Abstract
The hippocampus is strongly implicated in the psychotic symptoms of schizophrenia. Functionally, basal hippocampal activity (perfusion) is elevated in schizophrenic psychosis, as measured with positron emission tomography (PET) and with magnetic resonance (MR) perfusion techniques, while hippocampal activation to memory tasks is reduced. Subfield-specific hippocampal molecular pathology exists in human psychosis tissue which could underlie this neuronal hyperactivity, including increased GluN2B-containing NMDA receptors in hippocampal CA3, along with increased postsynaptic density protein-95 (PSD-95) along with augmented dendritic spines on the pyramidal neuron apical dendrites. We interpret these observations to implicate a reduction in the influence of a ubiquitous gene repressor, repressor element-1 silencing transcription factor (REST) in psychosis; REST is involved in the age-related maturation of the NMDA receptor from GluN2B- to GluN2A-containing NMDA receptors through epigenetic remodeling. These CA3 changes in psychosis leave the hippocampus liable to pathological increases in neuronal activity, feedforward excitation and false memory formation, sometimes with psychotic content.
Keywords: schizophrenia, REST, polychrome proteins, CA3, synaptic plasticity
A. INTRODUCTION
The role of the hippocampus in mammalian memory function is well established (117). Details of its anatomy, synaptic physiology and network functions which support learning and memory have been a fascinating chapter in modern neuroscience (9;34;81;92;95). Loss of hippocampal function with neurodegenerative diseases like Alzheimer’s disease is well established. But, increased hippocampal function, with its associated cognitive dysfunction, has been less well studied. Unmonitored increases in hippocampal activity could plausibly over-excite and confound memory processing (103). We reason here that psychosis could be associated with hippocampal hyperactivity and result from a pathological augmentation of normal learning and memory processes (95) resulting in inappropriate associations, illogical, and false memories, some with psychotic content.
Psychotic manifestations resemble human memories, including the spontaneous onset of the ideas themselves, the detail, content, stability over time, and the personal salience of the memory content (111). Individuals with chronic psychosis do not have continually new and different memory content populating their hallucinations or delusions. Their content is usually intensely personal and is often adverse (22). Psychotic experiences have an enduring content, with changes occurring only incrementally. Since memories are automatically encoded, as if through ‘unconscious’ mechanisms, a psychotic person would not be able to differentiate psychotic productions from normal thought. And, indeed, they cannot. “Loss of insight” (ie, the inability to distinguish psychotic experiences from real occurrences) is one of the cardinal features of schizophrenia, ubiquitous among patients, and one that many people who suffer with the illness cite as highly incapacitating. Based on this, in part, we propose that psychotic manifestations could be pathological memory productions (127).
In addition, in psychotic diseases like schizophrenia, declarative memory functions are not only altered, but there is a decrease in declarative memory performance in individuals with schizophrenia (121). The defect in declarative memory is not seen predominantly in new memory formation or retrieval; once normal memories are formed, the psychotic person can recall the memory. Rather, the defect in schizophrenia lies in the capacity to utilize the relational capacity of memory, ie, that a person with psychosis cannot manipulate, modify or flexibly use memories to make associations (127). Thus, alterations in relational memory processing could generate another cardinal characteristic in psychosis, namely, poor or ‘loose’ associations. We propose that these abnormal characteristics of declarative memory function also are linked mechanistically, in part, with psychosis.
These clinical characteristics of psychosis, along with changes (increases) in hippocampal basal activity measured with brain imaging techniques, motivated us to examine hippocampal structure (85), function (129) and synaptic physiology (126) in schizophrenia vs normal hippocampal tissue. We predicted that these clinical and imaging alterations would be accompanied by significant molecular and cellular changes in hippocampal subfield tissue in the disorder. Tissue studies in schizophrenia are particularly important because animal models of psychosis cannot be verified without language, leaving initial discovery of psychosis biology to come from human research. In the following sections, we will review findings in schizophrenia psychosis, first from in vivo brain imaging and then from human postmortem tissue analysis, comparing schizophrenia to healthy subjects. We have postulated that schizophrenia-associated tissue changes are due to alterations in repressor element-1 silencing transcription factor (REST) expression and to associated REST-associated proteins, mediated by altered epigenetic remodeling.
B. Hippocampal In Vivo Brain Imaging in Schizophrenic Psychosis
B.1 Hippocampal Volume
Hippocampal size is diminished in schizophrenia as measured with human brain imaging (10;12;14;123), more often for hippocampus than for any other brain region (51;119). Reduction in size is seen in the first psychotic episode (90;124) and progresses with the illness (23;135), independent of antipsychotic drug (99). It has been detected in non-psychotic siblings of schizophrenia individuals (130), in persons at risk for schizophrenia (65;134), and in psychotic bipolar disorder (122).
Hippocampal volume is reduced not only in schizophrenia, but across a spectrum of psychotic illnesses, including in individuals with schizophrenia (SZ), schizoaffective disorder (SAD), and psychotic bipolar disorder (BDP), compared to healthy controls (HC) (3;54). In examining hippocampal subfield volume in psychosis, we found reduced subfield volume in all three psychotic disorders. The most prominent structural difference between psychotic individuals and HC is in CA3 (82). The reduction in hippocampal subfield volumes were positively correlated with psychosis severity, declarative memory dysfunction, and overall poor cognitive performance (82). These findings show that the functional and molecular hippocampal changes highlighted below, have a correlate in altered hippocampal subfield volume.
