Summary
Impaired human oligodendrocyte progenitor cell (hOPC) differentiation likely contributes to failed remyelination in multiple sclerosis. The characterization of molecular pathways that regulate hOPC differentiation will provide means to induce remyelination. In this study, we determined the gene expression profile of PDGFαR+ hOPCs during initial oligodendrocyte commitment. Weighted gene coexpression network analysis was used to define progenitor and differentiation-specific gene expression modules and functionally important hub genes. These modules were compared with rodent OPC and oligodendrocyte data to determine the extent of species conservation. These analyses identified G-protein β4 (GNB4), which was associated with hOPC commitment. Lentiviral GNB4 overexpression rapidly induced human oligodendrocyte differentiation. Following xenograft in hypomyelinating shiverer/rag2 mice, GNB4 overexpression augmented myelin synthesis and the ability of hOPCs to ensheath host axons, establishing GNB4 as functionally important in human myelination. As such, network analysis of hOPC gene expression accurately predicts genes that influence human oligodendrocyte differentiation in vivo.
Keywords: oligodendrocyte progenitor, species conservation
Highlights
-
•
Transcriptional database of differentiating human oligodendrocyte progenitor cells
-
•
WGCNA reveals coordinated gene networks in oligodendrocyte specification
-
•
Dataset comparison identifies unique and shared cross-species gene networks
-
•
G-protein β4 (GNB4) expression accelerates human oligodendrocyte differentiation
Oligodendrocyte progenitor cell differentiation requires the coordinated regulation of many genes. Sim and colleagues capture the transcriptional profile during human primary oligodendrocyte specification. Using WGCNA and cross-species dataset comparisons, they identify jointly regulated genes, conserved across human and rodent species. Targeting highly regulated genes, they show that G-protein subunit β4 expression accelerates human oligodendrocyte differentiation.
Introduction
Oligodendrocytes produce myelin that facilitates saltatory conduction in the CNS and provide trophic support to axons (Lee et al., 2012, Morrison et al., 2013). In multiple sclerosis (MS), there is limited capacity for endogenous repair or remyelination (Franklin and Ffrench-Constant, 2008). As failed remyelination in animal models is associated with axonal degeneration (Irvine and Blakemore, 2008), the failure of remyelination in MS likely contributes to progressive disease (Trapp and Nave, 2008). The persistence of undifferentiated oligodendrocyte progenitor cells (OPCs) in MS lesions suggests that interventions that promote OPC commitment and differentiation may improve remyelination (Chang et al., 2000, Kuhlmann et al., 2008). However, it is not clearly understood which signaling cascades are active in human oligodendrocytes and which signals stimulate the early stages of differentiation, regulating the exit from cell cycle and commitment to oligodendrocyte fate.
The molecular processes that underpin oligodendrocyte differentiation in development and following demyelination have been extensively studied (reviewed in Bergles and Richardson, 2015, Wheeler and Fuss, 2016). This has led to the identification of several attractive targets for therapeutic intervention (reviewed in Franklin and Goldman, 2015). Recently, unbiased high-throughput screening methods have identified small molecules and pathways that increase oligodendrocyte differentiation in vitro (Deshmukh et al., 2013, Mei et al., 2014, Najm et al., 2015). One limitation of these screens has been the reliance on rodent primary cells. This is important, as the timing and scale of OPC generation are vastly different between humans and rodents and, in some cases, the responses to specific tropic signals differ substantially between species (reviewed in Dietz et al., 2016). We have taken an alternative approach to directly study human primary OPCs by isolation and transcriptomic analysis (Abiraman et al., 2015, Sim et al., 2011, Wang et al., 2013a).
In the current study, we examined the transcriptional processes underlying initial oligodendrocyte commitment of human OPCs (hOPCs) using weighted gene coexpression network analysis (WGCNA) (Zhao et al., 2010). WGCNA enables the identification of gene networks based on a pairwise Pearson correlation of all genes, thus clustering together genes with similar expression patterns. Genes with the greatest contribution or connectivity to these networks often represent functionally important genes and are a commonly referred to as hub genes. WGCNA was previously used to identify cell type-specific hub genes in brain (Miller et al., 2010, Oldham et al., 2008) and functionally relevant genes in glioma (Horvath et al., 2006). A further advantage is that WGCNA permits cross-species analysis to identify both conserved and divergent gene expression between humans and rodents (Miller et al., 2010, Oldham et al., 2006). As such, we also utilized WGCNA to re-analyze additional datasets from human (Abiraman et al., 2015) and rodent OPCs (Cahoy et al., 2008, Dugas et al., 2006) to define strongly species-conserved gene expression modules associated with oligodendrocyte differentiation. As a proof of concept, we confirmed the expression of several previously uncharacterized OPC- and oligodendrocyte-expressed transcripts and examined their function. We found that G-protein subunit β4 (Gβ4), GNB4, was highly upregulated during oligodendrocyte commitment, and that GNB4 overexpression induced oligodendrocyte differentiation of hOPCs. Importantly, Gβ4/GNB4 accelerated axonal ensheathment by hOPCs following transplantation. These data suggest that Gβ4/GNB4 alters the responsiveness of OPCs to promote differentiation, possibly by altering the effects of other inhibitory G-protein-coupled receptor pathways.
Results
Transcriptional Profiling of Human OPC Differentiation
To determine the transcriptional regulation of hOPC differentiation, we cultured freshly isolated human primary platelet-derived growth factor α receptor (PDGFαR)+ OPCs (Sim et al., 2011) and assessed their differentiation over 4 days in vitro. To distinguish between transcriptional changes associated simply with time spent in culture and oligodendrocyte commitment, we treated matched control cultures with PDGF-AA, a known OPC mitogen that delays oligodendrocyte differentiation. hOPCs were fixed on day 4 and assessed for oligodendrocyte differentiation (Figures 1A–1C). As expected, in the absence of PDGF-AA, many cells stained positively for O4 and exhibited a mature branched oligodendrocytic morphology (33.2% ± 4.8%, n = 4 individual tissue samples). A significant fraction of these also expressed myelin basic protein (MBP) (data not shown and Sim et al., 2011). Conversely, in PDGF-AA-containing medium, few cells differentiated as O4+ oligodendrocytes (6.2% ± 4.1%) (Figure 1C).
Figure 1.
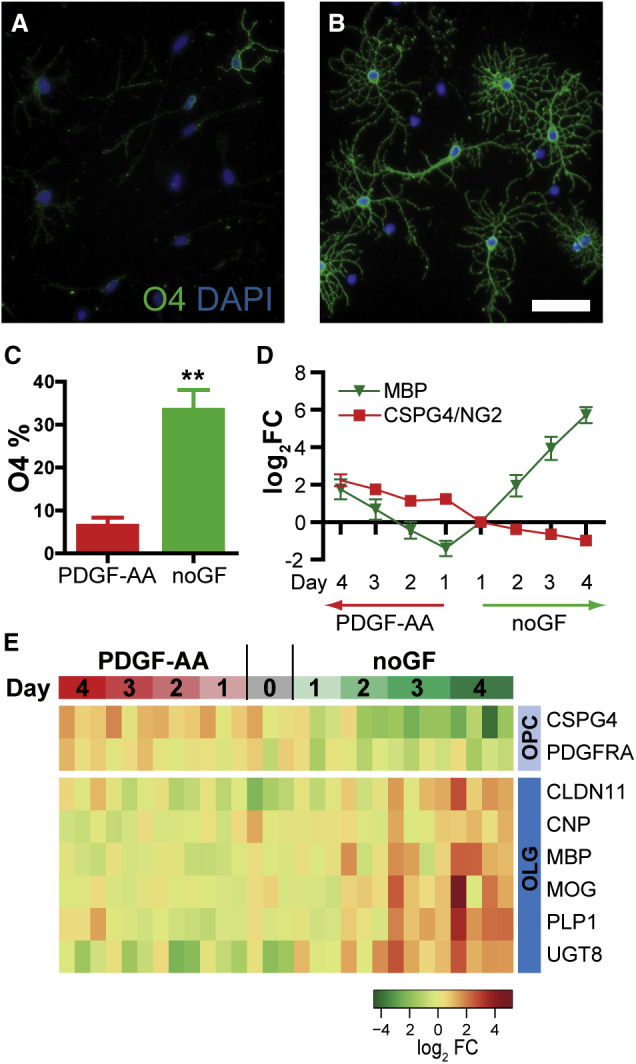
Human Oligodendrocyte Progenitor Cell Differentiation In Vitro
(A and B) Human PDGFαR + OPCs were cultured for 4 days in serum-free medium with PDGF-AA (A) or in the absence of mitogens (no growth factors [noGF]) (B). Cultures were stained for immature oligodendrocyte marker O4 (green). Scale bar, 25 μm.
