Abstract
Since the pioneering use of autologous rib cartilage for the reconstruction of microtia, there have been significant advances in surgical technique that have helped to ameliorate the psychological burden of microtia. To date, the use of rib cartilage for auricular reconstruction is one of the most enduring and ubiquitous techniques for microtia reconstruction as it provides excellent aesthetic results with lasting durability. In this review, the authors outline the most common methods of microtia reconstruction with a comparison of each technique and illustrative case examples.
Keywords: Brent, Nagata, autologous ear reconstruction, Tanzer, cartilage
There have been significant advances in reconstructive techniques since ear reconstruction was introduced in the Susruta Samhita in 600 B.C. 1 The most radical changes began when Gillies addressed microtia by burying carved cartilage under the mastoid skin and then separating it from the head with a cervical flap. 2 In 1930, Pierce modified the Gillies technique by lining the reconstructed sulcus with a skin graft and building the helix with a tubed flap. 3 In 1937, Gillies reported on his reconstructions of over 30 microtic ears with the use of maternal ear cartilage; however, long-term results were poor as the cartilage progressively resorbed. 4 5 Steffensen then used preserved rib cartilage and ran into the same problem as Gillies, that is, resorption of the framework. 6 7
In 1959, Tanzer made significant headway by using autologous rib cartilage. Lasting results were finally obtained without resorption. 8 9 In the late 1960s, Cronin used silicone ear frameworks; however, extrusion rates were persistently high. Since that time, the techniques pioneered by Brent, 10 11 Nagata, 12 Tanzer, 8 and Walton and Beahm 13 have served as the foundation for the techniques currently used. To date, the use of autogenous rib cartilage for auricular reconstruction is one of the most enduring techniques for microtia reconstruction as it provides excellent aesthetic results with lasting durability. 9 11 12 14 15 16 17 18 19 In this review, we will outline the most common methods of microtia reconstruction with a progression from a multistaged to a single-staged approach.
Epidemiology, Genetics, and Etiology of Microtia
The incidence of microtia is approximately 1/6,000 births with no difference between ethnicities. 20 21 The inheritance of microtia is multifactorial with a recurrence risk of 5.7%. 22 Most cases are unilateral; the right side is typically favored. The incidence is nearly 2 times higher in males. 10 19
The exact etiology for microtia is unclear, but one prevailing theory is that microtia can result from in utero tissue ischemia secondary to the obliteration of the stapedial artery or hemorrhage into the ear. 23 24 Certain medications (thalidomide, isotretinoin, clomiphene citrate, and retinoic acid among others) have been associated with the development of microtia. 25
Diagnosis and Classification of Microtia
There are three widely accepted ways of categorizing microtia: (1) auricular hypoplasia in descending severity, 26 (2) Tanzer's method by approach required for reconstruction, 27 and (3) Nagata's classification based on reconstructive techniques ( Table 1 ). 12
Table 1. Comparison of classification schemas of auricular hypoplasia.
| Classified by severity | Tanzer classification | Nagata classification |
|---|---|---|
| Microtia | Anotia | Anotia |
| Lop ear (folding of superior helix and scapha) | Complete hypoplasia (microtia) • With atresia of the external auditory canal • Without atresia of the external auditory canal |
Lobule type (cartilaginous remnant and vertical lobule without the meatus, concha, or tragus) |
| Cup or constricted ear with a deep concha and lack of superior helix and antihelical crura | Hypoplasia of the middle one-third of the auricle | Large conchal type (lobule, concha with or without the meatus, tragus, and intertragal notch present |
| Prominent/protruding ear | Hypoplasia of the superior one-third of the auricle • Constricted (cup and lop) ear • Cryptotia • Hypoplasia of the anterior superior one-third |
Small conchal type (similar to lobule type, but with an indentation in the region of the conchal bowl |
| Prominent ear | Atypical |
General Considerations
Microtia can cause significant psychological morbidity, and as such, deserves a reconstructive approach to treatment. 28 Furthermore, earlier reconstruction may lead to better psychological outcomes with a majority of patients being very satisfied with their reconstruction. 29 30
In the initial consultation with the family, it is important to describe the limitations of reconstruction so that the expectations of the surgeon, the family, and the patient align. If the family chooses autogenous reconstruction of the ear, the parents should be forewarned of the expected necessity for more than one procedure as well as the potential risks of the operation and the brief limitations on physical activity (up to 6 weeks) after harvesting costal cartilage. The general recommendation for the timing of reconstruction is between the ages of 7 and 10 before peer ridicule begins ( Figs. 1 , 2 ). Some surgeons prefer to begin at age 10, or when the chest circumference at the xiphoid is at least 60 cm.
