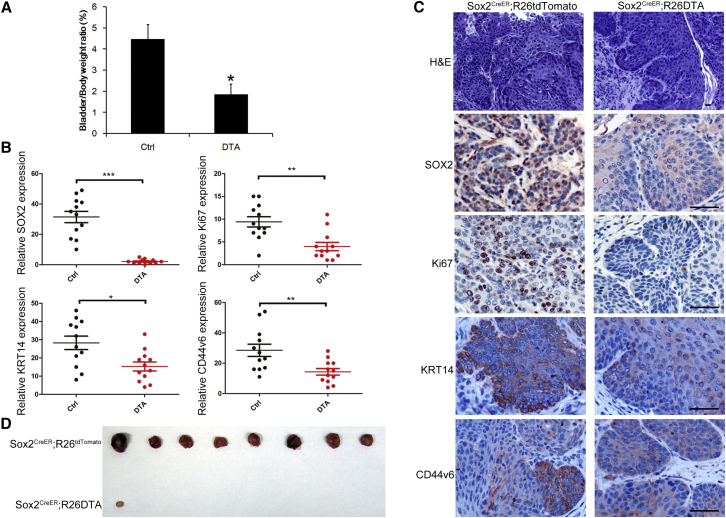
To further investigate the role of SOX2+ cells in BCa, we crossed Sox2CreER mice with a Rosa-DTA strain to evaluate the impact of SOX2+ cell lineage ablation on tumor growth and progression. We first induced BCa in Sox2CreER:Rosa-DTA mice using BBN administration for 26 weeks. To ablate the SOX2+ cells, we then injected the mice with tamoxifen for 3 consecutive days. Two weeks after the injections, we observed a marked regression in tumor size (n = 4, Figures 4A and 4C), along with a reduction in the KI-67+ cell ratio in the tumor lesion compared with the control group (Figures 4B and 4C). Moreover, while there were still KRT14+ and CD44v6+ cells remaining in the tumor lesion after Sox2 ablation (Figures 4B and 4C), they were present at a lower percentage than in the control mice. Furthermore, an equal number of cancer cells (1 × 107) from the induced BCa tissues of Sox2CreER:R26tdTomato and Sox2CreER:Rosa-DTA mice were transplanted into immune-deficient mice before treating them with tamoxifen for 3 consecutive days. After 3 weeks, all 8 recipients injected with BCa cells from Sox2CreER:R26tdTomato mice formed noticeable tumors (8/8, Figure 4D), while cells from Sox2CreER:Rosa-DTA mice generated only a single, small tumor in one nude mouse (1/8; Figure 4D). These results confirmed that SOX2 marks a population of tumor cells necessary for tumor growth and maintenance in vivo.
Figure 4.
Sox2 Lineage Ablation Leads to Regression of Pre-existing BCa
(A) The G/B ratio was calculated as (gross bladder weight/body weight) × 100% and analyzed with Student’s t test for both groups; n = 4, ∗p < 0.05.
(B) Quantitative measurement of SOX2, Ki67, KRT14, and CD44v6 expression in bladder tumor tissues (BCa) from Sox2CreER;R26tdTomato(Ctrl) or Sox2CreER;R26DTA(DTA) mice. Three different areas from each mouse (4 mice per group) were randomly picked, and the intensity of immunostaining was quantitatively measured using Image-Pro Plus 6.0 image analysis software. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p<0.001, Student’s t test.
(C) Representative histopathology and immunohistochemical staining of tumor tissues with indicated antibodies are shown.
(D) Tumor image showing secondary recipients 3 weeks after subcutaneous injection of the same number of tumor cells from Sox2 Tom or Sox2 DTA mice into immunodeficient mice.
Scale bars, 50 μm.
Discussion
Because cancers have a high hierarchical organization, mapping the fate of the stem and/or non-stem populations to determine the direction of hierarchies is important for cancer therapeutic implications. Many markers of BCa stem cells in vivo have been reported, which include CD44, Cytokeratin 5 (Krt5) (Chan et al., 2009, Van Batavia et al., 2014), Krt14 (Papafotiou et al., 2016), and sonic hedgehog (Shh) (Shin et al., 2014), all of which are expressed in the cells that arise from basal urothelium. Our findings suggest SOX2+ CSCs have a high coincidence rate with KRT14 and CD44v6+ cells, which indicates that the SOX2+ population in BCa may also be derived from the basal layer of the urothelium. However, lineage ablation of SOX2+ cells in BCa did not fully eliminate KRT14- and CD44-expressing cells, suggesting that SOX2+ stem cells may be a subpopulation of KRT14+ CSCs. In addition, CD44v6+/SOX2− cells also showed ability to formed spheres in vitro (although relative weaker than the SOX2+ cells), indicating it is possible that there are other SOX2− CSCs in BCa, which will require further investigation.
