Abstract
Congenital auricular anomalies are common sources of aesthetic concern and psychosocial distress for both children and their parents. Only one-third of these anomalies self-correct, leaving a large need for acceptable corrective methods. Otoplasty is often the standard treatment; however, newer nonsurgical methods, including splinting and molding in the neonatal period, have shown favorable results without the complications of surgical intervention and with the advantage of early intervention. These treatment options have not yet been widely adopted in Western countries due to delayed diagnosis of auricular deformities and confusion regarding treatment indications and technique.
Keywords: neonatal ear anomalies, auricular deformation, splinting, molding, nonsurgical management
There is no consensus among existing literature regarding the true incidence of congenital ear anomalies. There is wide variability on the spectrum of auricular anomaly, and many subtleties are missed by pediatricians and health care providers. 1 Historically, it was taught that these anomalies could resolve with time and that treatment by surgical intervention was reserved for moderate or severe anomalies. 2 Otoplasty is a viable option for many patients; however, it comes with the risks of any surgical procedure and may only be performed at an older age.
Nonsurgical management by splinting or molding has emerged as a favorable alternative to otoplasty for patients with mild or moderate ear deformations and is initiated in the neonatal period when the ear is still malleable. Early intervention avoids psychosocial issues that may happen as the child reaches school age, but necessitates early diagnosis of auricular deformations. 3 4
Anatomy
The human ear consists of an external, middle, and inner division, which form the periphery of the brain's auditory and vestibular apparatus. The external ear, composed of the auricle and the external auditory canal, functions as a protective structure for the middle ear's tympanic membrane, as well as a rudimentary filter for incoming sound stimuli. 5 The auricle, synonymous with the term “ear,” is the visible component of the external ear, and is perhaps most important as an aesthetic structure of the human face. 6
The auricle's characteristic three-tier topography consists of the helix and lobule, the antihelix and antitragus, and the concha ( Fig. 1 ).
Fig. 1.
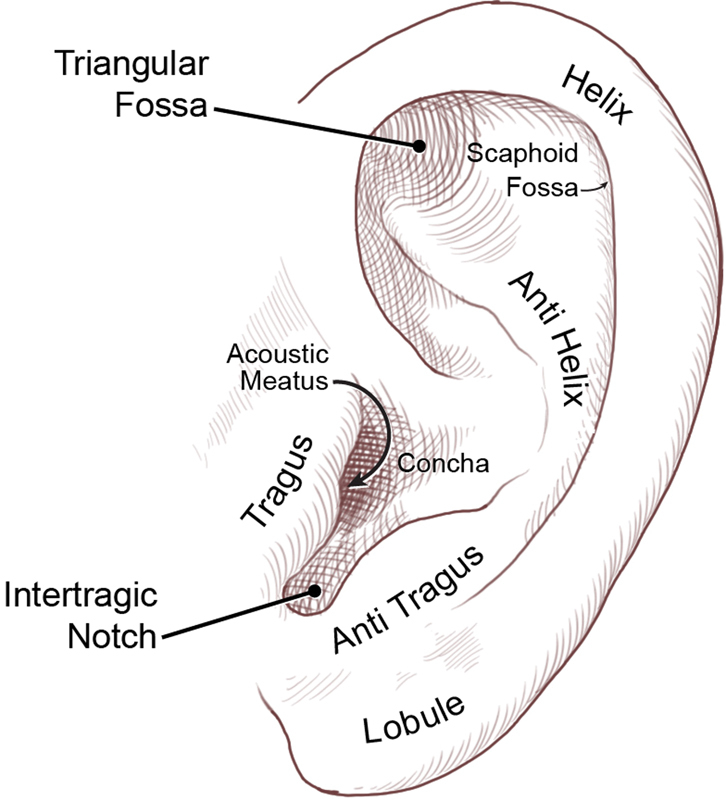
Auricular topography. (Reprinted with permission from Texas Children's Hospital.)
The most central portion of the auricle, the concha, is continuous with the cartilaginous external auditory canal. The conchal fossa houses the root of the ear's anterior helix. The helix, composed of an anterior and posterior division, is the auricle's peripheral boundary, extending posteriorly from the conchal fossa to the tubercle, and inferiorly from the tubercle to the fibrofatty lobule.