B.2 Brain Perfusion
One of the most critical observations made about hippocampus in schizophrenia, is its increased basal activity, most recently detected with high resolution MR methods. This increase in hippocampal activity is in contrast with other cortical regions implicated in SZ, which show hypoactivity in imaging studies (54;76). Increases in hippocampal perfusion in psychosis were identified in early PET studies (39;46;71;94;128) and later using MR approaches (47;79;86;88;102), including with recent high resolution cerebral blood volume (rCBV) measures (78;125). The studies indicate that perfusion is elevated in hippocampus in schizophrenia, particularly in medication-free individuals, possibly particularly in CA1, and may suggest that perfusion is partially “normalized” by antipsychotic treatment (86). The increase in regional perfusion correlates with the magnitude of psychosis in medication-free patients (61) making this hyperactivity pertinent to psychosis.
B.3 Memory Task Performance and Function
While hippocampal perfusion is increased in the illness, many studies show a reduction in hippocampal activation to memory tasks (1;37;47;48;50;56;67;96;131;137;138). In particular, a reduction in hippocampal activation has been observed during tasks assessing verbal memory with temporal context, shallow and deep word encoding, transitive inference with overlapping patterns, relationships between visual stimuli, arbitrary pair encoding, and word pair novelty, tasks which are difficult and which may involve relational memory (1;48;58;96;136). This reduction in activation accompanies the increase in basal perfusion, possibly because activation studies start with an already elevated activity baseline.
Relational memory performance in SZ is impaired, a defect which antipsychotic medication can rescue. Relational memory serves to binds together multiple components or features of an experience into an integrated memory, allowing memory generalization (28). It can be examined by contrasting learning of single events versus learning the relationship between jointly presented events. The medication effect is selective to the relational component of the task (38;48;58), and shows that capacity for generalization is a cognitive characteristic sensitive to dopamine receptor blockade and/or to psychosis modulation. The present findings are consistent with recent fMRI data demonstrating that hippocampal-midbrain interactions contribute to memory generalization, as well as with recent theories suggesting that disrupted hippocampal-midbrain function may be a key characteristic of schizophrenia (72).
Several investigations report that, among hippocampal subfields, dentate gyrus (DG) functions may be most critically altered in schizophrenia (127). Pattern separation (PS) is an aspect of cognition which helps distinguish a new perception from previously learned stimuli, even if the distinguishing stimulus characteristics are slight (27). PS is extensively impacted by dentate gyrus function. Recently, we showed that PS performance is degraded in schizophrenia compared to HC. Interestingly, the SZ group showed a significant decrement in PS performance relative to HC; whereas, the SZ did not differ on the memory retrieval performance task (27). These data indicate dentate gyrus dysfunction in schizophrenia, potentially contributing to declarative memory impairment in psychosis, a conclusion consistent with dentate gyrus molecular pathology in schizophrenia (see below).
C. Hippocampal Subfield Synaptic Pathology in SZ Psychosis
C.1 Human Dentate Gyrus in Schizophrenia
To understand the molecular and cellular basis for these functional hippocampal alterations in schizophrenia, we turned to human postmortem tissue (SZ vs HC). In the past, many molecular targets have been measured in the whole hippocampus, including synaptic plasticity markers (11;32;112), proteins associated with putative risk genes (31;63), glutamate receptors and their intracellular signaling markers (33;41;44;62) and other proteins associated with glutamate transmission (25;120); but, this approach has not uncovered any consistent changes in psychosis. Meanwhile, recent findings in animal and human memory research have associated distinct hippocampal subfields with distinct memory components. Differential functions of the hippocampal subfields––shown with electrophysiology (29), focal lesions in rodents (49), and regionally-selective genetically manipulated animals (49;57;84;89)—imply that distinct behavioral syndromes may accompany dysfunction of different subfields (59). Therefore, in these studies, we examined molecular targets in each hippocampal subfield in schizophrenia, especially in dentate gyrus (because of reduced ‘pattern separation’ in psychosis) and in CA3 (because of hyperassociative thinking in psychosis). Existing postmortem data make a cogent case for DG harboring prominent hippocampal molecular pathology in psychosis. (8;30;68;104;105). Since the dentate is the ‘gateway’ structure of the trisynaptic pathway, it is positioned to critically influence the downstream function of hippocampus proper, especially of CA3, its direct projection target. In an initial study of hippocampal dentate gyrus tissue, we identified a reduction in the N-methyl-D-aspartate (NMDA) receptor subunit GluN1 uniquely in DG in SZ (42) and confirmed this in subsequent examination (118). GluN1 is the critical subunit of the excitatory NMDA receptor (NMDAR) and therefore influences the receptor’s signaling characteristics. We suggest that this reduction in GluN1 will diminish signaling in the mossy fiber pathway to CA3.