Fig. 1.
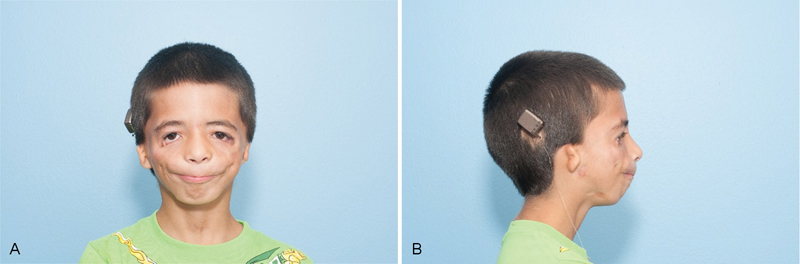
( A ) Preoperative frontal view of right microtia. ( B ) Preoperative lateral view of right microtia.
Fig. 2.
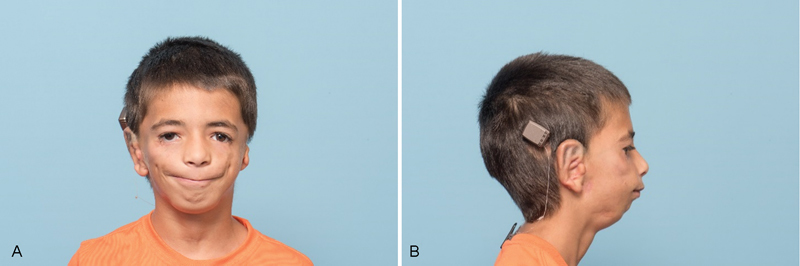
( A ) Postoperative frontal view of right microtia. ( B ) Postoperative lateral view of right microtia.
Brent's Method of Repair
Brent's technique of repair is based on multiple stages to achieve a detailed, lasting reconstruction. The first stage of Brent's method involves the harvesting of rib cartilage, constructing the framework, and inserting the framework in the pocket subcutaneously at the reconstructed ear location. The second stage involves lobule transposition. The third stage is separation of the ear from the head and surfacing the raw area of the reconstructed ear with a skin graft to create the auriculocephalic sulcus. The fourth stage involves tragal construction, conchal excavation, and simultaneous contralateral otoplasty.
In the first stage of reconstruction, the ear framework is usually obtained in one piece from the contralateral rib cartilage. A horizontal incision is made above the costal margin, and the external oblique and rectus muscles are divided ( Fig. 3 ). An ear template is placed on the rib cartilage to serve as a guide for the amount of rib cartilage needed. The rib cartilage used for the helical rim is obtained from the first free-floating rib, separately from the main ear. The synchondritic region of the sixth and seventh ribs serves as the body of the framework. Supraperichondrial dissection should be used to preserve the specimen. To decrease the likelihood of chest wall deformities, Brent preserves the rim of the upper margin of the sixth rib cartilage.
Fig. 3.
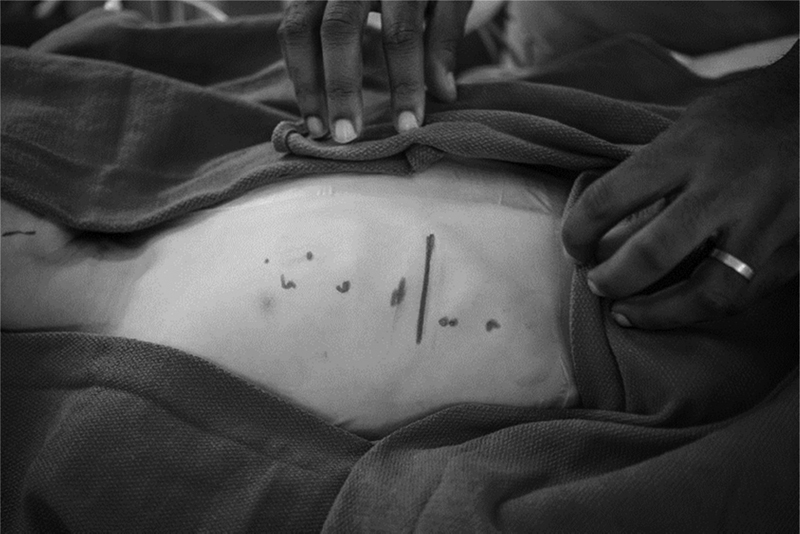
Surgical marking for chest incision.