(C) O4 quantification (mean ± SEM, n = 4 separate human samples). ∗∗p < 0.001, t test.
(D) qPCR of MBP and CSPG4 (log2 fold change relative to day 1 noGF, mean ± SEM, n = 4 human samples).
(E) Heatmap of OPC- and oligodendrocyte-enriched genes.
Total RNA was isolated on each day and qRT-PCR was performed for MBP and NG2 (CSPG4), a marker of OPCs. Consistent with oligodendrocyte differentiation, MBP mRNA was upregulated more than 300-fold in differentiating conditions (Figure 1D), but was not significantly increased in PDGF-AA-treated cultures (n = 4, one-way ANOVA). Conversely, CSPG4 mRNA was significantly downregulated in the differentiating conditions and was upregulated by PDGF-AA treatment (n = 4 individual tissue samples, p < 0.05, Tukey's post hoc test) (Figure 1D).
To characterize the transcriptional responses attendant with human oligodendrocyte differentiation, we performed microarray analysis. RNA was purified from matched cultures derived from four individual patient brain samples. We first examined markers of OPCs and oligodendrocyte differentiation. hOPCs cultured in differentiating conditions exhibited a time-dependent progressive increase in expression of myelin protein genes, including CLDN11, MBP, MOG, and PLP1, and a corresponding decrease in PDGFRA and CSPG4 (Figure 1E). These effects were effectively blocked by PDGF-AA, allowing us to discriminate between time- and differentiation-dependent effects.
Weighted Gene Coexpression Network Analysis of Human OPC Differentiation
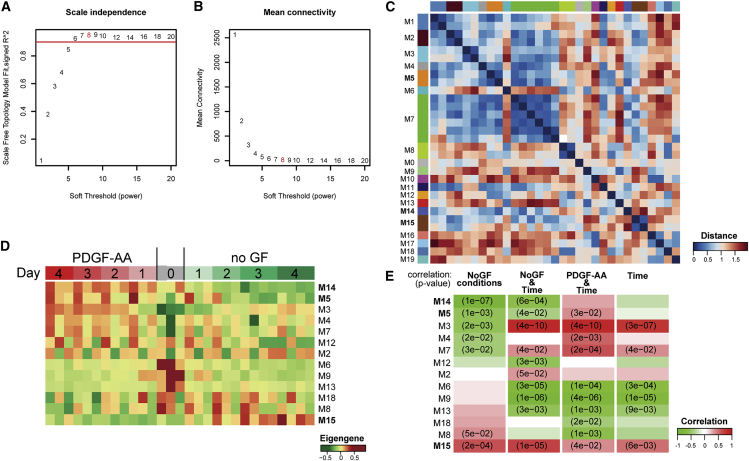
To take full advantage of time as a continuous parameter and to systematically analyze the gene expression profile of hOPC differentiation in an unsupervised fashion, we applied WGCNA. WGCNA is a network analysis-based technique that utilizes the extent of gene-gene coregulation to determine functionally relevant modules or sets of genes (Zhao et al., 2010). In brief, pairwise Pearson correlations were power-transformed to calculate adjacency values, which penalize weakly correlated genes. The power was selected to ensure that the resulting gene network conformed to a biologically relevant scale-free topology (Figure 2A) and maximized the connectedness of genes within the network, i.e., it maximized the net adjacency values (Figure 2B). Topological overlap, which quantifies the similarity in adjacency values associated with two genes, was used in unsupervised hierarchical gene clustering. As such, each gene was grouped into modules containing other highly coregulated and connected genes. Analysis of each module's first principal component, referred to as its eigengene, revealed several highly related modules (Figure 2C). As described previously (Oldham et al., 2008), we merged highly related modules to generate a final network composed of 20 distinct modules.
Figure 2.
Weighted Gene Coexpression Network Analysis of hOPC Differentiation
(A and B) Analysis of network topology to determine soft-thresholding power. (A) Fit of power-transformed gene-pairwise Pearson correlation to scale-free topology as a function of soft threshold. (B) Mean network connectivity as a function of soft threshold. Soft-thresholding power of 8 was selected to maximize the model fit.
(C) Heatmap of Pearson correlation between eigengenes of WGCNA modules.
(D) Expression profiles of merged module eigengene illustrate distinct expression patterns in final network.
(E) Correlation of module eigengenes with experimental variables: time, culture condition (PDGF-AA, noGF), and their interaction.
We examined the expression profile of each module's eigengene, which is considered representative of the gene expression profiles contained in that module (Horvath and Dong, 2008). Several module eigengene profiles were significantly correlated with experimental parameters, such as media conditions and time in vitro (Figure 2E). For example, M3 was highly correlated with time (r = 0.79, p = 1.3 × 10−7) but was negatively correlated with culture in differentiating conditions (no growth factors [noGF], r = −0.55, p = 0.002). Using this approach, we focused on modules with eigengenes that correlated with the pro-differentiating conditions (noGF) and time (Figures 2D and 2E). Of note, modules M14 (r = 0.81, p = 1 × 10−7) and M5 (r = 0.57, p = 0.001) were strongly correlated with progenitor growth conditions (PDGF-AA) but not dependent on time, consistent with maintenance of OPC fate. M15 represented the only module showing upregulation and positive correlation with differentiating conditions (r = 0.63, p = 2.0 × 10−4).
WGCNA Identifies Conserved Modules Expressed during Human and Rodent OPC Differentiation
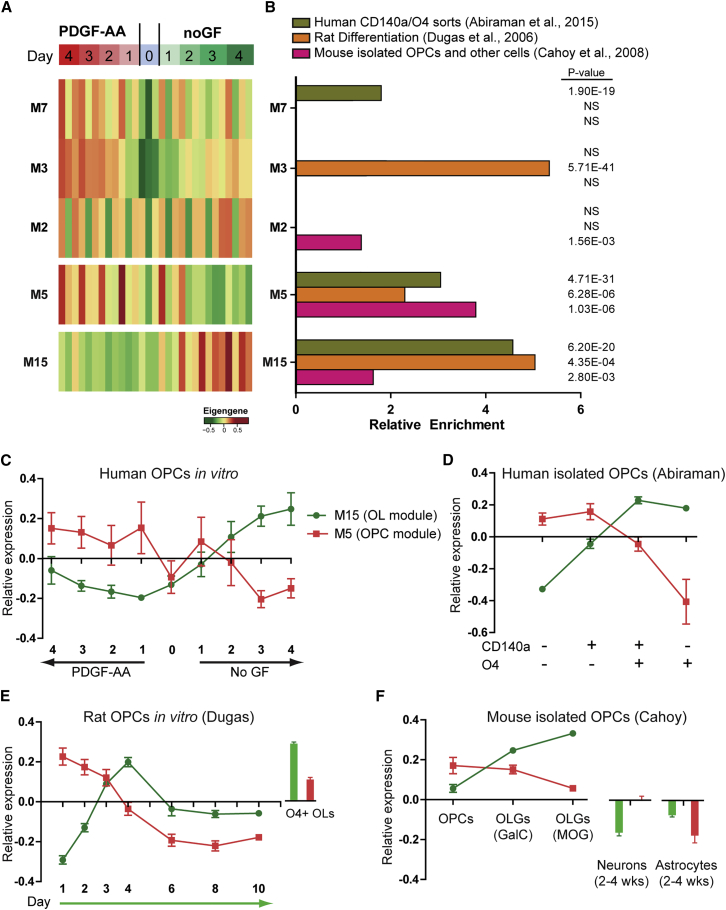
WGCNA provides a platform-independent approach to compare gene expression across experiments and species (Miller et al., 2010, Oldham et al., 2006). We performed WGCNA on three additional independent datasets that examined OPC transcriptional profiles. We reanalyzed (1) rat primary OPCs undergoing differentiation in vitro (Dugas et al., 2006); (2) primary mouse isolated OPCs and oligodendrocytes (Cahoy et al., 2008), and (3) primary human isolated OPCs and early oligodendrocytes (Abiraman et al., 2015). Following matched approaches for normalization and summarization, human homologs were found and annotated in the rodent datasets and WGCNA performed. We identified 19 modules in Dugas, 7 modules in Cahoy, and 10 modules in Abiraman datasets (Figure S1).
