Abstract
Aquaporins are membrane channels selectively permeated by water or water plus glycerol. Conflicting reports have described ion conductance associated with some water channels, raising the question of whether ion conductance is a general property of the aquaporin family. To clarify this question, a defined system was developed to simultaneously measure water permeability and ion conductance. The Escherichia coli water channel aquaporin-Z (AqpZ) was studied, because it is a highly stable tetramer. Planar lipid bilayers were formed from unilamellar vesicles containing purified AqpZ. The hydraulic conductivity of bilayers made from the total extract of E. coli lipids increased 3-fold if reconstituted with AqpZ, but electric conductance was unchanged. No channel activity was detected under voltage-clamp conditions, indicating that less than one in 109 transport events is electrogenic. Microelectrode measurements were simultaneously undertaken adjacent to the membrane. Changes in sodium concentration profiles accompanying transmembrane water flow permitted calculation of the activation energies: 14 kcal/mol for protein-free lipid bilayers and 4 kcal/mol for lipid bilayers containing AqpZ. Neither the water permeability nor the electric conductivity exhibited voltage dependence. This sensitive system demonstrated that AqpZ is permeated by water but not charged ions and should permit direct analyses of putative electrogenic properties of other aquaporins.
Water is the major component of all biological fluids, and the transport of water across cell membranes is fundamental to life. To withstand varying environmental and physiological stresses, all organisms must be able to absorb water, release water, and redistribute water between tissues with remarkable speed and precision. At the same time, the transport of water across cell membranes must be sufficiently selective to prevent the movement of other solutes, ions, and even protons (1). The discovery of aquaporin water channels provided a molecular explanation for these cellular processes (2). Multiple homologous proteins were soon identified, and their importance throughout nature is reflected by the existence of aquaporins in diverse life forms, including vertebrates, invertebrates, microbials, and plants (3).
Because of their low membrane water permeability, Xenopus laevis oocytes have been useful for functional analysis of aquaporins (2). Although AQP1 was found to be selectively permeable to water, other members of the protein family subsequently were found to have somewhat broader permeability. Thus, the mammalian aquaporins include a subset of proteins highly selective for water (orthodox aquaporins) and a subset permeated by water plus glycerol (aquaglyceroporins) (4). Interestingly, Escherichia coli has one member of each subgroup: aquaporin-Z (AqpZ) is permeable to water but not glycerol (5), whereas glycerol facilitator (GlpF) is permeable to both water and glycerol (6). The atomic structure of the human red cell AQP1 (7) provided detailed understanding of the aqueous pathway in each subunit of the tetramer. In addition, high-resolution structural analysis of GlpF revealed that the space at the fourfold axis of symmetry resembles the known structure of ion channels (8).
Several studies have suggested that some aquaporins may be permeated by ions, raising the question of whether this is a general property of aquaporins. Before molecular water channels were discovered, major intrinsic protein of lens (now known as AQP0) was reported to conduct anions in a voltage-dependent manner when added to planar lipid bilayers (9) but not when expressed in Xenopus oocytes (10). Nodulin 26 (Nod26), a nodule-specific symbiosome membrane from soybean, also showed anion conductance when added to planar lipid bilayers (11), whereas Nod26 in symbiosome membranes is permeated by water plus glycerol (12). AQP1 expressed in oocytes originally was shown to be permeable to water, but membrane currents were not detected (2). A subsequent report described cation conductance in oocytes expressing AQP1 after stimulation by forskolin (13) but this was not reproduced in multiple other laboratories (14). In a recent study, it was reported that cGMP stimulated ion conductance in oocytes expressing AQP1 but not AQP5 (15). AQP6 resides exclusively in intracellular vesicles of renal epithelia (16), but expression in Xenopus oocytes revealed anion conductance after pharmacological treatment with Hg2+ or exposure to low but physiologic pH environments (17).