C.2 Human CA3 in Schizophrenia
To understand the downstream consequences of DG pathology in SZ we focused on postmortem CA3 and CA1 tissue from healthy and schizophrenia cases and analyzed and contrasted the samples on molecular and anatomic markers of synaptic plasticity in the hippocampal subfields. The results of these studies show an increase in GluN2B-containing NMDA receptors (GluN2B/GluN1), an increase in BDNF expression ((126)) and, increases in the postsynaptic density protein-95 (PSD95) in CA3, but no change in these markers in CA1 (70). These are all synaptic plasticity markers, indicating an increase in synaptic strength in CA3. The GluN2B-containing NMDA receptors are the early developmental variant of the NMDA complex, implicating reduced maturation of the NMDA receptor in CA3 from GluN2B- to GluN2A-containing NMDA receptors. The CA3 GluN2B/GluN1 increase was present in schizophrenia cases with and without antipsychotic medication, thereby suggesting it as a disease (not medication) effect. These data point to an increase in excitatory signaling at the NMDA receptor in CA3 (126). The mossy fiber innervations in CA3 contact both the excitatory pyramidal neurons at thorny excrescences and inhibitory interneurons by way of en passant synapses, and these innervations have inverse effects on CA3 excitation (83). Thus, strengthened transmission at the pyramidal cell and reduced inhibitory control onto local interneurons could both together advantage feed-forward excitation in CA3 potentially generating run-away excitation in CA3 (64). Uncontrolled feed-forward excitation in CA3 might fuel overall hippocampal hyperactivity, possibly generating false memories and psychotic mental events.
Because the molecular outcomes suggest synaptic strengthening, Golgi staining was done on the SZ CA3 tissue to examine pyramidal cell spine number and morphology. The histological results document a substantial increase in spine density present in the stratum radiatum, on the apical trunk of pyramidal CA3 neurons, but not at the insertions of the recurrent collaterals in stratum oriens (70). The presence of greater spine density in stratum radiatum is consistent with and could represent the morphologic manifestation of increased GluN2B-containing NMDA receptors and PSD95 in CA3, as the GluN2B subunit augments long term potentiation (7;141). Increased spine density in CA3 is also compatible with elevated PSD95, as overexpression of this protein has been shown to elevate spine density in hippocampal cultures (35). Increased spine number is regularly observed following long term potentiation-mediated increases in synaptic strength at excitatory synapses (36;80). These changes could result in an increase in excitability and synaptic strength in CA3, promoted by a decrease in afferent mossy fiber stimulation from DG. The novel outcomes in CA3 are consistent with alterations in excitatory signaling in hippocampus in CA3 and we propose that the molecular mediators of the switch of GluN2B to GluN2A in the NMDA receptor (eg, REST and its associated proteins) could explain them.
Hippocampal pathology in SZ, including the decrease in GluN1 limited to DG, the increase in GluN2B-containing NMDA receptors in CA3, as well as the increase in PSD95 and the remarkable increase in spines on pyramidal cell neurons in CA3, all together provide evidence of a molecular substrate for increased pyramidal cell activity in CA3, plausibly fueling whole hippocampal hyperactivity in SZ. What could generate these specific kinds of molecular alterations? We have raised a hypothesis based on altered epigenetic remodeling.
D. Potential Association of Schizophrenia Psychosis with REST epigenetic signaling
D.1 REST is implicated in the switch in synaptic NMDAR phenotype
The repressor element 1 silencing transcription factor (REST/NRSF) is a gene silencing transcription factor that is widely expressed during embryogenesis in pluripotent stem cells and neural progenitors, where it acts via epigenetic remodeling to silence a large array of coding and noncoding neuron-specific genes important to synaptic plasticity and structural remodeling, including the NMDAR subunit GluN2B (6;97;109;116). In differentiated neurons REST is quiescent, but can be reactivated during normal postnatal development, driving the switch from immature to mature NMDARs (108) and in selectively-vulnerable hippocampal neurons in response to ischemic insults (21;40) and seizures (21;40;43;98). In human hippocampal and cortical neurons, REST is critical for normal ageing and loss of REST is a feature of Alzheimer’s disease(75). In Huntington’s disease, REST aberrantly accumulates in the nucleus of medium aspiny striatal neurons (142). Its characteristics in SZ have not yet been examined. Whereas REST was initially thought to function as a master transcriptional repressor of genes involved in neurogenesis and neuronal differentiation, loss of REST is critical for the acquisition of the neuronal phenotype (6). Our recent findings show that REST is activated during normal postnatal development and triggers experience–dependent chromatin remodeling and transcriptional repression of grin2b, the gene encoding the NMDAR subunit GluN2B. Failure to undergo the switch would result in a preponderance of GluN2B and NMDARs with an immature phenotype as observed in the CA3 of humans with SZ.
D.2 REST-dependent epigenetic remodeling drives the NMDARs switch
A fundamental mechanism by which REST silences target genes is that of epigenetic remodeling (15). Epigenetic modifications, such as DNA methylation or histone modifications, reflect environmental influences that are not “hard-wired” into the DNA sequence and represent a mechanism through which early life experience can modify brain development (13;106;133). In neural progenitors (5) and insulted adult neurons (93), REST binds the RE1 element within target genes and recruits CoREST (2;4) and mSin3A (15;45;52;91;109), corepressor platforms which recruit histone deacetylases (HDACs)-1 and 2. HDACs de-acetylate core histone proteins and effect dynamic and reversible gene silencing (6;97;109). REST mediates long-term gene silencing by association with the site-specific histone methyltransferase G9a, which promotes trimethylation of histone 3 at lysine 9 (H3K9me3) via CoREST-dependent (5;77) and independent (110) mechanisms, the site-specific histone demethylase LSD1, which removes methyl groups from lysine 4 of histone 3 (H3K4) (66;114) and methyl CpG binding protein 2 (MeCP2), a transcriptional repressor that reads epigenetic marks and is recruited to ‘hot-spots’ of methylated CpGs (5;77).