During fabrication of the framework, the helical rim and the details of the antihelical complex should be exaggerated as the skin covering will blunt the details of this region. Brent recommends anticipating framework warping and to use the warping to the surgeon's advantage. For the helix, intentional warping creates the flexion needed to attach it to the framework body. The helix is sutured to the construct with 4–0 clear nylon in a horizontal mattress fashion. Brent recommends against using stainless-steel wire sutures (unlike Nagata) to prevent wire extrusion.
For framework implantation, a cutaneous pocket is created by incising along the backside of the existing auricular tissue and raising a thin flap with sharp dissection ( Fig. 4 ). The skin should be dissected away from the vestigial cartilage, and the remnant cartilage can be discarded. Dissection continues 1 to 2 cm in each direction to the framework skin markings. The carved framework should be inserted into the pocket, and the slack skin should be displaced in the posterosuperior direction with the hairline just behind the rim. 8 Brent uses suction to coapt the skin flap to the framework, which prevents fluid collection and minimizes the risk for flap necrosis along the helical margin. 10 31 32 Active sports should be avoided for 6 weeks until the chest wound heals.
Fig. 4.
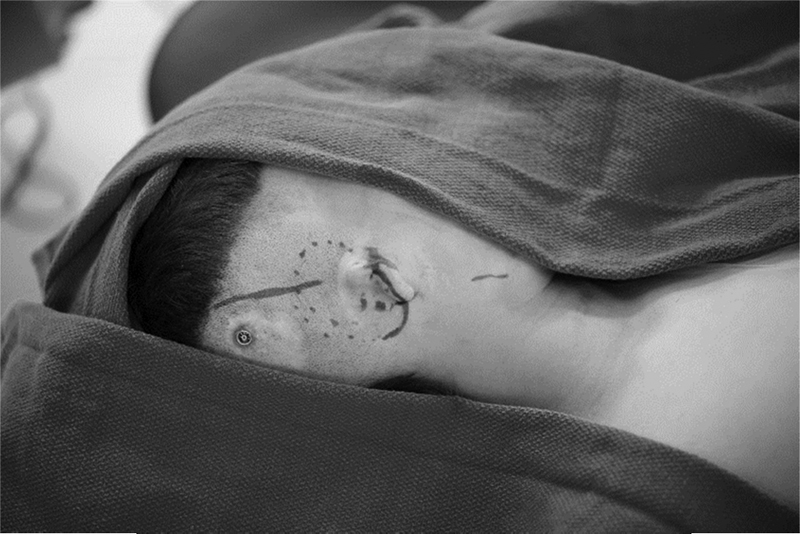
Surgical marking for ear construct.
Brent completes lobule transposition in a second stage to prevent scarring, decreased circulation and elasticity, and to lessen the risk of tissue necrosis that may accompany lobule transposition in the first stage. 32 33
In another operation or series of operations, Brent constructs the tragus, defines the concha, creates a false meatus, and elevates the reconstructed auricle from the head. The tragus is created by harvesting a composite chondrocutaneous graft from the contralateral auricle's anterolateral conchal surface and placing the elliptical graft beneath a J-shaped incision in the conchal region. 32 The straight limb of the J-incision is at the future tragal margin while the curved part of the J-incision serves as the intertragal notch. The concha is deepened by removing soft tissue beneath the tragal flap. Upon removal of the soft tissue behind the tragal flap, the tragus will cast a shadow that mimics a meatus.
The sulcus is created by elevating the ear from the head and covering the raw underside with a full-thickness skin graft. This step should not be undertaken until the final auricular details are defined, and edema has resolved. An incision several millimeters behind the rim should be made, and the retroauricular skin should be advanced so that the only raw surface needing a graft is the posterior surface of the ear. The full-thickness skin graft may be obtained from either the lower abdomen or the groin crease.