To clarify these apparent discrepancies, a defined system was used to form planar lipid bilayers from protein-free liposomes or proteoliposomes containing high concentrations of purified aquaporins (18). The shortest and simplest member of the aquaporin family, E. coli AqpZ, was studied because it can be expressed in the homologous system and purified in high concentrations (19). Moreover, the AqpZ protein is unusual, because it remains tetrameric even when solubilized in SDS; hence artefactual conductance resulting from subunit-subunit disassociation may be prevented. Using this system, we measured the osmotic water permeability of an aquaporin while simultaneously using a high sensitivity method to detect ion conductance.
Materials and Methods
Expression and Purification of AqpZ.
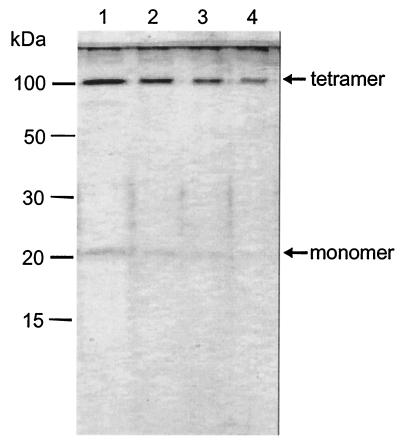
AqpZ protein was expressed in E. coli and purified (19). In brief, polyHis-containing recombinants were constructed by cloning the AqpZ DNA into the pTrc10HisEcoRI plasmid (20). 10-His-AqpZ was purified from the dodecylmaltoside-solubilized membrane fraction by Ni affinity chromatography. Aliquots of the eluted material were diluted in sample buffer (12% glycerol, 1% SDS, 140 mM β-mercaptoethanol, and 70 mM Tris⋅HCl, pH 6.8) and were analyzed by SDS/PAGE with 15% polyacrylamide slabs. A major molecular species, AqpZ tetramer migrating at ≈100 kDa, and a less-abundant species, AqpZ monomer at ≈20 kDa, were revealed by silver staining of the gels (Fig. 1). The purified protein was reconstituted into proteoliposomes by dialysis. A reconstitution mixture was prepared at room temperature by sequentially adding: 100 mM 3-(N-morpholino)propanesulfonic acid (Mops)-Na (Fluka; pH 7.5); 1.25% (wt/vol) octyl glucoside (Fluka); purified 10-His-AqpZ (final concentration 0.5 to 1 mg/ml); and preformed vesicles (50 mg/ml). From a chloroform solution of E. coli total lipid extract (acetone/ether preparation, Avanti Polar Lipids, Alabaster, AL), the solvent had previously been evaporated, leaving a thin lipid film on the wall of a round bottom flask. An appropriate volume of buffer [2 mM β-mercaptoethanol (Merck), 100 mM Mops-Na, pH 7.5] was added, and the solution was vortexed for 3 min. Large unilamellar vesicles were prepared by an extrusion technique (21) using the small-volume apparatus LiposoFast (Avestin, Ottawa) with filters of 100-nm pore diameter. One part of the liposome suspension was used for control experiments, the other part for protein reconstitution. The reconstitution mixture was loaded into SPECTRA/POR 2.1 dialysis tubing, molecular mass cut-off 15,000 (Spectrum Laboratories, Laguna Hills, CA), and dialyzed against 100 vol of assay buffer [50 mM Mops, 100 mM NaCl, 0.3 μM CaCl2 (all from Merck), adjusted to pH 7.5 with HCl] for 48 h at room temperature. Liposomes were harvested by centrifugation (60 min at 100,000 g) and resuspended into assay buffer at a concentration of 1 mg/ml.
Figure 1.
SDS/PAGE analysis of highly purified AqpZ protein after staining with silver reagent. Lane 1, 200 μg purified AqpZ was applied; lane 2, 100 μg; lane 3, 50 μg; and lane 4, 25 μg of protein. The method of Laemmli was used (44).
Planar Membranes.