Following cross-species annotation, the number of homologous genes shared between individual modules (M1–M15) and each of the modules identified in the other three datasets were calculated in a pairwise fashion. The significance of theses module-module relationships was determined by calculation of hypergeometric probabilities for each pair (Table S1). Intriguingly, only two modules identified in the analysis of hOPC differentiation in vitro were preserved across the other three datasets (false discovery rate [FDR]-corrected hypergeometric test) (Figure 3). Several modules were found to have significant overlap with only one dataset. For example, M3D, which we found to be regulated by time in vitro in hOPCs, was similarly regulated in rat OPCs in vitro (Figure S2), suggesting that this module represents an artifact of gene regulation governed by cell culture. M2C, which was only conserved in hOPC differentiation and the Cahoy mouse dataset (FDR-corrected hypergeometric test, p = 0.002), was instead characterized by genes expressed in cultured astrocytes (Figure S2). In contrast, both M5 and M15, which were preserved across all datasets, were found to have highly consistent eigengene profiles associated with high expression observed in OPCs and oligodendrocytes, respectively. This suggests that M5 and M15 comprise functionally important and conserved modules in the regulation of OPC commitment and differentiation.
Figure 3.
Conservation of OPC and Oligodendrocyte WGCNA Modules between Species
WGCNA was performed on rat oligodendrocytes (OLGs) (Dugas et al., 2006), isolated mouse OPCs/OLGs (Cahoy et al., 2008), and isolated hOPCs (Abiraman et al., 2015). Following module overlap analysis between these datasets, only five modules exhibited substantial and significant overlap (p < 0.05, FDR-corrected hypergeometric test).
(A) Heatmap of eigengene expression for the five modules.
(B–F) Relative enrichment of shared genes in each matching module (compared with random list) and corresponding hypergeometric test p value. Eigengene expression profiles of cross-species-conserved modules M15 and M5 in differentiating hOPCs (C), human isolated OPCs (D), rat differentiating OPCs (E), and isolated mouse OPCs/OLGs (F). Mean ± SEM.
Conserved OPC and Oligodendrocyte Modules Were Functionally Distinct
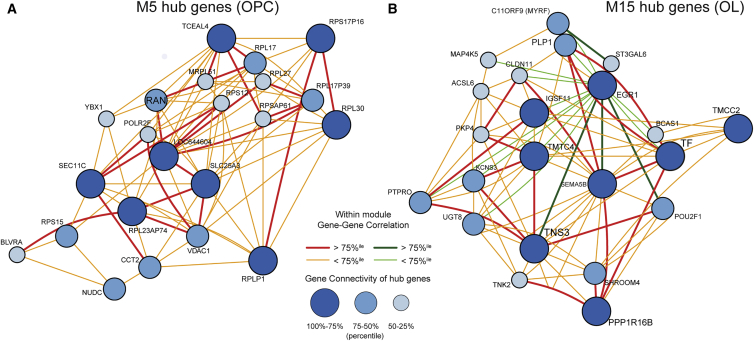
To determine the principal constituents of modules M5 and M15, we performed pathway analysis by determining the relative over-representation of individual genes within each module using gene ontology (GO) (Table S2). Module M5 and its homologs across the other datasets were enriched for GO terms associated with RNA synthesis including ribosomal subunit biogenesis and assembly. We defined hub genes, which have been shown to act as potential regulators of the entire module (Johnson et al., 2009, Oldham et al., 2008), using intra-module connectivity, i.e., the summation of all Pearson gene-gene correlations. Shared among the M5 hub genes (Figure 4A) and its homologs, we noted ribosomal proteins S5/S9/S15 and heterogeneous nuclear ribonucleoprotein A/B (HNRNPAB), which is involved in mRNA splicing and pre-mRNA processing and regulates gene expression in neurogenic progenitors (Lein et al., 2007), and may act to delay oligodendrocyte differentiation (Sinnamon et al., 2012). Both human networks contained several genes involved in mitochondrial electron transport (M5 and M5A, p < 0.001). We also noted PTPRZ1 in both M5 and M5D; PTPRZ1 is involved in hOPC self-renewal (McClain et al., 2012) and differentiation (Sim et al., 2006). Together, this suggested active roles for transcription and metabolism for module M5 that are conserved in human and rodent OPCs.
Figure 4.
Hub Genes of OPC and OLG Modules
Species-conserved OPC and oligodendrocyte differentiation-specific modules M5 (A) and M15 (B) were further examined to identify hub genes, which were expected to exert the largest influence on module constituents. The top 30 genes were selected based on the sum of intra-module connectivity between genes. The node size for each hub gene represents the relative hubness, and connection color indicates both the direction and degree of correlation with other hub genes.
Consistent with increased expression during differentiation across the four datasets, M15 was significantly enriched for genes involved in myelination (M15, p = 0.001; M15C, p < 0.001), and oligodendrocyte (M15C, p = 0.003) and glial (M15A, p = 0.001) differentiation. Importantly, the essential oligodendrocyte transcription factor, MYRF, was identified among the hub genes in M15 (human differentiation) (Figure 4B), M15C (mouse), and M15A (human FACS). (Note: the microarray used in the Cahoy dataset did not contain probes against MYRF). Likewise, several other known oligodendrocyte genes were identified in M15 and its homologs. Therefore, M15 was characterized as an oligodendrocyte differentiation module, which was conserved across species.