Projection of the reconstructed auricle is accomplished using a wedge of rib cartilage behind the elevated ear. During the first stage, an extra piece of rib cartilage is banked underneath the chest incision or underneath the scalp, posterior to the cutaneous pocket of the framework. 19 The cartilage should be split in situ so that a wider portion of the rib cartilage can be obtained for enhanced projection. Splitting the cartilage creates an advantageous warp in the wedge, providing a favorable shape for the posterior conchal wall. Brent, 19 Firmin, 34 and Weerda all cover the cartilage wedge with a turnover “book flap” of occipitalis fascia from behind the ear.
Nagata Technique
Nagata pioneered ear reconstruction in two stages. 14 15 16 17 The first stage consists of cartilage framework implantation, tragus construction, and lobule transposition. The second stage involves construct elevation from the head with projection of the reconstructed ear covered by fascial flap and skin graft.
In the first stage, the auricular framework is harvested from the sixth to ninth costal cartilages ( Fig. 5 ). The sixth and seventh costal cartilages are used for the base of the framework, while the ninth rib makes the antihelix as well as the superior and inferior crus. The eighth rib is used to construct the helical rim unit and the crus helicis. The conchal bowl pieces are derived from rib cartilage remnants. Nagata prefers the use of fine-gauge wire to hold the construct together ( Fig. 6 ). 35 Chest wall deformities are prevented by preserving the perichondrium at the donor site. 36 37 The remaining cartilage pieces are cut into 2 to 3 mm blocks and placed back in the perichondrial pockets.
Fig. 5.
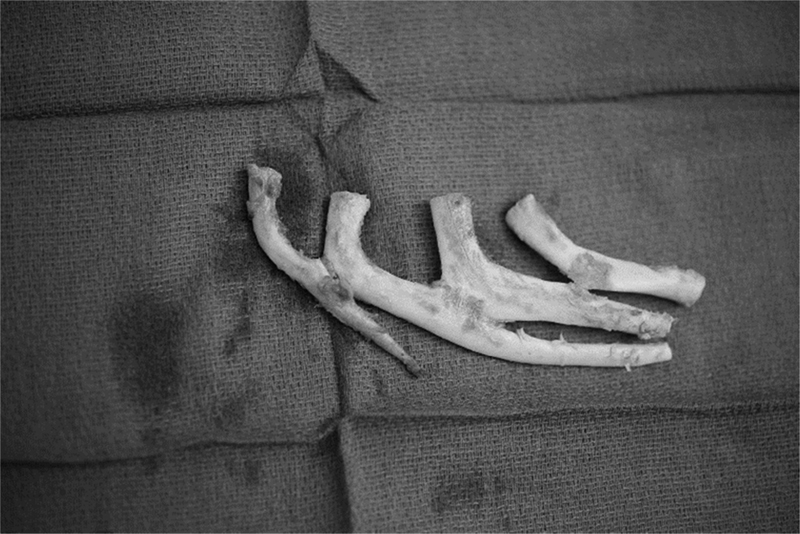
Harvested ribs with synchondrosis.
Fig. 6.
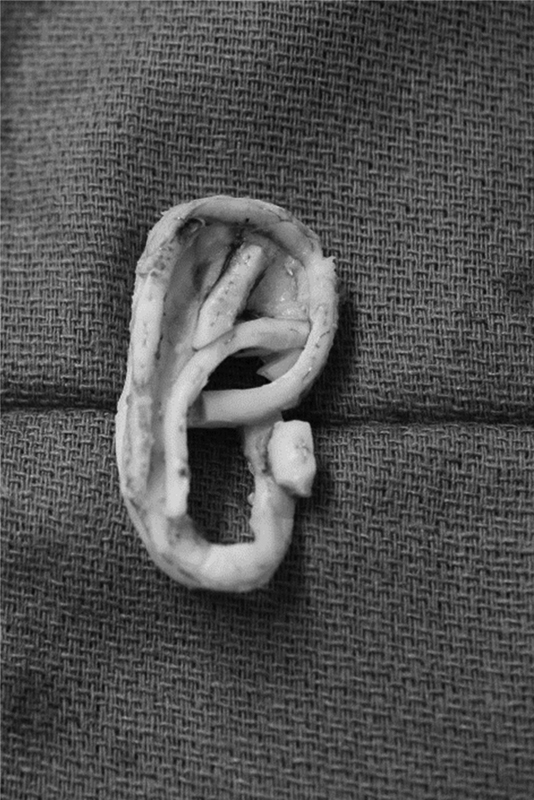
Cartilage construct using wires.