D.3 Polycomb repressive proteins coordinate with REST to remodel NMDARs
Polycomb group (PcG) proteins are gene-silencing factors that are abundantly expressed during embryogenesis and play an essential role in maintenance of stem cell pluripotency and self-renewal. In stem cells, PcG proteins actively repress genes important to embryonic development and cell fate. Unbiased epigenome-wide analysis has identified > 500 putative PcG targets (16). A fundamental mechanism by which polycomb group proteins promote gene silencing is via epigenetic remodeling of target genes (113;115;140). Polycomb proteins form large multimeric complexes known as polycomb repressive complex 1 (PRC1) and PRC2 (113;115;140). EZH2, the catalytically active component of PRC2, is a histone methyltransferase that confers a trimethylation mark on lysine 27 of core histone protein H3 (H3K27me3), a mark of strong gene repression (113;115;140) (Scheme 2). H3K27me3 recruits and stabilizes PRC1 at repressed regions of the genome (113;115;140). Polycomb proteins are recruited to RE1 sites by REST via HOTAIR (107;132). Our finding that EZH2 and its functional readout H3K27me3 are enriched at the grin2b promoter in the hippocampus together with REST at P15 implicates EZH2 in the developmental switch in NMDARs. An attractive scenario is that early in development, trimethylation of lysine 27 on H3 locks in the mature phenotype at synaptic sites. Our finding that H3K27me3 is depleted at the grin2b promoter at P15 in the hippocampus of maternally-deprived pups implicates polycomb proteins in early childhood adversity or neglect, a known environmental risk factor in schizophrenia.
D.4 CK1 phosphorylates and primes REST for ubiquitin-based proteasomal degradation
Casein kinase 1 (CK1) plays a pivotal role in membrane transport, cell division, DNA repair, and activation of transcription factors such as β-catenin and p53 (100;101). Recent studies indicate that REST is regulated at the level of protein stability via β-TrCP-dependent, ubiquitin-based proteasomal degradation in differentiated neurons under physiological conditions and identify CK1 as an upstream effector that bidirectionally regulates REST cellular abundance. This is significant in that dysregulation of CK1 is implicated in a number of brain disorders including narcolepsy, migraines, depression, Parkinson’s and Alzheimer’s disease. REST harbors two neighboring, but distinct, noncanonical degron motifs within its carboxy-terminus (139). Recent findings show that CK1 associates with and phosphorylates REST at these two degron motifs within the carboxy-terminus of REST essential to binding by the F-box protein β-TrCP, thereby priming REST for ubiquitin-based proteasomal degradation. Activation or overexpression of CK1 promotes REST ubiquitination and degradation in a β-TrCP-dependent manner. Inhibition or RNAi-mediated knockdown of CK1 promotes REST stability and increases REST cellular levels. These findings establish an inverse causal relation between CK1 and REST abundance.
D.5 Maternal deprivation and rescue strategies
Adverse experience early in life is associated with abnormal behaviors in later life. Maternal deprivation in mice during the first week of postnatal life has a profound and enduring impact on hippocampal development (73), neurogenesis (87) and NMDAR subunit composition (60). In addition, maternal separation increases anxiety (53), impairs maternal care (74), diminishes spatial navigation learning (53), and the ability of the hippocampus to respond to stress in adulthood (53). The dentate gyrus of adult rats exposed to brief maternal deprivation early in life exhibits reduced cell proliferation and increased production of immature neurons. Our recent findings show that adverse experience in the form of maternal deprivation early in life disrupts activation of REST, suppresses REST-dependent epigenetic remodeling of the grin2b promoter, and prevents acquisition of the mature NMDAR phenotype at hippocampal synapses. These findings support a novel and previously unappreciated role for REST-dependent epigenetic remodeling as a link between experience and synaptic function. Moreover, this observation motivates the hypothesis that early childhood trauma and neglect in humans is a risk factor for SZ based on the suppression of REST expression.
An important goal with clinical ramifications is that of rescuing the epigenetic changes caused by early maternal deprivation. One such rescue strategy is that of communal nesting. Communal nesting is a rearing strategy for rodents that is similar to their natural habitat and strongly regulates adult behavior (18;24). In this paradigm, three lactating females and their litters are housed together in a single nest and engage in shared care-giving from birth to weaning (P21), which increases both maternal and peer interactions. Communal nesting mimics social support (17;24) and can overcome chronic stress and anxiety associated with maternal deprivation. In adulthood, communally-reared offspring display reduced anxiety-like behavior (19), enhanced hippocampal release of BDNF (17;18) and increased social interaction and social competency (26). Whereas paradigms such as maternal care (55), neonatal handling (73) and exposure to an enriched environment (20;55) can reverse synaptic and behavioral deficits, at least in part, the ability to rescue the mature NMDAR phenotype at hippocampal synapses is, as yet, unknown. Parallel strategies in humans might rescue an individual with early childhood deprivation risks from a disease like SZ.