Planar lipid bilayers were formed from proteoliposomes (18). The technique is based on the finding that monolayers spontaneously form at the air- water interface of any vesicle suspension (22, 23). Two such monolayers were combined in an aperture of a 10-μm thick polytetrafluoroethylene (PTFE) septum separating the two aqueous phases of the chamber. The septum was pretreated with a hexadecane-hexane mixture (ratio by volume, 0.5:99.5). By raising the water levels in both compartments above the aperture, the two monolayers covered the PTFE septum and formed a bilayer within the aperture (24), similar to bilayer formation from solvent-spread monolayers (25). The membranes had a diameter of about 150 μm if not indicated otherwise. The bathing solution was agitated by magnetic stirrer bars.
Membrane Ion and Water Permeabilities.
Current-voltage relationships were measured by a patch-clamp amplifier (model EPC9, HEKA Electronics, Lambrecht/Pfalz, Germany). The effect of AqpZ on membrane conductance was studied to evaluate the impact of electrogenic transport modes that might occur during water transport.
Transmembrane water flux leads to solute concentration changes in the immediate membrane vicinity. Water passing through the membrane dilutes the solution it enters and concentrates the solution it leaves (26). The thickness δ of the stagnant water layer responsible for the polarization effect is defined in terms of the concentration gradient at the membrane water interface (27):
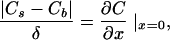 |
1 |
x is the distance from the membrane. Cb and Cs denote the solute concentrations in the bulk and at the interface, respectively. Within the unstirred layer adjacent to the membrane (−δ < x < δ) the solute concentration C is an exponential function of the distance x to the membrane (28):
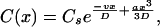 |
2 |
where D, v, and a are the diffusion coefficient, the linear velocity of the osmotic volume flow, and the stirring parameter, respectively. With the knowledge of v the transmembrane water permeability Pf can be calculated:
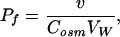 |
3 |
where Vw is the partial molar volume of water and Cosm the near-membrane concentration of the solute used to establish the transmembrane osmotic pressure difference. Because the diffusion coefficients of the osmolyte urea and sodium are very close, the following equation can be used to correct the urea bulk concentration, Cb,urea for dilution by water flux, i.e., to obtain Cosm (29):
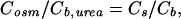 |
4 |
Obviously, v does also allow deriving of the transmembrane water flux jw:
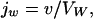 |
5 |
Polarization of membrane-impermeable sodium ions was used to determine water flux. The sodium-sensitive electrodes were made of glass capillaries containing mixture A of sodium ionophore II (Fluka) according to the procedure described by Amman (30). Their tips had a diameter of about 1–2 μm. Electrodes with a 90% rise time below 0.6 s were selected. Artifacts due to a very slow electrode movement are therefore unlikely. Nevertheless possible effects of time resolution or distortion of the unstirred layer were tested by making measurements while moving the microelectrode toward and away from the bilayer. Because no hysteresis was found it can be assumed that an electrode of appropriate time resolution was driven at a rate that is slow relative to the rate at which any electrode-induced disturbance of the unstirred layer reaches a “stationary” state. The osmotic gradient was induced by urea (Laborchemie Apolda, Apolda, Germany) added to one side of the membrane only.
The experimental arrangement was similar to the one described previously (31, 32). Voltage sampling was performed by an electrometer (model 617, Keithley) connected to a personal computer. Continuous motion of the microelectrode perpendicular to the surface of the lipid bilayer was handled by a hydraulic microdrive manipulator (Narishige, Tokyo). Touching the membrane was indicated by a steep potential change (33). Because the velocity of the electrode motion was known (1 μm⋅s−1) the position of the sodium sensor relative to the membrane could be determined at any instant of the experiment. The accuracy of the distance measurements was estimated to be ±5 μm.