Identification of Regulators of Human OPC Commitment and Differentiation
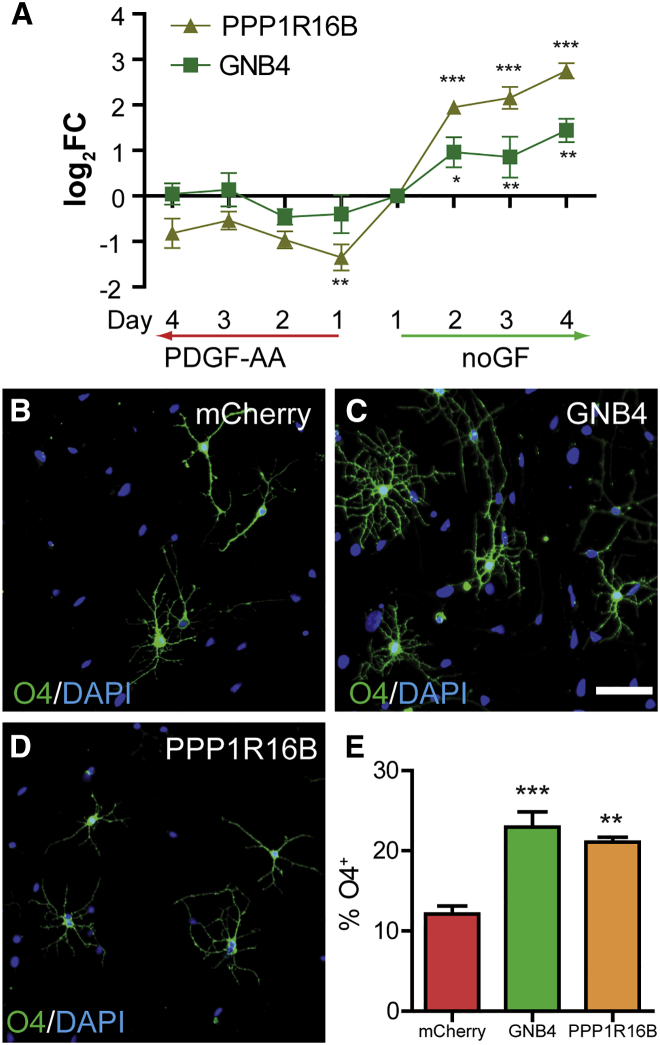
To provide proof of concept that WGCNA identified functionally relevant genes, we examined module M15 for genes with no known function in oligodendrocytes (Table 1). We focused on genes with differential upregulation in differentiated oligodendrocytes and those that were preserved across M15 homologs. The most highly upregulated was breast cancer amplified sequence 1, BCAS1, which was increased more than 50-fold in differentiating hOPCs and preserved in both M15 and M15C. Additionally, BCAS1 was significantly enriched in human O4+ oligodendrocytes (Abiraman et al., 2015) as well as in epiblast-derived oligodendrocytes (Najm et al., 2011). We confirmed that BCAS1 was significantly regulated using qPCR (Figure S3A) (n = 4 individual fetal samples, repeated-measures ANOVA, F(7,21) = 53.1, p < 0.0001). Consistent with the microarray data, BCAS1 was strongly and progressively upregulated with time in differentiating conditions (Tukey's post hoc test, p < 0.001 between each day). PPP1R16B, protein phosphatase 1 regulatory subunit 16B, was preserved only in the hOPC FACS dataset (M15A), but was also highly expressed by mouse MOG+ oligodendrocytes (Cahoy et al., 2008). qPCR confirmed upregulation of PPP1R16B in differentiation conditions (Figure 5A), as well as strong upregulation with time (Tukey's post hoc p < 0.001). Finally, we identified guanine nucleotide-binding protein subunit Gβ4 (GNB4) as the only M15 upregulated transcript whose expression was also preserved in M15D and M15A modules, but was also increased in mouse oligodendrocytes (Cahoy et al., 2008) and differentiating mouse Oli-Neu OPCs (Gobert et al., 2009). Consistent with the WGCNA predictions, GNB4 was significantly upregulated progressively each day during OPC commitment (Figure 5A) (p < 0.05).
Table 1.
Differentially Expressed Genes in Module M15
| Symbol | NCBI Entrez ID | Description | M15 Connectivity (Rank) | Day 4 versus Day 0 (No GF) |
Preserved in |
|||
|---|---|---|---|---|---|---|---|---|
| Log2 FC | q Value | M15D | M15C | M15A | ||||
| BCAS1 | 8537 | breast carcinoma amplified sequence 1 | 17.0 (22) | 5.80 | 3.29 × 10−6 | X | ||
| PPP1R16B | 26051 | protein phosphatase 1, subunit 16B | 21.0 (6) | 2.95 | 1.42 × 10−3 | X | ||
| GNB4 | 59345 | G protein β4 | 7.7 (119) | 2.43 | 2.09 × 10−4 | X | X | |
| HEXDC | 284004 | hexosaminidase D | 6.1 (159) | 2.08 | 2.88 × 10−3 | X | ||
| MAN2A2 | 4122 | mannosidase α class 2A2 | 5.8 (168) | 1.93 | 2.28 × 10−3 | |||
| POU2F1 | 5451 | POU class 2 homeobox 1 | 18.8 (12) | 1.78 | 2.04 × 10−3 | |||
| DUSP22 | 100134291 | similar to MAPK phosphatase x | 7.8 (118) | 1.72 | 7.92 × 10−4 | |||
| NPHP3 | 27031 | nephronophthisis 3 | 1.9 (368) | 1.58 | 1.56 × 10−3 | |||
Previously undescribed members of oligodendrocyte differentiation module M15 were identified that exhibited differential expression during oligodendrocyte differentiation, day 4 versus day 0 in differentiating conditions (log2 FC > 1, q value < 0.01). “X” indicates significant overlap between modules by FDR-corrected hypergeometric testing.
Figure 5.
Overexpression of PPP16R1B and GNB4 Induces hOPC Differentiation In Vitro
(A) qPCR of PPP16R1B and GNB4 in proliferating (PDGF-AA) or differentiating (noGF) conditions (mean ± SEM log2 fold change relative to day-1 conditions, n = 4 patient samples, Tukey's post test versus day-1 noGF conditions).
(B–D) hOPCs were infected with lentiviruses overexpressing GNB4 (C), PPP1R16B (D), or mCherry (control) (B). The extent of O4+ oligodendrocyte differentiation was assessed at 4 days post infection.
(E) Both PPP16R1B and GNB4 overexpression resulted in a significant increase in the proportion of O4+ cells (mean ± SEM, n = 4, repeated measures 1-way ANOVA, Dunnett's post hoc test).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, post test. Scale bar, 50 μm.
To determine whether the expression of these genes might be sufficient to drive oligodendrocyte differentiation from hOPCs, we overexpressed BCAS1, PPP1R16B, and GNB4 in hOPCs. For PPP1R16B and GNB4, hOPCs were infected with lentivirus and cultured for 4 days in the absence of PDGF-AA. We initially assessed the extent of O4+ oligodendrocyte differentiation. Interestingly, both GNB4 and PPR1R16B significantly increased the percentage of O4+ oligodendrocytes compared with mCherry controls (n = 4 fetal samples; 1-way ANOVA, F(2,8) = 18.4, Dunnett's post hoc test versus mCherry, p < 0.01) (Figures 5D and 5E). GNB4-infected cells developed into oligodendrocytes with highly complex and branched morphologies, often extending O4+ processes that contacted and extended along neighboring cells. In contrast, BCAS1 overexpression did not influence oligodendrocyte differentiation (Figures S3B and S3C).
The increased proportion of O4+ oligodendrocytes following PPP1R16B or GNB4 overexpression could be due to multiple effects on OPC fate. Therefore, we examined effects on cell number, lineage commitment, and proliferation. The increase in O4 percentage was not accompanied by changes in overall live cell number between groups (one-way ANOVA, F(2,8) = 0.2, p = 0.82) (Figure S4A).
We further analyzed the effects of GNB4 overexpression on OPC lineage commitment and proliferation. GNB4 overexpression did not alter the percentage of OLIG2+ cells (65% ± 11% versus 56% ± 8% mCherry; p = 0.55; n = 4 fetal samples) (Figures S4B–S4G). As an increase in O4+ cells could be caused by reduced OPC proliferation, we bromodeoxyuridine (BrdU)-pulsed matched cultures. The percentage of BrdU+ proliferating cells in GNB4-infected cells did not differ from mCherry cells (63% ± 12% versus 59% ± 13% mCherry; t test p = 0.42; n = 4 fetal samples). In contrast, BCAS1 overexpression substantially reduced BrdU incorporation (Figures S3D and S3E). Together, these data suggested that GNB4 and PPP1R16B overexpression were sufficient to drive OPC commitment to oligodendrocyte fate.