The next step in the first stage is coverage of the framework in the subcutaneous pocket with incisions in the shape of a “lazy W.” 35 The lobule is split to form anterior and posterior skin flaps. The posterior lobule flap remains attached to the mastoid skin flap, while the anteriorly based tragal flap is sutured to surface the external surface of the tragus. The middle limbs of the “W” meet to make the inverted cone and create the depth of the intertragal notch. The W flap and anterior lobule flap are transposed in z-plasty fashion. To optimize the vascularity of the W flap, the subcutaneous pedicle in the floor of the conchal bowl is preserved. The subcutaneous pocket is subsequently created by removing the vestigial auricular cartilage. The framework is then placed around the subcutaneous pedicle and the skin flaps are sutured together and secured over the framework with sutured bolsters that are left in place for 2 weeks. In a reported 273 cases performed by Nagata there was only 1 pneumothorax, 1 methicillin-resistant Staphylococcus aureus infection, and no chest wall deformities. 36 Firmin has reported partial necrosis of the posterior lobular flap using Nagata's technique in 13.9% of her 144 patients. 18
The second stage is undertaken after 6 months and consists of elevation of the reconstructed auricle. A skin incision is made 1 cm posterior to the helix. A crescent-shaped wedge of rib cartilage from the fifth rib should be harvested and placed under the ear to create projection of the reconstructed framework. A temporoparietal fascia flap is raised and tunneled subcutaneously toward the posterior aspect of the framework to cover the raw posterior surface of the ear as well as the newly harvested cartilage projection graft and the mastoid skin. Retroauricular skin should be advanced anteriorly and a full-thickness skin graft should be used to cover the remaining raw areas.
Chen et al have modified the second stage of the Nagata reconstruction to create continuous skin coverage by designing a leaf-like flap of split-thickness scalp skin graft in continuity with full-thickness skin of the anterior surface of the auricle. 38
Kurabayashi et al have modified the Nagata technique by avoiding use of the temporoparietal fascia flap and instead creating a pocket in the temporoparietal fascia and creating the “temporoparietal fascia pocket method.” 39 This method has been found to be less invasive, avoids the need for temporoparietal fascia flap elevation, and creates a superior and enduring temporoauricular sulci. 39
Park Method
Park's method is a 2-flap method completed in three stages. 40 In the first stage, a tissue expander is placed in a pocket underneath the fascial layer in the mastoid area. After 3 weeks, expansion begins and is continued for approximately 5 months or until the volume of the expander is 80 to 90 mL.
In the second stage, rib cartilage is harvested, and the framework is carved. The tissue expander is explanted, and the expanded facial layer and skin flap are subsequently separated with the framework to be placed in-between these pockets. Anteriorly, the skin is undermined so that the tragal aspect of the framework can fit. A medium-sized hole is made in the fascial flap for the crus helicis. The upper aspect of the base frame is placed between the skin and fascial flaps, and the lower part is placed into the earlobe envelope. The posterior aspect of the framework is covered by a fascial flap, while the anterior framework is covered by a skin flap. The raw surface of the fascial flap is covered by a skin graft from either the groin or scalp.
In the third stage, incisions are made over the anterior aspect of the framework so that the tragus, crus helicis, conchal floor, and intertragic notch can be shaped while a portion is hollowed to create a simulated external auditory meatus.