Results
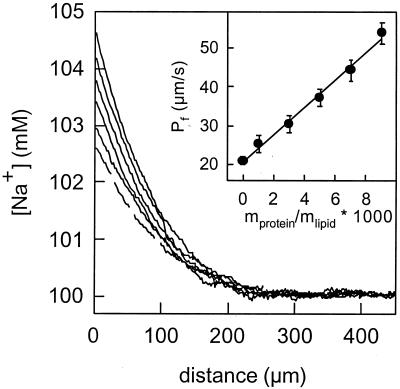
Planar lipid bilayers were formed from protein-free liposomes or proteoliposomes reconstituted with purified AqpZ protein. Because they were stable for hours, these membranes permitted measurement of steady-state water permeability in response to an osmotic gradient. Using microelectrodes, sodium concentration profiles were measured immediately adjacent to planar lipid bilayers, revealing a diffusion polarization that is much more pronounced in membranes containing AqpZ. As seen in Fig. 2, the osmotic water permeability (Pf) depended on the lipid-to-protein ratio, and Pf increased from a baseline of 20 μm/s to 54 μm/s at 23°C.
Figure 2.
Representative sodium concentration profiles at the hypotonic side of planar membranes. The solute polarization increased with an increase in the protein lipid ratio. (Inset) The corresponding increase in the water permeability is shown. At 23°C, it was equal to 20 μm/s in the absence of the protein (dashed line) and reached 54 μm/s after the reconstitution of AqpZ at a mass ratio of 1:100. The osmotic flux was driven by 1 M urea. pH was 7.5.
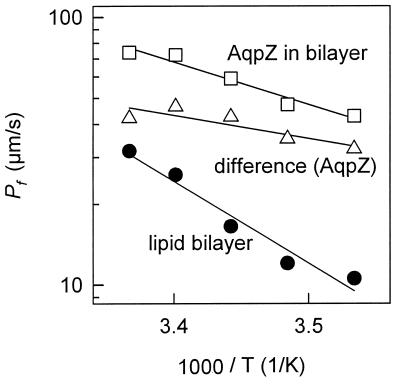
Unlike most aquaporins, AqpZ is not sensitive to mercurial compounds (5), so the presence of an aqueous pore was established by measuring the activation energy. Steady-state water flow was monitored across protein-free lipid bilayers (Schindler membranes) and bilayers containing AqpZ (Fig. 3). From the Arrhenius plot of the osmotic permeability as a function of temperature, the activation energy for protein-free lipid bilayers was calculated to be ≈14 kcal/mol (Fig. 3). The Arrhenius plot for lipid bilayers containing AqpZ represents the sum of water movements through the lipid bilayer plus water movements through AqpZ (Fig. 3). The incremental Pf caused by AqpZ corresponds to an activation energy of 4 kcal/mol.
Figure 3.
Arrhenius plot for water flow across protein-free lipid bilayers and bilayers reconstituted with AqpZ. An activation energy of about 14 kcal/mol was obtained for protein free lipid bilayers (●). AqpZ protein reconstitution (mass ratio of 1:70) decreased the activation energy (□). The incremental water permeability caused by AqpZ corresponds to an activation energy of 4 kcal/mol (▵).
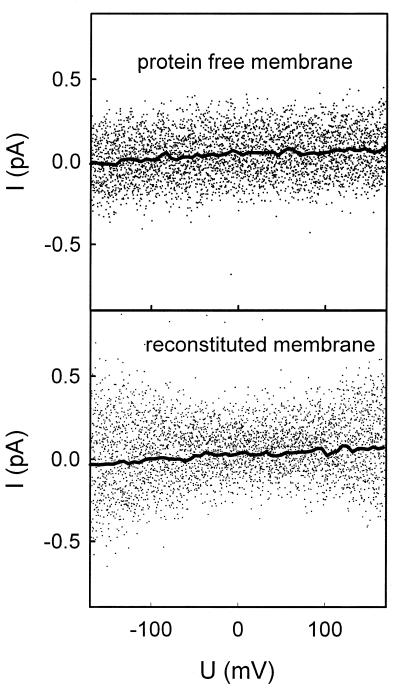
The ion conductivity of AqpZ was tested by measuring currents under voltage-clamp conditions. The current-voltage characteristics of a protein-free membrane and a membrane reconstituted with AqpZ were found to be equivalent at pH 7.5 (Fig. 4). At both physiological pH and acidic pH, the current-voltage dependence of lipid bilayers containing AqpZ was also indistinguishable from protein-free lipid bilayers, although the conductivity increased slightly with increasing proton concentration (not shown).