As the pro-differentiation effect of GNB4 overexpression was so pronounced, we sought to determine whether GNB4 expression was required for differentiation. Using three distinct and specific small interfering RNAi (siRNAi) sequences, we transfected proliferating hOPCs. Individual siRNA transfection was sufficient to knock down GNB4 mRNA by more than 90%, with the combined transfection reducing expression by >95% (98.2% ± 0.1% versus scrambled control, p < 0.0001, n = 3 fetal samples). Surprisingly, GNB4 knockdown of combined or individual siRNA sequences resulted in substantial cell death at 48 hr after transfection, characterized by process retraction, surface detachment, and reduced live cell number compared with matched cultures transfected with control non-targeting siRNA (Figure S5). Importantly, this effect was specific to GNB4, as other siRNAs did not influence cell survival (data not shown). Cell death following GNB4 knockdown was observed in OPCs maintained under both proliferating and pro-differentiation conditions. As such, these results indicate that Gβ4 expression is necessary for the survival of hOPCs.
GNB4/Gβ4 Overexpression Attenuates Inhibitory GPCR Signaling in Human OPCs
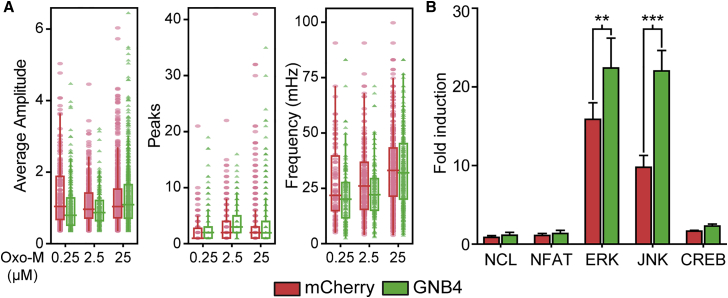
G-protein-coupled receptors (GPCRs) are key regulators of OPC differentiation in development and disease. For example, muscarinic receptor signaling inhibits oligodendrocyte differentiation and acts to delay myelin repair by transplanted hOPCs and remyelination in rodent models (Abiraman et al., 2015, Deshmukh et al., 2013, Mei et al., 2014). While all five β subunits were expressed in OPCs and differentiating oligodendrocytes (Figure S6), only GNB4 mRNA was upregulated during OPC differentiation and was enriched in OPCs relative to other neural cell types (Abiraman et al., 2015, Cahoy et al., 2008, Dugas et al., 2006). Individual Gβ subunits are known to influence specific GPCR effector signaling pathways (Khan et al., 2013). As GPCR muscarinic agonists block hOPC differentiation, we sought to establish whether GNB4 overexpression could influence muscarinic agonist-induced calcium oscillations mediated by the Gq-coupled M1 or M3 receptor. Using a lentiviral GCaMP6s to express a calcium-sensitive fluorescence reporter, we observed that the muscarinic agonist oxotremorine-M induced calcium waves in a dose-dependent manner with a typical duration of >4 min and frequency of 30–50 mHz. GNB4 overexpression did not alter the amplitude or frequency of calcium waves (Figure 6A) or the proportion of responding hOPCs (logEC50 = −5.9 ± 0.2 versus −6.5 ± 0.3 for mCherry and GNB4, respectively; Figure S7) compared with mCherry control hOPCs. Thus, GNB4 likely does not influence muscarinic receptor-mediated inhibition of oligodendrocyte differentiation.
Figure 6.
GNB4/Gβ4 Overexpression Induces MAPK Signaling
Fetal PDGFαR+ hOPCs were cultured and infected with intracellular Ca2+ reporter GCaMP6s. Following GNB4 or mCherry overexpression, time-lapse microscopy of Ca2+ response after Oxo-M treatment was recorded and analyzed.
(A) Individual responsive cell Ca2+ measurements for average spike amplitude (left), total number of peaks (center), and frequency (right), along with their box plot. GNB4 overexpression did not significantly change Ca2+ response (p > 0.05, n > 90 cells per condition, two-way ANOVA with Tukey’s HSD post test, n = 3).
(B) hOPC signaling was measured using luciferase-based reporter after infection with GNB4 or mCherry lentivirus. Activity is reported as fold change in luminescence compared with negative control. ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3), two-way ANOVA with Bonferroni post test.
To more broadly assess the mechanisms by which GNB4 regulates signaling in hOPCs, we determined the effects of GNB4 overexpression on several common signaling pathways using a panel of lentiviral luciferase reporters. GNB4 overexpression did not induce either cyclic AMP/protein kinase A (CREB) or protein kinase C/Ca2+ (NFAT) signaling, which is consistent with lack of effect on muscarinic signaling (Figure 6B). In serum-free conditions without mitogens, basal signaling was only detected in ERK (ELK-1/SRF) and JNK (AP-1) pathways (Figure 6B). Interestingly, both pathways were strongly activated following lentiviral GNB4 overexpression. While JNK signaling has not been associated with OPC differentiation, ERK1/2 activation is known to promote oligodendrocyte differentiation and myelin synthesis (Fyffe-Maricich et al., 2011, Ishii et al., 2012) and, therefore, may contribute to the observed effects of GNB4/Gβ4 expression on hOPC differentiation.
GNB4/Gβ4 Promotes Human Oligodendrocyte Differentiation and Axon Ensheathment
To further establish a functional role of Gβ4 in hOPC differentiation, we utilized the shiverer/rag2 model of hypomyelination that models stem cell-mediated myelin repair and enables assessments of human myelination in vivo (Abiraman et al., 2015, Wang et al., 2014). Following infection with either GNB4- or mCherry-expressing lentivirus, 105 hOPCs were transplanted directly into the corpus callosum of postnatal day 2 shiverer/rag2 pups. Recipient animals were euthanized at 8 weeks, at which time very few hOPCs have typically undergone differentiation into MBP-expressing oligodendrocytes (Abiraman et al., 2015, Sim et al., 2011). Human nuclear antigen (hNA)-positive cells were found in the corpus callosum at 8 weeks with a similar density and distribution in both groups (911 ± 275 versus 902 ± 121 hNA+ cells for mCherry and GNB4, respectively; n = 4–7 mice) (Figures 7A and 7B).
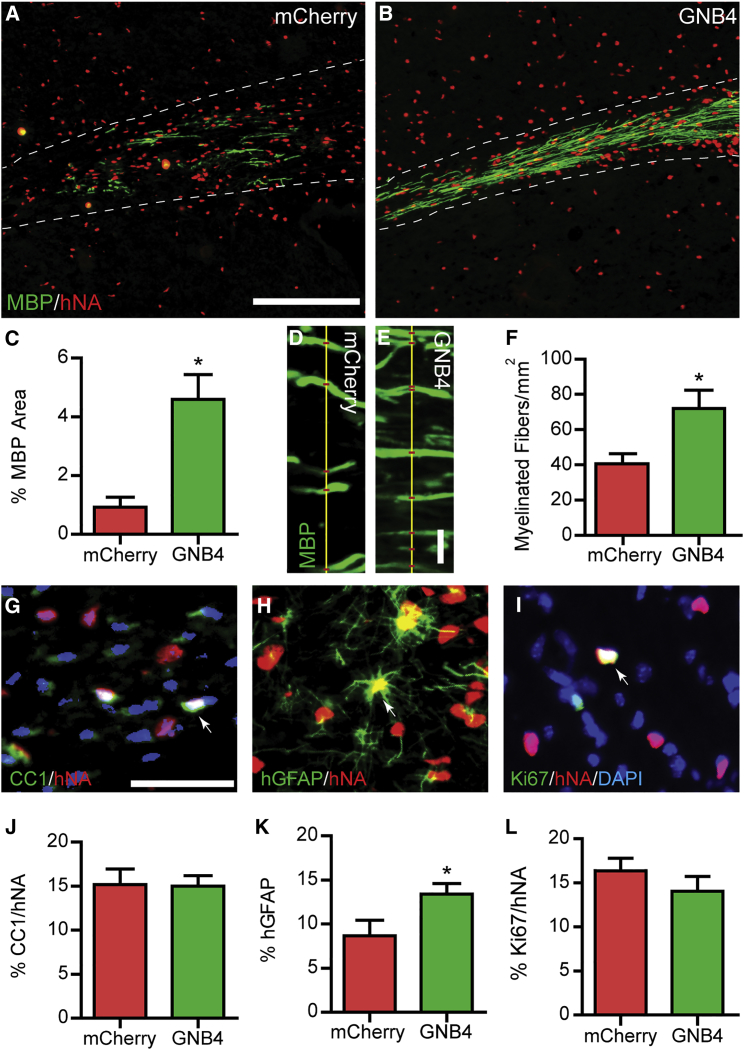
Figure 7.