Fisher's Modified Nagata Method
Fisher's modification of the Nagata technique, is a one-staged procedure. 41 It differs from the classical Nagata technique in that projection of the reconstructed auricle is included in the first (and only) stage. The costal cartilage is harvested, and the base frame (helix, antihelix, lobule, scaphoid fossa, posterior conchal wall) is made from the sixth and seventh rib cartilages. The costal cartilage from the eighth rib is used to create the helix that is attached to the framework using wire sutures placed at 3-mm intervals. The ninth costal cartilage is used to form the antihelix. When the rib is broad enough, Fisher attempts to create the entire antitragus from the ninth rib and excavates the triangular fossa from between the upper and inferior crura. When the ninth costal cartilage is not broad enough for the construction of the whole antihelix, the upper and lower crura are made separately with the antitragus and inferior crus comprising one piece while the upper crus is a second piece. To maintain projection of the tragus and add stability to the framework, Fisher modifies the tragus with a closing strut extending from the deep cephalic surface of the tragus to the deep surface of the base frame under the helical root. 41
The auricular incisions are made in the similar “Lazy W” fashion with the creation of three skin flaps: (1) bilobed W-flap, (2) anterior lobule flap, and (3) tragal flap. The framework is anchored to the mastoid fascia with 3–0 clear nylon sutures and the skin flaps are closed over the contours of the framework with the help of suction.
The primary advantage of this method is the avoidance of a second surgery while maintaining good aesthetic standards. Fisher found a reduced complication rate when switched from the 2-stage to a 1-stage procedure (22% to 15%) ( Table 2 ). 41
Table 2. Comparison of the techniques.
| Microtia repair technique | Advantages | Disadvantages |
|---|---|---|
| Nagata method (autologous costal cartilage graft in two stages) | High-fidelity reconstruction of the ear with excellent projection and minimal chest wall deformity. | Need to harvest costal cartilage twice. Risk of wire extrusion. Use of superficial vessel-containing fascial flap may cause hair thinning in the donor site. Use of a temporoparietal fascia flap limits options in case of future reconstruction. Soft tissue necrosis due to lobule transposition in the first stage 36 42 |
| Park method (subfascial tissue expansion with two flaps) | Good vascularization of the framework by the coaptation between the surfaces of the split flaps. Ability to create a deeper concha floor with a realistic simulation of the auditory meatus. All scars are hidden in the mastoid region. | Possibility for venous congestion after expansion and flap elevation. Need for multiple outpatient visits for expansion. Potential for depression of the mastoid bone secondary to expander pressure. |
| Fisher's method (1-staged Nagata method) | Good aesthetic results in one operation with decreased complication rates | Need for revision, possible loss of entire construct |
| Brent's method | Low complication rates (0.25%) with great detail of the reconstructed auricle. | Multiple stages |
Complications
Commonly encountered complications include pneumothorax, infection, cartilage framework exposure, lobule necrosis, and changes in framework size. If the pleura is violated, a catheter should be placed into the pleural defect, and the pleura should be closed while the catheter is placed to suction. As the anesthesiologist expands the lungs with positive pressure, the catheter can be removed. A chest X-ray is obtained in the operating room or postoperatively. If the patient develops a symptomatic pneumothorax postoperatively, a thoracostomy tube should be placed. Infection of the ear should be treated with either oral or intravenous antibiotics and judicious incision and drainage if necessary. If the cartilage framework is exposed, it should be kept clean, and application of 10% sulfamylon cream is suggested. If the exposed area is > 3 cm without any granulation tissue, a local flap or temporoparietal fascia flap may be required for coverage. Generally, the cartilage ear framework will grow along with the patient (48.1%) with some growing several millimeters larger than the unconstructed side and 10.3% growing several millimeters smaller. 10
Conclusion
Autologous reconstruction for microtia is one of the most challenging tasks of the reconstructive surgeon. The results can be excellent and reproducible. The advantages of the technique are multifold, compounded by the use of the body's own tissue. Significant advances have been pioneered over the years to bring about tremendous psychosocial benefits. As ever, current modifications seek to improve aesthetic results, decrease stages, and maintain the low complication rate. Experience and an artistic eye are both necessary for optimal outcomes.