E. INTERPRETATION AND DISCUSSION
The pathophysiology of psychosis has remained stubbornly elusive, despite many decades of research. Recent evidence suggests that alterations in NMDA receptor synaptic strength in CA3 could mediate hippocampal hyperactivity and that this hyperactivity could, in part, support psychotic thought processing. These human SZ findings implicate regulators of hippocampal cellular activity as plausible molecular substrates for psychotic processing. REST and its protein family are powerful regulators (repressors) of the normal developmental ‘switch’ from GluN2B-containing (2B) to GluN2A-containing (2A) NMDA receptors via epigenetic remodeling, with the immature 2B receptors favoring greater excitation. Moreover, findings have now demonstrated that this process of REST-mediated ‘switch’ from 2B- to 2A-NMDARs is sensitive to early environmental insult, a known risk factor in psychotic illness. These findings all implicate the involvement of REST and its corepressors, mediated by epigenetic remodeling, in the molecular bases of brain diseases with early environmental liability, especially if they involve GluN2B pathology.
The intriguing possibility that psychosis may be mediated or advantaged by a failure of a the normal developmental 2B→2A ‘switch’, leaving GluN2B-containing NMDARs to predominate in brain regions rich in NMDARs, is pointed up by the finding of increased 2B NMDARs in hippocampal CA3 in psychosis, supported by additional evidence consistent with CA3 hyperactivity, like increased BDNF (126), increased PSD95 and increased dendritic spine numbers in the stratum radiatum (70). This increase in CA3 pyramidal cell activity could generate hyper-associations, false memories, some with psychotic content. REST is one of the powerful regulators of the normal developmental switch from 2B to 2A NMDARs, therefore implicated here. The involvement of REST and its proteins is even more strongly supported by their sensitivity to early environmental adverse influences, also implicated in chronic psychiatric diseases with psychosis.
These observations motivate a novel model of hippocampal hyperactivity in schizophrenic psychosis. In this model, the DG harbors a glutamatergic lesion in DG, resulting in reduced glutamate transmission from DG onto CA3 pyramidal neurons (118). The possibility exists that diverse DG lesions, all resulting in granule cell hypo-function could be involved. This reduction in the afferent mossy fiber pathway activity would generate increased synaptic strength through synaptic homeostatic plasticity processes within CA3. This hypersensitivity would then become the substrate for other CA3 inputs to generate increased CA3 cellular activity (70), increased associational processing, which (when it exceeds the capacity of hippocampal memory mechanisms) could result in the generation of false memories, inappropriate associations and psychotic memory constructions (127). An attractive scenario is that elevated expression of GluN2B in the hippocampal CA3 of human subjects with schizophrenia arises as a consequence of impaired expression of the gene silencing protein REST. If true, such a mechanism would be expected to vary in an experience-dependent manner or in response to early childhood adversity. The associated vulnerabilities could be based on epigenetic changes in local gene expression. This latter is a testable hypothesis.
Clearly, new tools and added investigations are warranted to carry this psychosis construct further, the first being to establish that REST expression or that of its critical associated proteins is impaired in CA3 of schizophrenia and second, to establish a causal relation between impaired REST expression and elevated GluN2B expression in CA3. Recent findings that CA3 in schizophrenia exhibits elevated GluN2B (70) suggest the intriguing possibility that dysregulation of REST and REST-dependent epigenetic remodeling play a role in the hippocampal pathophysiology of schizophrenia (69). Future studies will test whether REST expression is impaired in the CA3 of human schizophrenia tissue. More human observations—correlations between psychosis intensity and hippocampal perfusion—are necessary; the demonstration that modulation of hippocampal hyperactivity would result in psychosis diminution will be important; and, more evidence is needed that genetic or environmental risks interact with hippocampal vulnerability in humans. Understanding how epigenetic remodeling dynamically alters synaptic NMDAR function in schizophrenia is likely to enhance our understanding of the neural networks for learning and memory, and how dysregulation of epigenetic remodeling causes psychotic disorders, including schizophrenia. We believe that future research to address these issues is warranted and will accelerate development of much needed new therapeutic strategies to treat the syndrome.
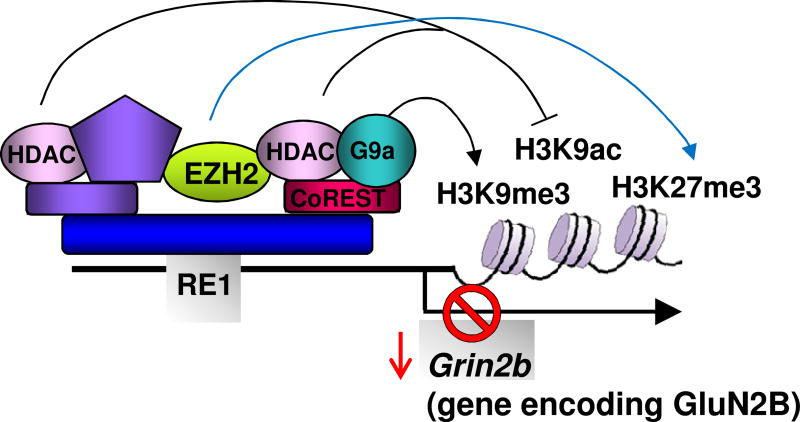
Figure 1. Model showing REST-dependent epigenetic remodeling of the grin2b promoter.