Figure 4.
Representative volt-ampere characteristics of a protein-free membrane and an AqpZ-containing membrane (mass ratio of 1:80). The conductances of the membranes with a diameter of 120 μm were equal to 0.25 and 0.29 pS, respectively. This difference is not significant because it is well within the range of the standard deviation usually obtained for different membranes (2.6 ± 0.6 nS/cm2). The pH was 7.5. The recording filter was a 4-pole Bessel with 3-dB corner frequency of 0.1 kHz. The acquired raw data were analyzed with the help of the tac software package (Bruxton, Seattle). Gaussian filters of 3 (points) and 0.02 Hz (spline line) were applied to reduce noise.
To obtain an upper estimate for ion/water permeability ratio, we assume that the measured membrane conductance, G, is caused by ion movement through AqpZ. G allows us to calculate the ion flux density, jion (34, 35):
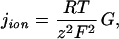 |
6 |
where R, T, F, and z are the gas constant, the absolute temperature, Faraday's constant, and the valence of the ion, respectively. According to Eq. 6 the maximum flux of ions in Fig. 4 (reconstituted protein) is about 7 × 10−16 mol⋅s−1⋅cm−2. The simultaneously measured incremental water flux density caused by AqpZ was equal to 4.3 μmol⋅s−1⋅cm−2 (Fig. 4). Consequently, less than one ion was transported per 109 water molecules.
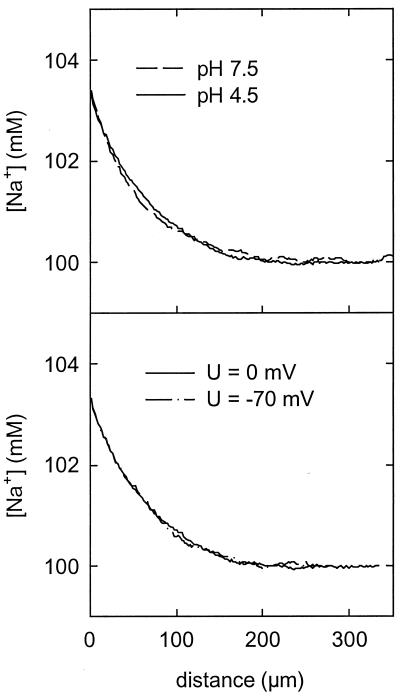
The water flux obtained from sodium concentration profiles according to Eqs. 2 and 5 did not depend on the transmembrane potential applied (Fig. 5). This observation was made both at physiological pH and acidic pH. A very weak dependence of the hydraulic conductivity on pH was observed (Fig. 5). Pf decreased from 50 ± 2 μm/s at pH 7.5 to 44 ± 2 μm/s at pH 4.5. Artifacts caused by interference between Na+ and H+ in the microelectrode are excluded by calibration at both pH values. Although reproducible, this decrease of less than 10% is of doubtful physiological importance.
Figure 5.
Sodium concentration profiles at different pH values and under voltage-clamp conditions. AqpZ was reconstituted in a protein/lipid mass ratio of 1:120. Pf decreased from 50 ± 2 μm/s at pH 7.5 to 44 ± 2 μm/s at pH 4.5. A dependence on the transmembrane potential applied has not been detected.
Discussion
Long before the discovery of aquaporins, it was predicted that putative water channels would be permeated by water but not by uncharged osmolytes or ions (1). Initial studies of human AQP1 expressed in oocytes showed that the protein conferred a 10- to 20-fold increase in osmotic water permeability without causing an increase in whole-cell currents (2). Studies of AQP1 purified from red cells and reconstituted into proteoliposomes also revealed a large increase in osmotic water permeability, but no increase in H+ flux was detected (36, 37). Thus, the studies of AQP1 supported the water-selectivity prediction.