To our knowledge, there is still not any direct evidence to support that Krt14 and CD44v6 are regulated by SOX2. However, SOX2+ cells have been identified as CSCs in the Shh+ subgroup of medulloblastoma (Vanner et al., 2014), and SOX2 controls the expression of the Shh in postnatal neural stem cells and the developing hippocampus (Favaro et al., 2009). Further, Sox2 is also reported to be regulated by shh signaling and contributes to the CSCs from non-small-cell lung cancer (Bora-Singhal et al., 2015). Shh signaling is also important in promoting the tumorigenicity and stemness of BCa (Islam et al., 2016). All these reports suggest a close correlation between Sox2-expressing cells and shh-expressing cells, and their relationship in the context of BCa stem cells awaits further study.
Our data suggest Sox2 expression is absent in normal urothelial cells of both human and mouse. Understanding how Sox2 is expression is activated in CSCs of BCa may be important to interpret the carcinogenesis of BCa in vivo. Genetic amplification of SOX2 has been reported in some cancers, including SCCs from lung and esophagus (Bass et al., 2009). According to the TCGA Bladder Urothelial Carcinoma (provisional raw data at the NCI), there are 65 BCa patients with amplification of the SOX2 location (chr3:3q26.33) in a survey of 412 patients (http://cancergenome.nih.gov/). For transcriptional regulation, SOX2 can be activated by STAT3 in embryonic cells (Foshay and Gallicano, 2008), and it cooperates with STAT3 to initiate malignant transformation in the forestomach (Liu et al., 2013). In BCa, STAT3 signaling is activated in CSCs and leads to BCa progression (Ho et al., 2012b). Our data also suggest a high co-localization of activated STAT3 with SOX2+ cells in BCa. Another possible mechanism of Sox2 activation is epigenetic regulation; SOX2 can be activated by JMJD2A (Bouzas et al., 2016), which is the demethylase of H3K9me3 and is often highly expressed in BCa (Kogure et al., 2013).
In conclusion, our studies shed light on Sox2 as a marker for stem-like tumor cells of BCa in vivo. How and when Sox2 expression becomes activated in BCa progression and whether it is involved in cancer cell fate determination will be the subject of future investigation.
Experimental Procedures
All animal procedures were performed under a protocol approved by the Laboratory Animal Center of Anhui Medical University and in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Clinical Sample
Twenty-two human BCa samples were obtained from the Department of Urology, Huadong Hospital, Fudan University, with patients' informed consent. The pathological condition of the BPH samples was determined by experienced urologists at Huadong Hospital. This study was conducted under the approval of Ethics Committee of Huadong Hospital affiliated to Fudan University.
Statistics
Data are presented as the means ± SD or SE. All of the statistical analyses were performed using Excel (Microsoft, Redmond, WA) or Prism (GraphPad Software, La Jolla, CA). The two-tailed Student's t test or one-way ANOVA was used and a p value <0.05 was considered significant.
Author Contributions
F.Z., Y. Liang, and M.W. did the experiments; H. Z. and W.Q. provided and analyzed the clinical samples; Y. Li designed the study; Y.Z., X.Z., and Q.G. prepared the figures. Y. Li wrote the main manuscript text, and all authors reviewed the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81672776 and 81200975 to Y. Li, 31501838 to X.Z.). The authors thank Dr. John M. Szymanski for help with the preparation of the manuscript.
Published: August 8, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.07.004.
