Abstract
Reconstruction of partial ear defects represents one of the most challenging areas within reconstructive surgery of the head and neck. Each case of auricular reconstruction is unique and warrants a systematic approach that accounts for defect size and location, the quality of the surrounding skin, patient preference, and operator experience. In this article, the authors outline different reconstructive approaches for defects of the upper-, middle-, and lower-third of the auricle. The relevant anatomy is discussed in detail. Successful outcomes in auricular reconstruction rely on the surgeon's careful analysis of the defect as well as knowledge of the different reconstructive options available.
Keywords: auricular reconstruction, ear reconstruction, partial ear defects
Reconstruction of partial ear defects represents one of the most formidable challenges in reconstructive surgery of the head and neck. The exposed position of the auricle makes it a frequent site of neoplastic and traumatic injury that can result in deformity and tissue loss. A deformity or partial defect of the auricle can detract from facial aesthetics and cause significant psychological morbidity for the patient. 1 Indeed, the psychosocial benefit of reconstruction, whether congenital or acquired, is well-documented. 1 2
Acquired deformities of the ear are most frequently caused by bite injuries (35–72%) 3 4 5 and less commonly by blunt trauma, tumors, thermal injury, and complications after otoplasty. In children, dog bites are the most common bite injuries with infection the most common overall complication. 6 7 In cases of blunt trauma, hematoma is the most common complication and requires prompt evacuation with bolster dressing application to prevent the dreaded cauliflower deformity. 8 Thermal injuries include both frostbite and burns, with an estimated 90% of facial burns involving the external ear. 9 Chondritis is the most common early complication after a burn of the auricle and can be severe enough to destroy the cartilage completely. 10
Approximately 5 to 8% of skin cancers occur on the external ear, 11 with squamous and basal cell carcinomas being most common. 12 In comparison, auricular melanoma is relatively rare and represents only 1% of all cutaneous melanomas. 13 Approximately 45 to 55% of auricular skin cancers are located on the helical rim. 14 They are most often seen in men due to hairstyles and vocations that leave the external ear exposed to ultraviolet radiation.
Each case of auricular reconstruction is unique and warrants a systematic approach that accounts for not only the defect size and location, but also the quality of the surrounding skin, patient preference, and surgeon experience. The various convexities and concavities of the external ear coupled with concomitant differences in rigidity and elasticity account for the wide variety of partial defects observed. In this article, we present an overview of the reconstructive options for partial auricular defects that are most commonly performed.
Embryology of the External Ear
The external ear is formed by six mesenchymal hillocks on the first (mandibular) and second (hyoid) branchial arches ( Fig. 1 ). 15 The tragus, root of the helix, and superior helix arise from the first branchial arch. The antihelix, antitragus, and lobule arise from the second branchial arch. These hillocks fuse by the twelfth week of gestation and development is complete by the twentieth week. As fusion of the hillocks is complicated, developmental anomalies of the auricle are common and are frequently associated with other congenital abnormalities. The external auditory meatus is derived from the first branchial cleft and is separated from the tympanic cavity by the tympanic membrane. 15
Fig. 1.
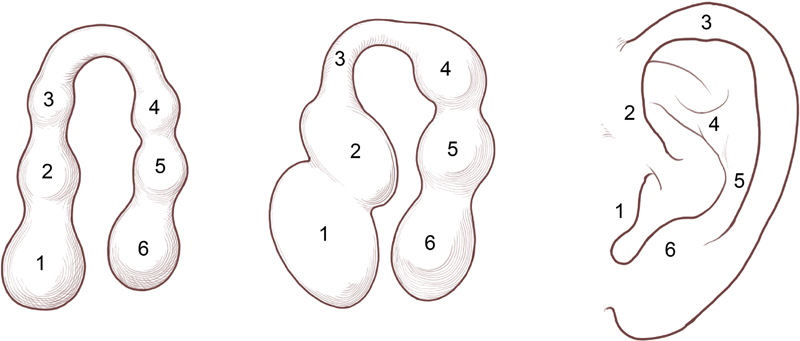
Embryology of the external ear. (Reprinted with permission from Texas Children's Hospital.)
The auricle is initially located in the lower neck region of the embryo. As the mandible and maxilla develop, the auricular areas are displaced dorsolaterally. It reaches its adult location by week 20. 15 16
Anatomy of the External Ear
The skeletal structure of the external ear is composed of fibrocartilage that provides mechanical support and allows for elasticity and shape retention. Cartilage is present in only the upper two-thirds of the ear with the lobule composed of fibrofatty tissue. 11
The cutaneous coverage of the anterolateral surface of the auricle differs from the posteromedial surface. The anterolateral skin lacks subcutaneous tissue and is tightly adherent to the underlying perichondrium. 17 In contrast, the posteromedial aspect of the auricle has a thick layer of adipose tissue between the skin and perichondrium, allowing the skin to be less adherent in this area. As such, the medial auricular skin can more readily be used for skin grafts and flaps.