Given the relatively large membrane conductivity of oocytes (1.5 μA/V), rare electrogenic events may be undetected. Subsequent analyses of oocytes injected with AQP1 cRNA led to the claim that AQP1 is an ion channel when activated by forskolin (13); however, this finding was not reproduced in several other laboratories (14). Subsequently, the investigators reported that oocytes injected with high concentrations of AQP1 cRNA exhibit ion channels that are directly activated by cGMP (15). Using the technique described here, we recently have examined AQP1 in planar bilayers and confirmed that a tiny fraction of the proteins conduct ions (38). When oocytes expressing other aquaporins were examined by microelectrode puncture for possible membrane currents, only AQP6 was found to exhibit membrane conductances (17). Surprisingly, the AQP6 conductances were induced by Hg2+ or low pH, although the physiological importance and general significance remain to be established, and the fraction of AQP6 proteins that conducts ions has not been determined.
Planar black lipid bilayers offer an exquisitely sensitive method for detecting membrane currents; however, these may be fraught with difficulties because protein conformations may be affected by the reconstitution procedure. By fusion of proteoliposomes with decane containing black lipid membranes, AQP0 (then known as major intrinsic protein of lens) was shown to produce voltage-dependent ion channels (9), even though these currents were not detected in oocytes expressing AQP0 (10). In addition, Nod26, a protein whose sequence is closely related to aquaporins, also produced voltage-dependent currents when symbiosomes from soybean nodules were fused with preformed black lipid membranes (11). A serious drawback of the method fusing membrane vesicles with preformed lipid bilayers is that only a relatively few membrane proteins can be introduced into the membrane. Thus, it would be impossible to assess whether a small number of water channel proteins have retained their normal conformation and water permeability.
A different method for preparing planar lipid bilayers from proteoliposomes has been developed that permits introduction of a large number of membrane proteins (18). In this case, the osmotic water permeability of the planar lipid bilayer may then be measured by determining the dilution of Na+ ions within the hypotonic compartment immediately adjacent to the membrane or the increase of Na+ concentration within the hypertonic compartment. Previously, we have used this method to characterize the water permeability of protein-free membranes (28) as well as membranes containing gramicidin (39, 40). It is important to note that microelectrode-aided measurements do not only allow the determination of water flux but also the estimation of the flux of ions that are dragged by water across the channels. In the case of gramicidin, the ion flux determined simultaneously by current measurements was identical (40).
Here we have used Schindler's method to incorporate large numbers of AqpZ water channel proteins into planar lipid bilayers. The measurements of membrane osmotic water permeability indicate that AqpZ protein remains biologically active after reconstitution into the membrane. As a simple calculation reveals, the hydraulic permeability coefficient of a single protein channel, Pf, in the planar membrane is very close to the one determined in stopped-flow experiments (19). Pf is equal to the absolute hydraulic conductivity of all channels divided by the number of channels, n(1); n is anticipated to be equal to the total number of lipid molecules, L, in the bilayer divided by the molar lipid-to-protein ratio, r:
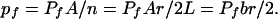 |
7 |
L is derived from two times the membrane area, A (because the membrane has two leaflets), divided by the area per lipid molecule, b. We did not measure the composition of the vesicles after dialysis; however, previous investigators have achieved protein incorporation of about 50% efficiency (41). Assuming the same level of efficiency and dividing the slope of the regression line of Fig. 2 (Inset) by the molecular mass of the AqpZ subunit (23 kDa) and multiplying the result by the molecular mass of the lipid (800 Da), Pf was calculated to be 2 × 10−14 cm3/subunit per s for b = 78 A2 (37). We calculate that more than 107 water channels are incorporated. Pf agrees closely to the value determined with AqpZ containing proteoliposomes (19). Thus, unit water permeability of the E. coli AqpZ is comparable to the mammalian homologs (42) or narrow model peptide channels that also transport water in a single file (39, 40). Our failure to detect ion channels in membranes reconstituted with an extremely high concentration of functional AqpZ water channels leads us to conclude that the protein is electrically silent.