GNB4/Gβ4 Overexpression Enhances Human Myelination by Transplanted hOPCs
GNB4 or mCherry overexpressing hOPCs were transplanted into the corpus callosum of 2- to 3-day shiverer/rag2 mice and euthanized at 8 weeks to assess engraftment and myelination.
(A and B) Immunostaining for human nuclear antigen (hNA) and MBP to assess engraftment and oligodendrocyte differentiation (corpus callosum outlined by dotted lines).
(C) Quantification of MBP area as a proportion of corpus callosum (mean ± SEM, n = 3–6 mice).
(D and E) Confocal microscopy of MBP+ ensheathed axons.
(F) Density of myelinated axons was quantified within engrafted regions (mean ± SEM, n = 3–4).
(G–L) Cell fate was assessed by immunofluorescence for CC1+hNA+ human oligodendrocytes (G), hGFAP+hNA+ human astrocytes (H), and Ki-67+hNA+ dividing human cells (I). Arrows indicate double-positive human cells in each panel. The proportion is quantified in (J) to (L). GNB4 did not significantly effect oligodendrocyte maturation (J) or proliferation (L) among transplanted cells, but increased the proportion of differentiated astrocytes (K), compared with mCherry controls.
∗p < 0.05, n = 3–6 mice per group, unpaired t test. Scale bars, 100 μm (A; applies also to B), 10 μm (E; applies also to D), and 25 μm (G; applies also to H and I).
To determine the effect of GNB4 overexpression on oligodendrocyte maturation, we assessed MBP immunohistochemistry which is absent in shiverer mice. Strikingly, animals transplanted with GNB4-expressing hOPCs exhibited a greater than 3-fold increase in MBP staining within the corpus callosum (p = 0.003) (Figures 7A–7C). Consistent with improved myelination by GNB4-expressing transplanted cells, the density of ensheathed axons increased from 40.5 ± 5.1 fibers/mm by mCherry control cells to 71.8 ± 9.8 fibers/mm by GNB4-expressing cells, representing a >75% increase in axonal ensheathment (p = 0.028; n = 4) (Figures 7D–7F). Thus, GNB4-expressing hOPCs more rapidly synthesized MBP and ensheathed host axons, consistent with accelerated donor-derived myelin synthesis.
Surprisingly, the observed increase in myelination was not accompanied by an increase in the proportion of hOPCs undergoing oligodendrocyte differentiation, as the percentage of CC1+ oligodendrocytes was not altered (Figures 7G and 7J). The increase in extent of MBP expression and axon ensheathment was, therefore, not due to a substantial increase in oligodendrocyte number, but rather an increased rate of oligodendrocyte maturation. In addition, we observed that GNB4 expression increased the proportion of human glial fibrillary protein (GFAP)+ astrocytes (p < 0.05; n = 4–7) (Figures 7H and 7K). Finally, GNB4 expression did not alter the proportion of Ki-67+ proliferating cells compared with mCherry control cells (Figures 7I and 7L). Taken together, these data establish that Gβ4 expression differentially regulates hOPC fate in vitro and in vivo and, importantly, GNB4/Gβ4 was sufficient to substantially improve the production of myelin proteins and axonal ensheathment by hOPCs in vivo.
Discussion
The transcriptional processes involved in the initial stages oligodendrocyte differentiation have been examined in detail using rodent primary and stem cell-derived progenitors (Dugas et al., 2006, Gobert et al., 2009, Najm et al., 2011, Zhang et al., 2014). However, the extent to which these pathways and mechanisms are conserved during human development is less understood. We previously compared the transcriptional profile of freshly isolated human cells to their rodent homologs, with hOPCs isolated from both fetal (Abiraman et al., 2015, Sim et al., 2011) and adult (Sim et al., 2006, Sim et al., 2009) human brain. The aim of this study was to establish the extent of conservation between human and rodent programs of initial oligodendrocyte differentiation using a network-based analytic approach. Using a longitudinal experimental design, we could analyze the gene expression of matched OPCs isolated from individual patient samples and predict the function of genes previously undescribed in hOPC differentiation.
Rather than profile the transcriptome of pluripotent stem cell-derived OPCs, which may not accurately represent the phenotype of endogenous progenitors, we isolated primary OPCs from fetal human brain using PDGFαR-based cell sorting at a developmental stage during which OPCs undergo massive expansion in the forebrain (Sim et al., 2011). Similar to primary rat OPCs (Dugas et al., 2006), following the initiation of oligodendrocyte differentiation we observed an exponential increase in MBP mRNA expression in hOPCs occurring over the first 4 days. Indeed, our transcriptional analysis captured an expression profile of early myelin-enriched genes very similar to that defined previously (Dugas et al., 2006), and as such was suitable for comparisons between species. Furthermore, we incorporated PDGF-AA treatment to distinguish between genes whose expression was regulated by the process of oligodendrocyte differentiation and not simply a consequence of time spent in vitro.
Using this experimental approach and WGCNA, we identified several modules containing genes with highly related functions. Five modules were associated with progenitor maintenance and function, while only a single module was associated with differentiation. Interestingly, each of the OPC upregulated eigengene modules had distinct functional annotations. For example, M3 was associated with DNA replication and cell-cycle processes and M14 with proteasome function and G1 cycle checkpoint regulation. The species-conserved module M5 was associated with ribosome function and translation, and was highly enriched in MYC target genes (Enrichr/ChEA analysis) (Kuleshov et al., 2016, Lachmann et al., 2010). As such, WGCNA segregates genes associated with biochemically distinct biological processes that were associated with progenitor maintenance.
The observation that only a single WGCNA module was associated with differentiation suggests that the initiation of differentiation involves a largely unitary coordinated gene expression program. Among the M15 hub genes, we found known transcription factors that regulate positive (MYRF, SOX8) and negative (EGR1, EGR2, ID4) aspects of oligodendrocyte differentiation. Intriguingly, an analysis of M15 hub genes by Enrichr/ChEA chromatin immunoprecipitation enrichment analysis identified M15 gene expression as likely regulated by a combination of well-characterized oligodendrocyte transcription factors (OLIG2, Brg1/SMARCA4), as well as MITF, WT1, and SMAD4. This is consistent with the known transcription factor hierarchy in which OLIG2/SMARCA4 regulates SOX and MYRF factors to initiate differentiation (Yu et al., 2013), and BMP/SMAD regulation of OLIG2/ID4 to conversely inhibit differentiation (Samanta, 2004). In addition, this suggested a previously undescribed role for MITF or other members of the TFE family in oligodendrocyte differentiation. It is noteworthy that these factors physically interact with one another (Laurette et al., 2015, Wang et al., 2016) and thereby could compete to generate a bistable transcriptional switch.
To better determine whether WGCNA could predict functionally relevant genes, we selected candidate genes based on their expression patterns in human and rodent systems and M15 module membership. We selected three candidates for functional validation: BCAS1, PPP1R16B, and GNB4. While BCAS1 had no effect on differentiation, both PP1R16B and GNB4 induce precocious O4+ oligodendrocyte differentiation when overexpressed in vitro. BCAS1 overexpression reduced hOPC proliferation but did not affect oligodendrocyte differentiation. Interestingly, BCAS1 null mice were recently described and display a very mild hypomyelinating phenotype in the CNS, suggesting a non-essential role in oligodendrocyte development (Ishimoto et al., 2017).