REST binds to the RE1 element within the promoter of its target gene grin2b and recruits mSin3A and CoREST, HDACs-1/2, G9a and MeCP2. The REST-corepressor complex promotes epigenetic remodeling of core histone proteins at the grin2b promoter. Recruitment of EZH2 in the promoter of grin2b confers trimethylation on lysine 27 of core histone protein H3. These may play a crucial role in acquisition of the mature NMDAR phenotype during postnatal development. Modified with permission from Noh et al, Proc Natl Acad Sci USA 109:E962-7.
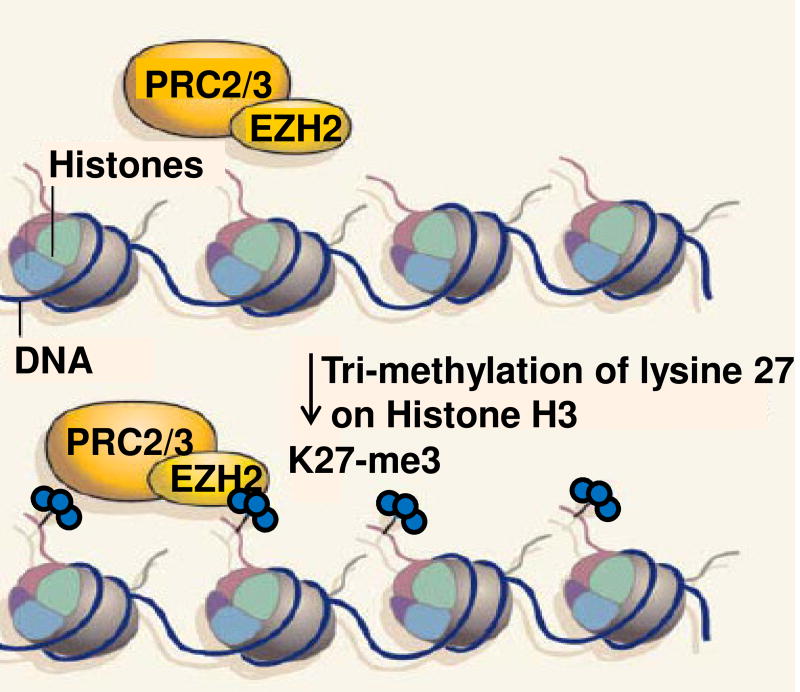
Figure 2. The Polycomb group protein EZH2 confers epigenetic marks of gene repression.
The Polycomb group protein EZH2 acts as part of the Polycomb Repressive Complexes 2 and 3 (PRC2/3), which add methyl groups to histone proteins. EZH2 is recruited to the promoter region of target genes. such as grin2b, where it confers three methyl marks to lysine 27 in histone 3. This mark is possibly the most enduring mark of gene represson. EZH2 may also have a role in recruiting other repressive proteins such as DNMT1methyl-binding domain protein. Adapted from Viré et al, Nature 439:794–795.
HIGHLIGHTS.
Hippocampus shows in vivo hyperactivity in schizophrenic psychosis
In schizophrenia, CA3 shows synaptic strengthening, which are not present in CA1
Golgi staining reveals increased dendritic spine density in CA3 in schizophrenia
These findings can be explained by reduced activity of REST
REST is responsible for the GluN2B→GluN2A maturational switch during development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Achim AM, Bertrand MC, Sutton H, Montoya A, Czechowska Y, Malla AK, et al. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64(9):999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- 2.Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, et al. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci U S A. 1999;96(17):9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold SJ, Ivleva EI, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, et al. Hippocampal Volume Is Reduced in Schizophrenia and Schizoaffective Disorder But Not in Psychotic Bipolar I Disorder Demonstrated by Both Manual Tracing and Automated Parcellation (FreeSurfer) Schizophr Bull. 2015;41(1):233–249. doi: 10.1093/schbul/sbu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, et al. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 5.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121(4):645–57. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15(5):500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106(11):4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker T, Elmer K, Schneider F, Schneider M, Grodd W, Bartels M, et al. Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res. 1996;67:135–143. doi: 10.1016/0925-4927(96)03002-8. [DOI] [PubMed] [Google Scholar]
- 11.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, et al. Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res. 1995;17:47–58. doi: 10.1016/0920-9964(95)00028-k. [DOI] [PubMed] [Google Scholar]
- 13.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 14.Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35(1):1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- 15.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60(6):961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 17.Branchi I, Curley JP, D'Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38(4):522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branchi I, D'Andrea I, Fiore M, Di FV, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60(7):690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Branchi I, D'Andrea I, Gracci F, Santucci D, Alleva E. Birth spacing in the mouse communal nest shapes adult emotional and social behavior. Physiol Behav. 2009;96(4–5):532–539. doi: 10.1016/j.physbeh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118(2):571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- 21.Calderone A, Jover T, Noh K-M, Tanaka H, Yokota H, Lin Y, et al. Ischemic insults de-repress the gene silencer rest in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter WT, Jr, Buchanan RW. Schizophrenia. N Engl J Med. 1994;330(10):681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 23.Chakos MH, Schobel SA, Gu H, Gerig G, Bradford D, Charles C, et al. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry. 2005;186:26–31. doi: 10.1192/bjp.186.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33(4):593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Society of Biological Psychiatry. 2000;48:381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- 26.D'Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183(1):60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Das T, Ivleva EI, Wagner AD, Stark CE, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159(1):193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davachi L, Wagner AD. Hippocampal Contributions to Episodic Encoding: Insights From Relational and Item-Based Learning. J Neurophysiol. 2002;88(2):982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 29.Diba K, Buzsaki G. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J Neurosci. 2008;28(50):13448–13456. doi: 10.1523/JNEUROSCI.3824-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eastwood SL, Burnet PW, Harrison PJ. Decreased hippocampal expression of the susceptibility gene PPP3CC and other calcineurin subunits in schizophrenia. Biol Psychiatry. 2005;57(7):702–710. doi: 10.1016/j.biopsych.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73(2–3):159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Eastwood SL, McDonald B, Burnet PWJ, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Mol Brain Res. 1995;29:211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- 34.Eichenbaum H. A cortical-hippocampal system for declarative memory. Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 35.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 36.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 37.Eyler-Zorrilla LT, Jeste DV, Paulus MP, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res. 2002;59:187–198. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- 38.Farkas M, Polgar P, Kelemen O, Rethelyi J, Bitter I, Myers CE, et al. Associative learning in deficit and nondeficit schizophrenia. NeuroReport. 2008;19(1):55–58. doi: 10.1097/WNR.0b013e3282f2dff6. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher P. The missing link: a failure of fronto-hippocampal integration in schizophrenia. News and Views. 1998;1(4):266–267. doi: 10.1038/1078. [DOI] [PubMed] [Google Scholar]