The possibility that under certain conditions some aquaporins behave as ion channels again surfaced because of recent high-resolution structural studies. Analysis of the atomic model for human AQP1 protein deduced from electron crystallography revealed the transmembrane aqueous channel in the center of each subunit of the AQP1 tetramer (7). Moreover the atomic structure of GlpF, the sequence-related glycerol transporting protein from E. coli, revealed the pathway for glycerol and water as well an unexpected cavity at the 4-fold axis of symmetry, which resembled the structure of ion channels (8). We are unaware of any published information suggesting that GlpF behaves as an ion channel. Nevertheless, the GlpF tetramer appears to be less tightly associated than other aquaporins (6). In contrast, AqpZ appears to be a remarkably rigid tetramer that does not disassociate even when solubilized in SDS (19). Hence subunit-subunit disassociation may be avoided with AqpZ but could be detected when other aquaporin proteins are examined.
In summary, water transport across planar lipid bilayers containing AqpZ is shown to be electrically completely silent and voltage-independent. Along with the development of expression systems for the purification of large quantities of modified aquaporins (19, 43), water channel reconstitution into planar bilayers should allow investigation of the molecular basis for the extremely high water selectivity, because both water and ion movement can be measured simultaneously. Importantly, this method should permit resolution of contradictory studies reporting putative ion permeation of aquaporins studied in other systems.
Acknowledgments
We thank Olaf Andersen (Cornell Weill) and Christopher Miller (Brandeis) for thoughtful suggestions and critical readings of the manuscript. Financial support from the Deutsche Forschungsgemeinschaft (Po 533/2–3 and Po 533/7–1) and the National Institutes of Health (HL33991, HL48268, and EY11239) is gratefully acknowledged.
Abbreviations
- AqpZ
aquaporin-Z
- GlpF
glycerol facilitator
- Mops
3-(N-morpholino)propanesulfonic acid
References
- 1.Finkelstein A. Water Movement Through Lipid Bilayers, Pores, and Plasma Membranes. New York: Wiley; 1987. [DOI] [PubMed] [Google Scholar]
- 2.Preston G M, Carroll T P, Guggino W B, Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 3.Borgnia M J, Nielsen S, Engel A, Agre P. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Agre P, Bonhivers M, Borgnia M J. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- 5.Calamita G, Bishai W R, Preston G M, Guggino W B, Agre P. J Biol Chem. 1995;270:29063–29066. doi: 10.1074/jbc.270.49.29063. [DOI] [PubMed] [Google Scholar]
- 6.Borgnia M J, Agre P. Proc Natl Acad Sci USA. 2001;98:2888–2893. doi: 10.1073/pnas.051628098. . (First Published February 20, 2001, 10.1073/pnas.051628098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann J B, Engel A, Fujiyoshi Y. Nature (London) 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 8.Fu D, Libson A, Miercke L J, Weitzman C, Nollert P, Krucinski J, Stroud R M. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- 9.Ehring G R, Zampighi G, Horwitz J, Bok D, Hall J E. J Gen Physiol. 1990;96:631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulders S M, Preston G M, Deen P M T, Guggino W B, van Os C H, Agre P. J Biol Chem. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- 11.Weaver C D, Shomer N H, Louis C F, Roberts D M. J Biol Chem. 1994;269:17858–17862. [PubMed] [Google Scholar]
- 12.Rivers R L, Dean R M, Chandy G, Hall J E, Roberts D M, Zeidel M L. J Biol Chem. 1997;272:16256–16261. doi: 10.1074/jbc.272.26.16256. [DOI] [PubMed] [Google Scholar]
- 13.Yool A J, Stamer W D, Regan J W. Science. 1996;273:1216–1218. doi: 10.1126/science.273.5279.1216. [DOI] [PubMed] [Google Scholar]
- 14.Agre P, Lee M D, Devidas S, Guggino W B, Sasaki S, Uchida S, Kuwahara M, Fushimi K, Marumo F, Verkman A S, et al. Science. 1997;275:1490–1491. [Google Scholar]