PPP1R16B overexpression increased hOPC-oligodendrocyte differentiation. PPP1R16B is a regulatory subunit of protein phosphatase 1 (PP1) that may act to target the catalytic subunit of PP1 to specific membrane-associated proteins (Ito et al., 2004). The complex of PP1 with regulatory subunits provides target specificity and compartmentalization within the cell (Cohen, 2002). In the CNS, PPP1R16B mRNA is highly enriched in early differentiating human and mouse oligodendrocytes and localized to spinal cord white matter in postnatal day 4 spinal cord (Allen Brain Atlas). As PPP1R16B is regulated by cyclic AMP/protein kinase A in endothelial cells and has been shown to block PTEN function (Obeidat et al., 2014), it is possible that PP1R16B may act to potentiate phosphatidylinositol 3-kinase/Akt signaling in newly generated oligodendrocytes and thereby promote the maturation of progenitors to oligodendrocytes (Flores et al., 2008).
Heterotrimeric G proteins composed of Gα and Gβγ subunits are key signaling transducers that are activated by GPCRs and modulate a variety of intracellular signaling cascades. While Gα subunits have clearly defined roles, the role of specific Gβγ dimers and individual Gβ subunits is less clear (Khan et al., 2013). Gβ4/GNB4 is one of five Gβ proteins in humans, sharing greatest homology with Gβ1. While all except GNB3 mRNA were expressed in oligodendrocyte lineage cells, only GNB4 mRNA was upregulated during oligodendrocyte differentiation and was enriched compared with neurons and astrocytes. GNB1/2 mRNAs were quantitatively more abundant in hOPCs than GNB4, suggesting that upregulation of Gβ4 may interfere with signal transduction mediated by Gβ1/2 as well as promote specific Gβ4-coupled signaling.
We found that overexpression of GNB4 increased oligodendrocyte formation in vitro and potentiated oligodendrocyte maturation and axonal ensheathment following transplantation. This suggests that GNB4 expression is rate limiting during oligodendrocyte differentiation and that increased expression could be employed to promote the differentiation of OPCs. Intriguingly, genomic mutations in GNB4 have been associated with dominant intermediate Charcot-Marie-Tooth disease (Soong et al., 2013). As Gβ4 protein is localized to peripheral nervous system myelin, this suggests a critical role for GNB4 in Schwann cells and peripheral myelination. In hOPCs, GNB4 siRNAi induced rapid cell death, indicating that this subunit is also required for OPC survival. The observed effects of GNB4 overexpression on hOPC-mediated myelin repair suggest that GNB4 accelerates differentiation in vitro and promotes myelin maturation in vivo. After transplantation, while we did not observe an effect of GNB4 on CC1+ oligodendrocyte density, a far greater number of myelinated internodes was produced. As such, driving GNB4 expression in OPCs derived from pluripotent stem cells might represent a useful strategy to potentiate the formation of oligodendrocytes and accelerate the otherwise protracted protocols that are necessary (Douvaras et al., 2014, Wang et al., 2013b). Indeed, strategies to increase GNB4 expression might be envisaged to augment transplant-mediated myelin repair without affecting overall cell density or reducing the progenitor pool.
In conclusion, we have defined the gene expression profile of hOPCs undergoing oligodendrocyte differentiation. By using WGCNA on several datasets obtained in human, mouse, and rat primary cells, we identified gene expression modules containing highly coordinated genes that were expressed across species. This approach identified GNB4/Gβ4 as a previously undescribed gene involved in OPC differentiation and as a target to improve myelin repair by transplanted progenitors. This database includes several other important candidates likely to regulate important aspects of human oligodendrocyte commitment and differentiation (www.FindDb.org). We anticipate that these data will provide a valuable resource for analyzing oligodendrocyte differentiation in rodents and as a point of reference for comparing primary and pluripotent stem cell-derived progenitors and their differentiated progeny.
Experimental Procedures
Preparation of Human PDGFαR+ OPCs
Fetal brain samples (18–22 weeks gestational age) were obtained from patients who consented to tissue use under protocols approved by the SUNY at Buffalo HSIRB. Tissue was dissociated and PDGFαR+ hOPCs prepared as previously described (Sim et al., 2011) (see Supplemental Experimental Procedures).
Immunocytochemistry, Real-Time RT-PCR, Calcium Imaging, and Luciferase Analysis
hOPC culture and immunocytochemistry were performed as described by Abiraman et al. (2015). Antibody details are provided in Table S3. For assessment of gene expression, RNA was extracted (Omega Bio-Tek) and real-time RT-PCR performed (see Table S4 for list of primers). Calcium imaging in response to muscarinic agonist was performed following infection with GCaMP6s-expressing lentivirus using a motorized epifluorescence microscope (Olympus) and analyzed using R software (complete description in Supplemental Experimental Procedures). Luciferase reporter assays were performed according to the manufacturer's instructions following infection of hOPCs with Cignal Lentiviral reporters (Qiagen) and quantified using Bright-Glo (Promega).
Gene Expression and WGCNA
Gene expression analysis was performed using Illumina whole-genome arrays and analyzed using R/Bioconductor (see Supplemental Experimental Procedures for complete details). WGCNA was performed on each dataset (Langfelder and Horvath, 2008). Following identification of human homologs, species-conserved modules were identified by hypergeometric testing and corrected by false discovery rate.
Viral Expression and siRNA
GNB4, PPP1R16B, and GCaMP6s overexpression lentiviruses were generated by subcloning into pTRIP-EF1α (a gift from A. Benraiss, University of Rochester). Retroviral BCAS1-IRES-GFP and control viral plasmids were provided by M. Petryniak (Oregon Health & Science University). GNB4 siRNA (Thermo Fisher Scientific) was transfected as per manufacturer's instructions (100 μM).
Transplantation
All experiments using shiverer/rag2 mice were performed according to protocols approved by the University at Buffalo Institutional Animal Care and Use Committee. As previously described (Wang et al., 2014), 105 hOPCs were implanted into shiverer/rag2 neonatal corpus callosum and euthanized at 8 weeks post implantation. Confocal and wide-field immunofluorescence (Supplemental Experimental Procedures and Table S3) was performed to assess hOPC engraftment, cell fate, and axonal ensheathment.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism (San Diego, CA). Data were compared by Student's t test, one-way ANOVA, or two-way ANOVA, where appropriate; significance was considered at p < 0.05.
Author Contributions
S.U.P., J.J.P., R.A.S., M.A.O., H.J.S., K.C.D., and F.J.S. performed experiments and analyzed data. S.U.P., J.J.P., R.A.S., and F.J.S. provided intellectual contributions. S.U.P., J.J.P., K.C.D., and F.J.S. wrote the paper.
Acknowledgments
This work was supported by the NIH (NCATS, UL1TR001412), the National Multiple Sclerosis Society (RG 5505-A-2), the Kalec Multiple Sclerosis Foundation, the Change MS Foundation, the Skarlow Memorial Trust, and the Empire State Stem Cell Fund through New York State Department of Health Contract (C028108). We thank Dr. J. Conroy of Roswell Park Cancer Institute for assistance with Illumina Beadarray techniques. The microarray profiling was supported by grants from the NIH/National Cancer Institute, P30 CA016056 (RPCI Cancer Center support grant). We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo. We thank Dr. Magdalena A. Petryniak, Department of Pediatrics, Oregon Health & Science University, for assistance with BCAS1 retrovirus. We thank David Bratton and Aberlee J. Milliron for technical assistance.
Published: August 8, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.07.007.