- 40.Formisano L, Noh KM, Miyawaki T, Mashiko T, Bennett MV, Zukin RS. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci USA. 2007;104(10):4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157(7):1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 42.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157(7):1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 43.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9(11):1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 44.Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11(5):569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- 45.Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL, et al. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem. 2000;275(13):9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- 46.Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978;87(2):226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- 47.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DLFAJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 48.Heckers S, Zalesak M, Weiss A, Ditman T, Titone D. Hippocampal Activation During Transitive Inference in Humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- 49.Hoang LT, Kesner RP. Dorsal hippocampus, CA3, and CA1 lesions disrupt temporal sequence completion. Behav Neurosci. 2008;122(1):9–15. doi: 10.1037/0735-7044.122.1.9. [DOI] [PubMed] [Google Scholar]
- 50.Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57(9):1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 51.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Myers SJ, Dingledine R. Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci. 1999;2(10):867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 53.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950(1–2):52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 54.Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwata E, Kikusui T, Takeuchi Y, Mori Y. Fostering and environmental enrichment ameliorate anxious behavior induced by early weaning in Balb/c mice. Physiol Behav. 2007;91(2–3):318–324. doi: 10.1016/j.physbeh.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn K-U, et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- 57.Kent K, Hess K, Tonegawa S, Small SA. CA3 NMDA receptors are required for experience-dependent shifts in hippocampal activity. Hippocampus. 2007;17(10):1003–1011. doi: 10.1002/hipo.20332. [DOI] [PubMed] [Google Scholar]
- 58.Keri S, Nagy O, Kelemen O, Myers CE, Gluck MA. Dissociation between medial temporal lobe and basal ganglia memory systems in schizophrenia. Schizophr Res. 2005;77(2–3):321–328. doi: 10.1016/j.schres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Molecular Neurobiology. 2009;39(1):24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- 60.Ku HY, Huang YF, Chao PH, Huang CC, Hsu KS. Neonatal isolation delays the developmental decline of long-term depression in the CA1 region of rat hippocampus. Neuropsychopharmacology. 2008;33(12):2847–2859. doi: 10.1038/npp.2008.36. [DOI] [PubMed] [Google Scholar]
- 61.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 62.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. NeuroReport. 2001;12(13):2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 63.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16(2):129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26(11):631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Society of Biological Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- 66.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437(7057):432–5. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 67.Leube DT, Rapp A, Buchkremer G, Bartels M, Kircher TTJ, Erb M, et al. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia - an fMRI study. Schizophr Res. 2003;64:83–85. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- 68.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54(4):583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W, Ghose S, Gleason K, Begovic A, Perez J, Bartko J, et al. Synaptic Proteins in Schizophrenia Hippocampus Indicate Increased Neuronal Activity in CA3. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Ghose S, Gleason KA, Begovic A, Perez J, Bartko JJ, et al. Synaptic Proteins in Schizophrenia Hippocampus Indicate Increased Neuronal Activity in CA3. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2014.14010123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 72.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 74.Lovic V, Gonzalez A, Fleming AS. Maternally separated rats show deficits in maternal care in adulthood. Dev Psychobiol. 2001;39(1):19–33. doi: 10.1002/dev.1024. [DOI] [PubMed] [Google Scholar]
- 75.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, et al. REST and stress resistance in ageing and Alzheimer's disease. Nature. 2014 doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lui S, Yao L, Xiao Y, Keedy SK, Reilly JL, Keefe RS, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45(1):97–108. doi: 10.1017/S003329171400110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J, et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298(5599):1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- 78.Malaspina D, Corcoran C, Schobel SA, Small SA, Kimby D, Lewandowski N. Hippocampal hyperactivity is associated with positive symptoms. Schizophr Bull. 2009;35(suppl_1):160. [Google Scholar]
- 79.Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychology. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283(5409):1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 81.Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46(4):959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- 82.Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, et al. Medial Temporal Lobe Structures and Hippocampal Subfields in Psychotic Disorders: Findings From the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. JAMA Psychiatry. 2014;71(7):769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- 83.McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res. 2008;169:225–240. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 85.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 86.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;(11):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 87.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7(8):841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 88.Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39(2):117–127. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319(5867):1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 90.Narr KL, Thompson PM, Szeszko PR, Robinson D, Jang S, Woods RP, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Naruse Y, Aoki T, Kojima T, Mori N. Neural restrictive silencer factor recruits mSin3 and histone deacetylase complex to repress neuron-specific target genes. Proc Natl Acad Sci USA. 1999;96(24):13691–13696. doi: 10.1073/pnas.96.24.