References
- 1.Bhishagratna K KL. Calcutta: Wilkins Press; 1907. An English Translation of the Susruta Samhita. [Google Scholar]
- 2.Gillies H. London: Frowde, Hodder & Stoughton; 1920. Plastic Surgery of the Face. [Google Scholar]
- 3.Pierce G W. Reconstruction of the external ear. Surg Gynecol Obstet. 1930;50:601. [Google Scholar]
- 4.Converse J M.The absorption and shrinkage of maternal ear cartilage used as living homografts: Follow-up report of 21 of Gillies' patientsIn: John M. Converse, ed.Reconstructive and Plastic Surgery. 2nd ed Philadelphia, PA: Saunders; 1977308 [Google Scholar]
- 5.Gillies H D. Reconstruction of the external ear with special reference to the use of maternal ear cartilage as the supporting structure. Rev Chir Structive. 1937;7:169. [Google Scholar]
- 6.Steffensen W H. Comments on total reconstruction of the ear. Plast Reconstr Surg (1946) 1952;10(03):186–190. doi: 10.1097/00006534-195207000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Steffensen W H. Comments on reconstruction of the external ear. Plast Reconstr Surg (1946) 1955;16(03):194–200. doi: 10.1097/00006534-195509000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Tanzer R C. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. 1959;23(01):1–15. doi: 10.1097/00006534-195901000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tanzer R C. Microtia--a long-term follow-up of 44 reconstructed auricles. Plast Reconstr Surg. 1978;61(02):161–166. doi: 10.1097/00006534-197802000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brent B.Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases Plast Reconstr Surg 19929003355–374., discussion 375–376 [PubMed] [Google Scholar]
- 11.Brent B.Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases Clin Plast Surg 20022902257–271., vii [DOI] [PubMed] [Google Scholar]
- 12.Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993;92(02):187–201. doi: 10.1097/00006534-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Walton R L, Beahm E K.Auricular reconstruction for microtia: Part II. Surgical techniques Plast Reconstr Surg 200211001234–249., quiz 250–251, 387 [DOI] [PubMed] [Google Scholar]
- 14.Nagata S.Modification of the stages in total reconstruction of the auricle: Part I. Grafting the three-dimensional costal cartilage framework for lobule-type microtia Plast Reconstr Surg 19949302221–230., discussion 267–268 [PubMed] [Google Scholar]
- 15.Nagata S.Modification of the stages in total reconstruction of the auricle: Part II. Grafting the three-dimensional costal cartilage framework for concha-type microtia Plast Reconstr Surg 19949302231–242., discussion 267–268 [PubMed] [Google Scholar]
- 16.Nagata S.Modification of the stages in total reconstruction of the auricle: Part III. Grafting the three-dimensional costal cartilage framework for small concha-type microtia Plast Reconstr Surg 19949302243–253., discussion 267–268 [PubMed] [Google Scholar]
- 17.Nagata S.Modification of the stages in total reconstruction of the auricle: Part IV. Ear elevation for the constructed auricle Plast Reconstr Surg 19949302254–266., discussion 267–268 [PubMed] [Google Scholar]
- 18.Firmin F. Ear reconstruction in cases of typical microtia. Personal experience based on 352 microtic ear corrections. Scand J Plast Reconstr Surg Hand Surg. 1998;32(01):35–47. doi: 10.1080/02844319850158930. [DOI] [PubMed] [Google Scholar]
- 19.Brent B.Technical advances in ear reconstruction with autogenous rib cartilage grafts: personal experience with 1200 cases Plast Reconstr Surg 199910402319–334., discussion 335–338 [DOI] [PubMed] [Google Scholar]
- 20.Grabb W C. The first and second branchial arch syndrome. Plast Reconstr Surg. 1965;36(05):485–508. doi: 10.1097/00006534-196511000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Aase J M, Tegtmeier R E.Microtia in New Mexico: evidence for multifactorial causation Birth Defects Orig Artic Ser 197713(3A):113–116. [PubMed] [Google Scholar]
- 22.Takahashi H, Maeda K. Survey of familial occurrence in 171 microtia cases. Jpn J Plast Surg. 1982;15:310. [Google Scholar]