- 15.Anthony T L, Brooks H L, Boassa D, Leonov S, Yanochko G M, Regan J W, Yool A J. Mol Pharmacol. 2000;57:576–588. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- 16.Yasui M, Kwon T H, Knepper M A, Nielsen S, Agre P. Proc Natl Acad Sci USA. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasui M, Hazama A, Kwon T H, Nielsen S, Guggino W B, Agre P. Nature (London) 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 18.Schindler H. Methods Enzymol. 1989;171:225–253. doi: 10.1016/s0076-6879(89)71014-4. [DOI] [PubMed] [Google Scholar]
- 19.Borgnia M J, Kozono D, Calamita G, Maloney P C, Agre P. J Mol Biol. 1999;291:1169–1179. doi: 10.1006/jmbi.1999.3032. [DOI] [PubMed] [Google Scholar]
- 20.Tamai E, Fann M C, Tsuchiya T, Maloney P C. Protein Expression Purif. 1997;10:275–282. doi: 10.1006/prep.1997.0754. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald R C, MacDonald R I, Menco B P M, Takeshita K, Subbarao N K, Hu L R. Biochim Biophys Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- 22.Pattus F, Desnuelle P, Verger R. Biochim Biophys Acta. 1978;507:62–70. doi: 10.1016/0005-2736(78)90374-7. [DOI] [PubMed] [Google Scholar]
- 23.Schindler H. Biochim Biophys Acta. 1979;555:316–336. doi: 10.1016/0005-2736(79)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Schindler H. FEBS Lett. 1980;122:77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- 25.Montal M, Mueller P. Proc Natl Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fettiplace R, Haydon D A. Physiol Rev. 1980;60:510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- 27.Dainty J, House C R. J Physiol (London) 1966;182:66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pohl P, Saparov S M, Antonenko Y N. Biophys J. 1997;72:1711–1718. doi: 10.1016/S0006-3495(97)78817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohl P, Saparov S M, Antonenko Y N. Biophys J. 1998;75:1403–1409. doi: 10.1016/S0006-3495(98)74058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amman D. Ion-Selective Microelectrodes: Principles, Design, and Application. Berlin: Springer; 1986. [Google Scholar]
- 31.Antonenko Y N, Denisov G A, Pohl P. Biophys J. 1993;64:1701–1710. doi: 10.1016/S0006-3495(93)81542-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohl P, Antonenko Y N, Rosenfeld E H. Biochim Biophys Acta. 1993;1152:155–160. doi: 10.1016/0005-2736(93)90242-r. [DOI] [PubMed] [Google Scholar]
- 33.Antonenko Y N, Bulychev A A. Biochim Biophys Acta. 1991;1070:279–282. doi: 10.1016/0005-2736(91)90089-q. [DOI] [PubMed] [Google Scholar]
- 34.Hodgkin A L. Biol Rev. 1951;26:339–365. [Google Scholar]
- 35.Walter A, Hastings D, Gutknecht J. J Gen Physiol. 1982;79:917–933. doi: 10.1085/jgp.79.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeidel M L, Ambudkar S V, Smith B L, Agre P. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- 37.Zeidel M L, Nielsen S, Smith B L, Ambudkar S V, Maunsbach A B, Agre P. Biochemistry. 1994;33:1606–1615. doi: 10.1021/bi00172a042. [DOI] [PubMed] [Google Scholar]
- 38.Saparov, S. M., Kozono, D., Rothe, U., Agre, P. & Pohl, P. (2001) J. Biol. Chem., in press. [DOI] [PubMed]
- 39.Pohl P, Saparov S M. Biophys J. 2000;78:2426–2434. doi: 10.1016/S0006-3495(00)76786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saparov S M, Antonenko Y N, Koeppe R E, Pohl P. Biophys J. 2000;79:2526–2534. doi: 10.1016/S0006-3495(00)76493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zampighi G A, Hall J E, Kreman M. Proc Natl Acad Sci USA. 1985;82:8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang B, Verkman A S. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 43.Coury L A, Mathai J C, Prasad G R, Brodsky J L, Agre P, Zeidel M L. Am J Physiol. 1998;43:F34–F42. doi: 10.1152/ajprenal.1998.274.1.F34. [DOI] [PubMed] [Google Scholar]
- 44.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]