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 93.Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1121568109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O'Shora-Celaya L, et al. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15(6):541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- 95.Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 96.Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 97.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8(7):544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 98.Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18(4):1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Panenka WJ, Khorram B, Barr AM, Smith GN, Lang DJ, Kopala LC, et al. A longitudinal study on the effects of typical versus atypical antipsychotic drugs on hippocampal volume in schizophrenia. Schizophr Res. 2007;94(1–3):288–292. doi: 10.1016/j.schres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Polakis P. Casein kinase 1: a Wnt'er of disconnect. Curr Biol. 2002;12(14):R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 101.Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20(4):399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 102.Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162(10):1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, et al. Creating a false memory in the hippocampus. Science. 2013;341(6144):387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 104.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 105.Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):290–299. doi: 10.1007/s00406-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 106.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 107.Ren X, Kerppola TK. REST interacts with Cbx proteins and regulates polycomb repressive complex 1 occupancy at RE1 elements. Mol Cell Biol. 2011 doi: 10.1128/MCB.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodenas-Ruano A, Chavez AE, Cossio MJ, Castillo PE, Zukin RS. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci. 2012;15(10):1382–1390. doi: 10.1038/nn.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roopra A, Huang Y, Dingledine R. Neurological disease: listening to gene silencers. Mol Interv. 2001;1(4):219–228. [PubMed] [Google Scholar]
- 110.Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell. 2004;14(6):727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 111.Saks ER. The Center Cannot Hold: My Journey Through Madness. Hyperion. 2007 [Google Scholar]
- 112.Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, et al. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2005;62(3):263–272. doi: 10.1001/archpsyc.62.3.263. [DOI] [PubMed] [Google Scholar]
- 113.Schwartz YB, Pirrotta V. Polycomb complexes and epigenetic states. Curr Opin Cell Biol. 2008;20(3):266–273. doi: 10.1016/j.ceb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Shi YJ. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19:1–8. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 115.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10(10):697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 116.Singh SK, Kagalwala MN, Parker-Thornburg J, Adams H, Majumder S. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature. 2008;453(7192):223–227. doi: 10.1038/nature06863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A. 1996;93(24):13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Molecular Psychiatry. 2014 doi: 10.1038/mp.2014.54. [DOI] [PubMed] [Google Scholar]
- 119.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 120.Steffek AE, Haroutunian V, Meador-Woodruff JH. Serine racemase protein expression in cortex and hippocampus in schizophrenia. NeuroReport. 2006;17(11):1181–1185. doi: 10.1097/01.wnr.0000230512.01339.72. [DOI] [PubMed] [Google Scholar]
- 121.Stone WS, Hsi X. Declarative memory deficits and schizophrenia: problems and prospects. Neurobiol Learn Mem. 2011;96(4):544–552. doi: 10.1016/j.nlm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57(6):633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 123.Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR, Jr, Weinberger DR. Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry. 1989;146(4):464–472. doi: 10.1176/ajp.146.4.464. [DOI] [PubMed] [Google Scholar]
- 124.Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, et al. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- 125.Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clinical. 2014;5:359–364. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate Dysfunction in Hippocampus: Relevance of Dentate Gyrus and CA3 Signaling. Schizophr Bull. 2012;38(5):927–935. doi: 10.1093/schbul/sbs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 128.Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 129.Tamminga CA, Thomas BP, Chin R, Mihalakos P, Youens K, Wagner AD, et al. Hippocampal novelty activations in schizophrenia: Disease and medication effects. Schizophr Res. 2012;138(2–3):157–163. doi: 10.1016/j.schres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 130.Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Society of Biological Psychiatry. 2003;54:1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 131.Thermenos HW, Seidman LJ, Poldrack RA, Peace NK, Koch JK, Faraone SV, et al. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61(4):564–574. doi: 10.1016/j.biopsych.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 132.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 134.van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61(4):346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- 135.Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 136.Weiss AP, Goff D, Schacter DL, Ditman T, Freudenreich O, Henderson D, et al. Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biol Psychiatry. 2006;60(11):1268–1277. doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 137.Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, et al. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53(1):48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- 138.Weiss A, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Society of Biological Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 139.Weissman AM. How much REST is enough? Cancer Cell. 2008;13(5):381–383. doi: 10.1016/j.ccr.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 140.Whitcomb SJ, Basu A, Allis CD, Bernstein E. Polycomb Group proteins: an evolutionary perspective. Trends Genet. 2007;23(10):494–502. doi: 10.1016/j.tig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 141.Zhao JP, Constantine-Paton M. NR2A−/− mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci. 2007;27(50):13649–13654. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35(1):76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]