The antihelix is a Y-shaped structure that separates the concha from the helix. Its two crura extend superiorly toward the helix into the scaphoid and triangular fossae, while the body extends inferiorly toward the lobule into the antitragus. The antitragus is separated from the tragus, located anterior to the external auditory canal, by the intertragic notch. 6
The three extrinsic muscles of the auricle, the anterior, superior, and posterior auricularis, and the six intrinsic muscles of the auricle, the tragicus, antitragicus, helicis major, helicis minor, intrinsic transverse, and intrinsic oblique muscles, serve as a vestigial function in the adult, but may influence early development of the ear's cartilage framework. The literature suggests that deformational auricular anomalies are due in part to aberrant auricular muscle insertion. 6 7 8
Embryology
Development of the external ear begins early in the embryonic period and extends well past the fetal period into puberty. By the fifth week of gestation, the human embryo has four well-defined pairs of pharyngeal arches, which contribute to multiple developing structures in the head and neck. 6
The first and second pharyngeal arches, or mandibular and hyoid arches, are the structural foundations from which the external ear takes shape. 5 The arches' ventral segments give rise to the tragus, antitragus, intertragic notch, lobule, helical root, and anterior helix, while their dorsal segments give rise to the concha, triangular fossa, inferior crus, and body of the antihelix.
In the sixth gestational week, six foci of mesenchymal proliferation, termed hillocks , emerge from the first and second pharyngeal arches at the level of the embryonic neck ( Fig. 2 ), and migrate cranially as the mandible develops. The auricular hillocks mark the region from which the ear develops; however, there is disagreement as to which adult structures these foci form. 5 6
Fig. 2.
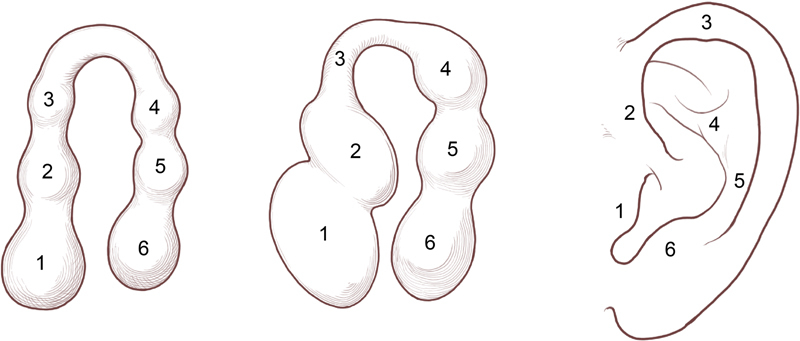
Ear development with six hillocks present in early fetus and corresponding external ear structures in adulthood. (Reprinted with permission from Texas Children's Hospital.)
In the third month of gestation, the developing ear begins to protrude outward, and the remainder of the helix, scaphoid fossa, and superior crus take shape from a free ear fold. By the end of the sixth month, the peripheral helix begins to curl, the antihelix folds, and the antihelical crura take shape. 8 Postnatally, the auricle, apart from the concha, intertragic notch, and helical brim, continues to grow apart. 85% of this growth is within the child's first 3 years of life. 8
Classification of Ear Anomalies
Various classification systems exist to simplify diagnosis and guide treatment of auricular anomalies; however, these were developed to direct surgical treatment of severe anomalies, and do not consider contemporary treatment options. 9 10 Many auricular anomalies do not fit a single category by current classifications; therefore, it is best to simply classify congenital auricular anomalies as malformations or deformations.
Auricular Malformations
Malformations are abnormalities of fetal development that occur due to an error in gestational weeks 5 through 8. These anomalies are characterized by deficient or supernumerary chondrocutaneous structures and require surgical correction. The cause of auricular malformations is often a combination of circumstances, which may include single gene mutations, fetal exposure to harmful environmental agents, or interference in chondrogenesis. They are often seen in conjunction with various craniofacial anomalies as part of a syndrome. 1
Examples of malformational anomalies include anotia—a complete lack of external ear structures—and microtia—a partial or nearly complete absence of external ear structures, cleft ear, polyotia, and preauricular tags or sinuses. 6
Auricular Deformations
Deformations are abnormalities secondary to an external force on a normally developed primary structure, and contributing errors may occur at any point from the ninth week of gestation into the neonatal period. Infants with auricular deformations typically possess a full complement of auricular components. Normal surface topography can often be transiently achieved through simple digital manipulation, making these anomalies most amenable to nonsurgical correction. 1 11
Examples of deformational anomalies include prominent or protruding ear, Stahl's or Spock ear, constricted ear, and cryptotia.