Supplemental Information
References
- Abiraman K., Pol S.U., O'Bara M.A., Chen G.D., Khaku Z.M., Wang J., Thorn D., Vedia B.H., Ekwegbalu E.C., Li J.X. Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J. Neurosci. 2015;35:3676–3688. doi: 10.1523/JNEUROSCI.3510-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles D.E., Richardson W.D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S., Xing Y., Lubischer J.L., Krieg P.A., Krupenko S.A. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Nishiyama A., Peterson J., Prineas J., Trapp B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P.T. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Deshmukh V.A., Tardif V., Lyssiotis C.A., Green C.C., Kerman B., Kim H.J., Padmanabhan K., Swoboda J.G., Ahmad I., Kondo T. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K.C., Polanco J.J., Pol S.U., Sim F.J. Targeting human oligodendrocyte progenitors for myelin repair. Exp. Neurol. 2016;283:489–500. doi: 10.1016/j.expneurol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P., Wang J., Zimmer M., Hanchuk S., O'Bara M.A., Sadiq S., Sim F.J., Goldman J., Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas J.C., Tai Y.C., Speed T.P., Ngai J., Barres B.A. Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A.I., Narayanan S.P., Morse E.N., Shick H.E., Yin X., Kidd G., Avila R.L., Kirschner D.A., Macklin W.B. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R.J., Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin R.J., Goldman S.A. Glia disease and repair-remyelination. Cold Spring Harb. Perspect. Biol. 2015;7:a020594. doi: 10.1101/cshperspect.a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich S.L., Karlo J.C., Landreth G.E., Miller R.H. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J. Neurosci. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert R.P., Joubert L., Curchod M.L., Salvat C., Foucault I., Jorand-Lebrun C., Lamarine M., Peixoto H., Vignaud C., Fremaux C. Convergent functional genomics of oligodendrocyte differentiation identifies multiple autoinhibitory signaling circuits. Mol. Cell. Biol. 2009;29:1538–1553. doi: 10.1128/MCB.01375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Dong J. Geometric interpretation of gene coexpression network analysis. PLoS Comput. Biol. 2008;4:e1000117. doi: 10.1371/journal.pcbi.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Zhang B., Carlson M., Lu K.V., Zhu S., Felciano R.M., Laurance M.F., Zhao W., Qi S., Chen Z. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc. Natl. Acad. Sci. USA. 2006;103:17402–17407. doi: 10.1073/pnas.0608396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K.A., Blakemore W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Ishii A., Fyffe-Maricich S.L., Furusho M., Miller R.H., Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 2012;32:8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T., Ninomiya K., Inoue R., Koike M., Uchiyama Y., Mori H. Mice lacking BCAS1, a novel myelin-associated protein, display hypomyelination, schizophrenia-like abnormal behaviors, and upregulation of inflammatory genes in the brain. Glia. 2017;65:727–739. doi: 10.1002/glia.23129. [DOI] [PubMed] [Google Scholar]
- Ito M., Nakano T., Erdodi F., Hartshorne D.J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Johnson M.B., Kawasawa Y.I., Mason C.E., Krsnik Z., Coppola G., Bogdanovic D., Geschwind D.H., Mane S.M., State M.W., Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.M., Sleno R., Gora S., Zylbergold P., Laverdure J.P., Labbe J.C., Miller G.J., Hebert T.E. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol. Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T., Miron V., Cui Q., Wegner C., Antel J., Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q., Wang Z., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A., Xu H., Krishnan J., Berger S.I., Mazloom A.R., Ma'ayan A. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26:2438–2444. doi: 10.1093/bioinformatics/btq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurette P., Strub T., Koludrovic D., Keime C., Le Gras S., Seberg H., Van Otterloo E., Imrichova H., Siddaway R., Aerts S. Transcription factor MITF and remodeller BRG1 define chromatin organisation at regulatory elements in melanoma cells. Elife. 2015;4:e06857. doi: 10.7554/eLife.06857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein E.S., Hawrylycz M.J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A.F., Boguski M.S., Brockway K.S., Byrnes E.J. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- McClain C.R., Sim F.J., Goldman S.A. Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J. Neurosci. 2012;32:15066–15075. doi: 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Fancy S.P., Shen Y.A., Niu J., Zhao C., Presley B., Miao E., Lee S., Mayoral S.R., Redmond S.A. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.A., Horvath S., Geschwind D.H. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc. Natl. Acad. Sci. USA. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B.M., Lee Y., Rothstein J.D. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 2013;23:644–651. doi: 10.1016/j.tcb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F.J., Zaremba A., Caprariello A.V., Nayak S., Freundt E.C., Scacheri P.C., Miller R.H., Tesar P.J. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nat. Methods. 2011;8:957–962. doi: 10.1038/nmeth.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F.J., Madhavan M., Zaremba A., Shick E., Karl R.T., Factor D.C., Miller T.E., Nevin Z.S., Kantor C., Sargent A. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat M., Li L., Ballermann B.J. TIMAP promotes angiogenesis by suppressing PTEN-mediated Akt inhibition in human glomerular endothelial cells. Am. J. Physiol. Ren. Physiol. 2014;307:F623–F633. doi: 10.1152/ajprenal.00070.2014. [DOI] [PubMed] [Google Scholar]
- Oldham M.C., Horvath S., Geschwind D.H. Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc. Natl. Acad. Sci. USA. 2006;103:17973–17978. doi: 10.1073/pnas.0605938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham M.C., Konopka G., Iwamoto K., Langfelder P., Kato T., Horvath S., Geschwind D.H. Functional organization of the transcriptome in human brain. Nat. Neurosci. 2008;11:1271–1282. doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- Sim F.J., Lang J.K., Waldau B., Roy N.S., Schwartz T.E., Pilcher W.H., Chandross K.J., Natesan S., Merrill J.E., Goldman S.A. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann. Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Sim F.J., Windrem M.S., Goldman S.A. Fate determination of adult human glial progenitor cells. Neuron Glia Biol. 2009;5:45–55. doi: 10.1017/S1740925X09990317. [DOI] [PubMed] [Google Scholar]
- Sim F.J., McClain C.R., Schanz S.J., Protack T.L., Windrem M.S., Goldman S.A. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnamon J.R., Waddell C.B., Nik S., Chen E.I., Czaplinski K. Hnrpab regulates neural development and neuron cell survival after glutamate stimulation. RNA. 2012;18:704–719. doi: 10.1261/rna.030742.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong B.W., Huang Y.H., Tsai P.C., Huang C.C., Pan H.C., Lu Y.C., Chien H.J., Liu T.T., Chang M.H., Lin K.P. Exome sequencing identifies GNB4 mutations as a cause of dominant intermediate Charcot-Marie-Tooth disease. Am. J. Hum. Genet. 2013;92:422–430. doi: 10.1016/j.ajhg.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Nave K.A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Wang J., O'Bara M.A., Pol S.U., Sim F.J. CD133/CD140a-based isolation of distinct human multipotent neural progenitor cells and oligodendrocyte progenitor cells. Stem Cells Dev. 2013;22:2121–2131. doi: 10.1089/scd.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Bates J., Li X., Schanz S., Chandler-Militello D., Levine C., Maherali N., Studer L., Hochedlinger K., Windrem M. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Pol S.U., Haberman A.K., Wang C., O'Bara M.A., Sim F.J. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proc. Natl. Acad. Sci. USA. 2014;111:E2885–E2894. doi: 10.1073/pnas.1408295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao L., Su Q., Fan X., Wang Y., Gao S., Wang H., Chen H., Chan C.B., Liu Z. Phosphorylation of MITF by AKT affects its downstream targets and causes TP53-dependent cell senescence. Int. J. Biochem. Cell Biol. 2016;80:132–142. doi: 10.1016/j.biocel.2016.09.029. [DOI] [PubMed] [Google Scholar]
- Wheeler N.A., Fuss B. Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp. Neurol. 2016;283:512–530. doi: 10.1016/j.expneurol.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Chen Y., Kim B., Wang H., Zhao C., He X., Liu L., Liu W., Wu L.M., Mao M. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Langfelder P., Fuller T., Dong J., Li A., Hovarth S. Weighted gene coexpression network analysis: state of the art. J. Biopharm. Stat. 2010;20:281–300. doi: 10.1080/10543400903572753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.