- 23.McKenzie J, Craig J. Mandibulo-facial dysostosis (Treacher Collins syndrome) Arch Dis Child. 1955;30(152):391–395. doi: 10.1136/adc.30.152.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poswillo D. The pathogenesis of the first and second branchial arch syndrome. Oral Surg Oral Med Oral Pathol. 1973;35(03):302–328. doi: 10.1016/0030-4220(73)90070-4. [DOI] [PubMed] [Google Scholar]
- 25.Brent B. The pediatrician's role in caring for patients with congenital microtia and atresia. Pediatr Ann. 1999;28(06):374–383. doi: 10.3928/0090-4481-19990601-09. [DOI] [PubMed] [Google Scholar]
- 26.Rogers B O. Microtic, lop, cup and protruding ears: four directly inheritable deformities? Plast Reconstr Surg. 1968;41(03):208–231. doi: 10.1097/00006534-196803000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tanzer R C. The constricted (cup and lop) ear. Plast Reconstr Surg. 1975;55(04):406–415. [PubMed] [Google Scholar]
- 28.Horlock N, Vögelin E, Bradbury E T, Grobbelaar A O, Gault D T. Psychosocial outcome of patients after ear reconstruction: a retrospective study of 62 patients. Ann Plast Surg. 2005;54(05):517–524. doi: 10.1097/01.sap.0000155284.96308.32. [DOI] [PubMed] [Google Scholar]
- 29.Johns A L, Lucash R E, Im D D, Lewin S L. Pre and post-operative psychological functioning in younger and older children with microtia. J Plast Reconstr Aesthet Surg. 2015;68(04):492–497. doi: 10.1016/j.bjps.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Akter F, Mennie J C, Stewart K, Bulstrode N. Patient reported outcome measures in microtia surgery. J Plast Reconstr Aesthet Surg. 2017;70(03):416–424. doi: 10.1016/j.bjps.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Cronin T D. Use of a silastic frame for total and subtotal reconstruction of the external ear: preliminary report. Plast Reconstr Surg. 1966;37(05):399–405. doi: 10.1097/00006534-196605000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Brent B. The correction of mi-rotia with autogenous cartilage grafts: I. The classic deformity.? Plast Reconstr Surg. 1980;66(01):1–12. doi: 10.1097/00006534-198007000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Brent B. The correction of microtia with autogenous cartilage grafts: II. Atypical and complex deformities. Plast Reconstr Surg. 1980;66(01):13–21. doi: 10.1097/00006534-198007000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Firmin F. Ear reconstruction in cases of typical microtia. Personal experience based on 352 microtia corrections. Scand J Plast Reconstr Surg Hand Surg. 1998;32(01):35–47. doi: 10.1080/02844319850158930. [DOI] [PubMed] [Google Scholar]
- 35.Nagata S.Total auricular reconstruction with a three-dimensional costal cartilage framework Ann Chir Plast Esthet 19954004371–399., discussion 400–403 [PubMed] [Google Scholar]
- 36.Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: part I. Avoidance and prevention of intraoperative and postoperative complications and problems. Plast Reconstr Surg. 2006;117(06):2011–2018. doi: 10.1097/01.prs.0000210015.28620.1c. [DOI] [PubMed] [Google Scholar]
- 37.Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: part II. Evaluation and analysis of the regenerated costal cartilage. Plast Reconstr Surg. 2007;119(01):308–315. doi: 10.1097/01.prs.0000244880.12256.7c. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z-C, Goh R C, Chen P K-T, Lo L-J, Wang S-Y, Nagata S. A new method for the second-stage auricular projection of the Nagata method: ultra-delicate split-thickness skin graft in continuity with full-thickness skin. Plast Reconstr Surg. 2009;124(05):1477–1485. doi: 10.1097/PRS.0b013e3181babaf9. [DOI] [PubMed] [Google Scholar]
- 39.Kurabayashi T, Asato H, Suzuki Y, Kaji N, Mitoma Y. A temporoparietal fascia pocket method in elevation of reconstructed auricle for microtia. Plast Reconstr Surg. 2017;139(04):935–945. doi: 10.1097/PRS.0000000000003228. [DOI] [PubMed] [Google Scholar]
- 40.Park C. Subfascial expansion and expanded two-flap method for microtia reconstruction. Plast Reconstr Surg. 2000;106(07):1473–1487. doi: 10.1097/00006534-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Baluch N, Nagata S, Park C et al. Auricular reconstruction for microtia: A review of available methods. Plast Surg (Oakv) 2014;22(01):39–43. [PMC free article] [PubMed] [Google Scholar]
- 42.Brånemark R, Brånemark P I, Rydevik B, Myers R R. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38(02):175–181. [PubMed] [Google Scholar]