Supplemental Information
References
- Bass A.J., Watanabe H., Mermel C.H., Yu S., Perner S., Verhaak R.G., Kim S.Y., Wardwell L., Tamayo P., Gat-Viks I. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B., Blanpain C. Unravelling cancer stem cell potential. Nat. Rev. Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- Bora-Singhal N., Perumal D., Nguyen J., Chellappan S. Gli1-mediated regulation of sox2 facilitates self-renewal of stem-like cells and confers resistance to egfr inhibitors in non-small cell lung cancer. Neoplasia. 2015;17:538–551. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Bouzas S.O., Marini M.S., Torres Zelada E., Buzzi A.L., Morales Vicente D.A., Strobl-Mazzulla P.H. Epigenetic activation of Sox2 gene in the developing vertebrate neural plate. Mol. Biol. Cell. 2016;27:1921–1927. doi: 10.1091/mbc.E16-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.S., Espinosa I., Chao M., Wong D., Ailles L., Diehn M., Gill H., Presti J., Jr., Chang H.Y., van de Rijn M. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.S., Volkmer J.P., Weissman I. Cancer stem cells in bladder cancer: a revisited and evolving concept. Curr. Opin. Urol. 2010;20:393–397. doi: 10.1097/MOU.0b013e32833cc9df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R., Valotta M., Ferri A.L., Latorre E., Mariani J., Giachino C., Lancini C., Tosetti V., Ottolenghi S., Taylor V. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Ferone G., Song J.Y., Sutherland K.D., Bhaskaran R., Monkhorst K., Lambooij J.P., Proost N., Gargiulo G., Berns A. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell. 2016;30:519–532. doi: 10.1016/j.ccell.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foshay K.M., Gallicano G.I. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev. 2008;17:269–278. doi: 10.1089/scd.2007.0098. [DOI] [PubMed] [Google Scholar]
- Hatina J., Schulz W.A. Stem cells in the biology of normal urothelium and urothelial carcinoma. Neoplasma. 2012;59:728–736. doi: 10.4149/neo_2012_089. [DOI] [PubMed] [Google Scholar]
- Ho P.L., Kurtova A., Chan K.S. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat. Rev. Urol. 2012;9:583–594. doi: 10.1038/nrurol.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P.L., Lay E.J., Jian W., Parra D., Chan K.S. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutz K., Mejias-Luque R., Farsakova K., Ogris M., Krebs S., Anton M., Vieth M., Schuller U., Schneider M.R., Blum H. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35:942–950. doi: 10.1093/carcin/bgt410. [DOI] [PubMed] [Google Scholar]
- Islam S.S., Mokhtari R.B., Noman A.S., Uddin M., Rahman M.Z., Azadi M.A., Zlotta A., van der Kwast T., Yeger H., Farhat W.A. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol. Carcinog. 2016;55:537–551. doi: 10.1002/mc.22300. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Torigoe T., Hirohashi Y., Asanuma H., Inoue R., Nishida S., Tanaka T., Fukuta F., Masumori N., Sato N. Prognostic impact of the expression of ALDH1 and SOX2 in urothelial cancer of the upper urinary tract. Mod. Pathol. 2013;26:117–124. doi: 10.1038/modpathol.2012.139. [DOI] [PubMed] [Google Scholar]
- Kogure M., Takawa M., Cho H.S., Toyokawa G., Hayashi K., Tsunoda T., Kobayashi T., Daigo Y., Sugiyama M., Atomi Y. Deregulation of the histone demethylase JMJD2A is involved in human carcinogenesis through regulation of the G(1)/S transition. Cancer Lett. 2013;336:76–84. doi: 10.1016/j.canlet.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Leis O., Eguiara A., Lopez-Arribillaga E., Alberdi M.J., Hernandez-Garcia S., Elorriaga K., Pandiella A., Rezola R., Martin A.G. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354–1365. doi: 10.1038/onc.2011.338. [DOI] [PubMed] [Google Scholar]
- Liu K., Jiang M., Lu Y., Chen H., Sun J., Wu S., Ku W.Y., Nakagawa H., Kita Y., Natsugoe S. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg I.V., Edin S., Eklof V., Oberg A., Palmqvist R., Wikberg M.L. SOX2 expression is associated with a cancer stem cell state and down-regulation of CDX2 in colorectal cancer. BMC Cancer. 2016;16:471. doi: 10.1186/s12885-016-2509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafotiou G., Paraskevopoulou V., Vasilaki E., Kanaki Z., Paschalidis N., Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun. 2016;7:11914. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J., Wei B., Xu Z., Yang S., Zhou Y., Yu M., Liang J., Jin K., Huang X., Lu P. Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med. Oncol. 2013;30:445. doi: 10.1007/s12032-012-0445-z. [DOI] [PubMed] [Google Scholar]
- Santini R., Pietrobono S., Pandolfi S., Montagnani V., D'Amico M., Penachioni J.Y., Vinci M.C., Borgognoni L., Stecca B. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697–4708. doi: 10.1038/onc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Lim A., Odegaard J.I., Honeycutt J.D., Kawano S., Hsieh M.H., Beachy P.A. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 2014;16:469–478. doi: 10.1038/ncb2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Van Batavia J., Yamany T., Molotkov A., Dan H., Mansukhani M., Batourina E., Schneider K., Oyon D., Dunlop M., Wu X.R. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 2014;16:982–991. doi: 10.1038/ncb3038. 981-985. [DOI] [PubMed] [Google Scholar]
- Vanner R.J., Remke M., Gallo M., Selvadurai H.J., Coutinho F., Lee L., Kushida M., Head R., Morrissy S., Zhu X. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26:33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P.D., Lee J.K., Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.