In the prominent or protruding ear, the upper ear protrudes farther than normal due to excess conchal cartilage, abnormal cartilage positioning, or absent cartilage folding. 6 12 The antihelix often fails to hold, resulting in a characteristic widened conchoscaphal angle and flattening of one or both crura. 8
Stahl's or Spock ear has a pointed appearance due to the presence of extra cartilage along the upper ear. This fold forms a third crus, flattened helix, and malformed scaphoid fossa. 6
The constricted ear is a more broad category of auricular deformation, which includes cup ear and crinkled ear, and is characterized by a “folded over,” tightened appearance of the superior helix due to abnormal chondrocutaneous distribution. The cup ear, also known as the lop or lidding ear, has a folded over appearance with a malformed helix, scapha, and antihelix and excess of conchal cartilage. The crinkled or crumpled ear has “crumpled” skin overlying the malformed helix, scapha, and antihelix even when the cartilage framework is returned to a length and width within normal limits. 13 Recently, studies have reclassified constricted ears into groups or types based on severity or axis of deformation to better guide management decisions. 9 14
Cryptotia is a deformational anomaly in which cartilage of the upper ear has formed, but is hidden underneath the skin of the scalp, creating the illusion of an absent auricle. This deformity will never resolve spontaneously and requires more extensive nonsurgical management than the previously mentioned deformations. 7 15 16
Initial Assessment
Nonsurgical correction of neonatal ear anomalies is time sensitive and most effective when initiated within the first 2 weeks of life. 6 For this reason, early identification of the candidate for intervention is critical to success: 75% of auricular deformities are noticed by the child's parents before a physician. Unfortunately, by the time most of these children are evaluated in a plastic surgery clinic, they have surpassed the age of effective nonsurgical treatment. 2 17
Early protocols recommended that neonatologists consult craniofacial nurses for any neonate with a suspected auricular deformity; however, many anomalies were overlooked or were not considered severe enough to contact a specialist for potential intervention. Recent literature suggests training audiologists and audiology technicians to thoroughly examine and recognize common ear deformations during newborn hearing screens. 4 Any nonhypoplastic auricular deformity patient is a potential candidate for nonsurgical correction. 17
Methods of Nonsurgical Intervention
Advantages
Splinting and molding are two current methods of nonsurgical auricular management. These less invasive methods avoid the complications of surgical otoplasty, such as hematoma, scarring, and the need for additional surgery, and are significantly less expensive. 18 Nonsurgical treatment also has the advantage of initiation in the newborn period. Neonatal intervention prevents the potential psychosocial harm and physical discomfort that children with uncorrected auricular deformities face. There is a significant association with higher levels of teasing, inferior cognitive performance, social avoidance, immaturity, and diminished self-confidence in these children and adolescents as well as a societal pressure on parents to seek surgical treatment for their children. 3 4 19
Otoplasty is typically delayed until after the patient is 5 or 6 years old to avoid deformity recurrence and the need for successive procedures. 16 20 Although new literature suggests that surgery in patients younger than 3 years of age has similar recurrence rates to those performed in older children, there is developing concern regarding patient autonomy and informed consent in younger children undergoing invasive surgery with a primarily cosmetic purpose. 21 Splinting and molding sidestep these psychological and ethical issues as well as the risks of surgery and general anesthesia.
Timing
Appropriate timing of nonsurgical auricular intervention is crucial to a successful outcome. The ideal treatment of auricular deformations is initiated within the first 2 weeks of life and may continue until the infant is 3 months old. 6 17 Levels of circulating maternal estrogen and tissue hyaluronic acid, which give the newborn ear malleability, peak in the first 3 days of life, and start to taper dramatically after. By 6 weeks of age, estrogen levels have normalized and the firm, elastic ear is less amenable to splinting or molding treatments. 2
Although it is best to begin management as soon as a deformity is identified, some advocate holding intervention for 3 days to allow intrinsic auricular muscle action and possible spontaneous correction. 4 6 7 15 Premature infants may require extended periods of intervention and the age of effective nonsurgical auricular correction should be extended in congruence with weeks of prematurity. 11 Injectable chondroplasty is a potential new procedure that would allow nonsurgical management far past the ideal 3-month period, but additional studies are necessary to prove its safety and efficacy in practice. 22
Splinting
Splinting is the treatment of choice for neonatal Lop and Stahl's ear deformations. Prominent ears often require a longer duration of treatment due to the horizontal axis of deformation. 14 16 23 Ideally, splints should be light, inexpensive, quick to fabricate, adjustable to complicated cartilage contours, simple to apply and remove, and easy to reform. 23 Satisfactory results have been reported with both wire within a silicone tube and thermoplastic materials. The splinting process involves positioning of the splint material within the groove of the helix and antihelix and securing with adhesive skin closure strips. Adjustments are made each week as an outpatient procedure either by remodeling of the wire core segment or application of heat to the thermoplastic splint. 4 6 14 24 An effective length of treatment may be as few as 2 weeks in newborns with mild deformations, but the average period of splitting is 4 to 6 weeks. Reported complications of treatment are minimal and include superficial skin necrosis, transient skin irritation, and difficulty keeping the splints in place.
Rigid Molding
The Earwell Infant Corrective System (Becon Medical Ltd.) is one of the nonsurgical molding devices currently available for the correction of neonatal ear deformations. The molding process involves the application of a rigid cradle that surrounds the patient's auricle and adheres firmly to the skin. The anterior cradle, posterior cradle, retractor, and conchal former components of the mold enact three key forces on the developing auricle. The anterior conformer, curved to match the natural curvature of the patient's helical rim, flattens the conchal cruise and corrects the conchal-mastoid angle. The posterior conformer, in direct alignment with the antihelix, guides misplaced folds of the antihelix into the superior limb of the triangular fossa, and the helical rim retractor allows auricular expansion if necessary ( Fig. 3 ). 15
Fig. 3.
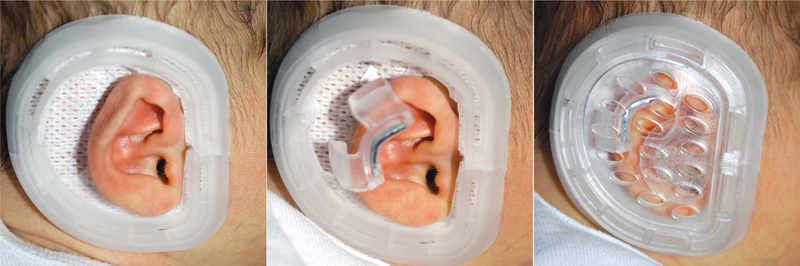
Earwell System application process (Earwell Infant Corrective System from Becon Medical Ltd.).
Molding has shown superior outcomes to stents, tapes, glues, and even otoplasty for all infant deformities with the exception of cryptotia. 15 Complications have a low incidence and include localized skin excoriation and ulceration. 15 17
Injectable Chondroplasty
Injectable chondroplasty is a developing treatment option that provides minimally invasive alteration to cartilage structure. In the 1950s, papain protease injection was found to deplete components of the cartilage matrix and was later cited as a possible future medical therapy in the correction of auricular deformities. 24 25 Recently, studies have pursued research in percutaneous delivery of selective protease enzymes. 22 24 A proteolytic solution of hyaluronidase, pronase, and collagenase II effectively softens the cartilage matrix and would allow the use of splinting or molding in children beyond the ideal window of treatment.
Summary
Splinting and molding are gaining recognition as favorable methods of cosmetic auricular correction. These nonsurgical options provide natural results with significantly fewer risks and complications than a surgical otoplasty. Nonsurgical management must begin within the first few weeks of life to take advantage of the malleable newborn ear and to prevent the childhood psychosocial harm associated with ear deformity. There continues to be a dearth of knowledge regarding available auricular shaping methods, and education efforts will be necessary to ensure these management techniques become a standard of care. The next steps in the nonsurgical management of auricular deformity will involve increased publicity of current techniques and further studies on injectable protease as an option for older patients.
References
- 1.Leonardi A, Bianca C, Basile E et al. Neonatal molding in deformational auricular anomalies. Eur Rev Med Pharmacol Sci. 2012;16(11):1554–1558. [PubMed] [Google Scholar]
- 2.Chang C S, Bartlett S P. A simplified nonsurgical method for the correction of neonatal deformational auricular anomalies. Clin Pediatr (Phila) 2017;56(02):132–139. doi: 10.1177/0009922816641368. [DOI] [PubMed] [Google Scholar]
- 3.Litschel R, Majoor J, Tasman A J. Effect of protruding ears on visual fixation time and perception of personality. JAMA Facial Plast Surg. 2015;17(03):183–189. doi: 10.1001/jamafacial.2015.0078. [DOI] [PubMed] [Google Scholar]
- 4.Petersson R S, Recker C A, Martin J RK, Driscoll C LW, Friedman O. Identification of congenital auricular deformities during newborn hearing screening allows for non-surgical correction: a Mayo Clinic pilot study. Int J Pediatr Otorhinolaryngol. 2012;76(10):1406–1412. doi: 10.1016/j.ijporl.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Wright C G. Development of the human external ear. J Am Acad Audiol. 1997;8(06):379–382. [PubMed] [Google Scholar]
- 6.Porter C JW, Tan S T. Congenital auricular anomalies: topographic anatomy, embryology, classification, and treatment strategies. Plast Reconstr Surg. 2005;115(06):1701–1712. doi: 10.1097/01.prs.0000161454.08384.0a. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K, Hayashi R, Kiyono M, Hirose T, Netsu Y. Nonsurgical correction of congenital auricular deformities. Clin Plast Surg. 1990;17(02):383–395. [PubMed] [Google Scholar]
- 8.Tan S T, Gault D T. When do ears become prominent? Br J Plast Surg. 1994;47(08):573–574. doi: 10.1016/0007-1226(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Tanzer R C. The constricted (cup and lop) ear. Plast Reconstr Surg. 1975;55(04):406–415. [PubMed] [Google Scholar]
- 10.Nagata S. Alternative surgical methods of treatment for the constricted ear. Clin Plast Surg. 2002;29(02):301–315. doi: 10.1016/s0094-1298(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 11.Daniali L N, Rezzadeh K, Shell C, Trovato M, Ha R, Byrd H S. Classification of newborn ear malformations and their treatment with the EarWell Infant Ear Correction System. Plast Reconstr Surg. 2017;139(03):681–691. doi: 10.1097/PRS.0000000000003150. [DOI] [PubMed] [Google Scholar]
- 12.Lindford A J, Hettiaratchy S, Schonauer F.Postpartum splinting of ear deformities BMJ 2007334(7589):366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallach S G, Argamaso R V. The crumpled-ear deformity. Plast Reconstr Surg. 2001;108(01):30–37. doi: 10.1097/00006534-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Schonauer F, La Rusca I, Molea G. Non-surgical correction of deformational auricular anomalies. J Plast Reconstr Aesthet Surg. 2009;62(07):876–883. doi: 10.1016/j.bjps.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 15.Byrd H S, Langevin C J, Ghidoni L A. Ear molding in newborn infants with auricular deformities. Plast Reconstr Surg. 2010;126(04):1191–1200. doi: 10.1097/PRS.0b013e3181e617bb. [DOI] [PubMed] [Google Scholar]
- 16.van Wijk M P, Breugem C C, Kon M. Non-surgical correction of congenital deformities of the auricle: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2009;62(06):727–736. doi: 10.1016/j.bjps.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Doft M A, Goodkind A B, Diamond S, DiPace J I, Kacker A, LaBruna A N. The newborn butterfly project: a shortened treatment protocol for ear molding. Plast Reconstr Surg. 2015;135(03):577e–583e. doi: 10.1097/PRS.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 18.Calder J C, Naasan A. Morbidity of otoplasty: a review of 562 consecutive cases. Br J Plast Surg. 1994;47(03):170–174. doi: 10.1016/0007-1226(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury E T, Hewison J, Timmons M J. Psychological and social outcome of prominent ear correction in children. Br J Plast Surg. 1992;45(02):97–100. doi: 10.1016/0007-1226(92)90165-t. [DOI] [PubMed] [Google Scholar]
- 20.Gosain A K, Kumar A, Huang G. Prominent ears in children younger than 4 years of age: what is the appropriate timing for otoplasty? Plast Reconstr Surg. 2004;114(05):1042–1054. doi: 10.1097/01.prs.0000135334.13796.9d. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe S A.Timing of otoplasty for prominent ears Plast Reconstr Surg 200611702680–681., author reply 680–681 [DOI] [PubMed] [Google Scholar]
- 22.Gandy J R, Foulad A, Chao K K, Wong B J.Injectable chondroplasty: Enzymatic reshaping of cartilage grafts Eur Ann Otorhinolaryngol Head Neck Dis 2017pii: S1879-7296(17)30015-7 [DOI] [PubMed] [Google Scholar]
- 23.Yotsuyanagi T, Yokoi K, Sawada Y.Nonsurgical treatment of various auricular deformities Clin Plast Surg 20022902327–332., ix [DOI] [PubMed] [Google Scholar]
- 24.Tan S T, Abramson D L, MacDonald D M, Mulliken J B. Molding therapy for infants with deformational auricular anomalies. Ann Plast Surg. 1997;38(03):263–268. doi: 10.1097/00000637-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Thomas L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956;104(02):245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]