The superficial temporal (STAs) and posterior auricular arteries (PAAs) are the major blood supply of the auricle, and both arise from the external carotid artery. 17 The PAA makes a more prominent contribution to the auricle and supplies both of its anterior and posterior surfaces. 18 Between 3 to 7 posterior artery perforators 19 enter the ear at the medial aspect of the triangular fossa, cymba conchae, cavum conchae, helical root, and earlobe. 20 Zilinsky et al 19 noted that a row of constant perforators emerged together in a longitudinal line 1-cm posterior to the tragus. In approximately 7% of the population, the occipital artery supplies the posterior auricular skin. 21
The STA gives off three auricular branches (upper, middle, and lower) and supplies the lateral surface of the auricle ( Fig. 2 ). 17 20 Recent anatomical studies suggest that the helical rim is supplied primarily by an arterial arcade formed by the upper and lower branches of the STA. 22 The multiple anastomoses between the STA and PAA provide the anatomical basis for the surplus of different auricular flaps used in reconstruction and account for auricular survival after sustaining posttraumatic defects. 19
Fig. 2.
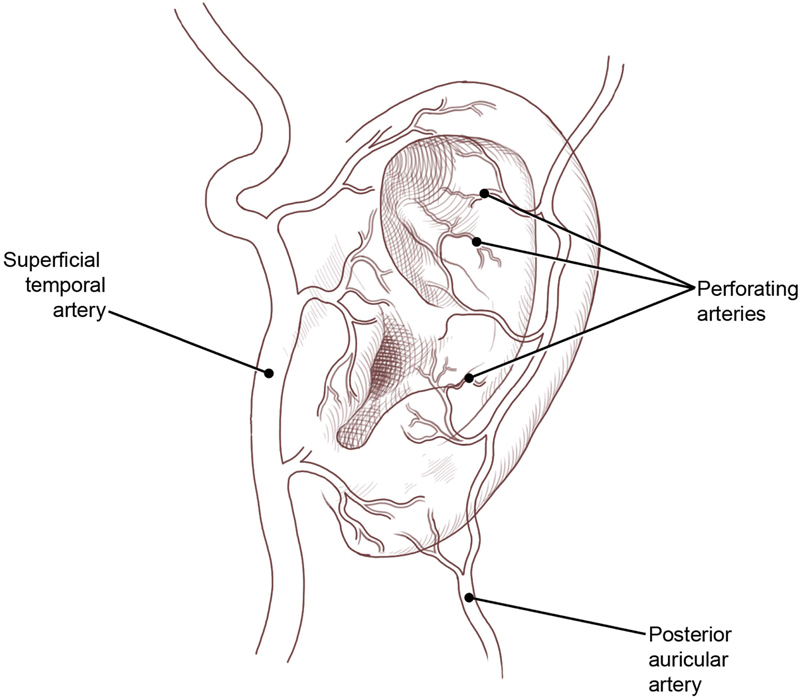
Blood supply of the external ear. (Reprinted with permission from Texas Children's Hospital.)
The innervation of the external ear is derived from several sources ( Fig. 3 ). The great auricular nerve (C2–3) supplies the lower lateral portion (i.e., lobule) and inferior cranial surface. The McKinney point, 6.5 cm inferior to the external auditory canal, has been described as a landmark for great auricular nerve identification. 23 There is a classically described bifurcating (anterior, posterior) branching pattern, but a lobular branch has been identified, frequently originating as a trifurcation. 24
Fig. 3.
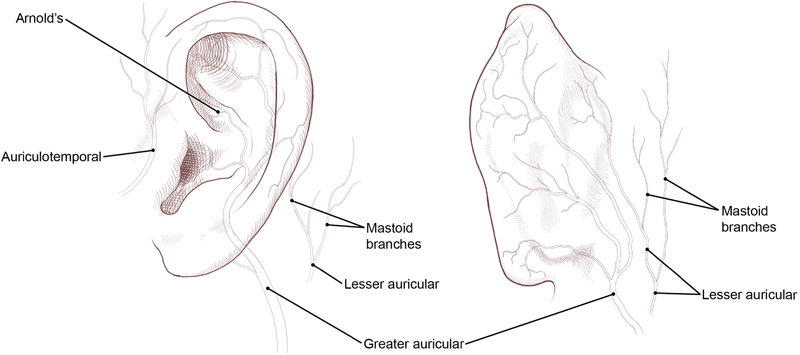
Innervation of the external ear. (Reprinted with permission from Texas Children's Hospital.)
The auriculotemporal nerve, a branch of the third division of the trigeminal nerve (CN V 3 ), provides sensation to the tragus and anterior portion of the auricle, along with the posterior portion of the temple. 17 25 It can often divide into more than one branch as it moves over the posterior portion of the zygomatic arch and travels cranially within the layers of temporoparietal fascia. 25 In up to one-third of patients, the auriculotemporal nerve may cross or be intertwined with the STA. 25
The lesser occipital nerve supplies the superior cranial surface of the ear and arises from the lateral branch of the dorsal rami of C2–3. 17 Anatomical studies have traced its emergence at approximately 60 to 70 mm from the midline and 40 to 60 mm inferior to a line drawn between the lowest points of the external auditory canals. 26
Arnold's nerve, also called the auricular branch of the vagus nerve (CN X), supplies the posterior inferior external auditory canal and meatus, and inferior conchal bowl. 17 Clinically, this nerve is not anesthetized with the typical auricular ring blockade and requires direct infiltration. 27
The lymphatic drainage of the external ear correlates with its embryological development. The concha and meatus drain to the parotid and infraclavicular nodes, and the external canal and cranial surface of the auricle drain to the mastoid and infra-auricular cervical nodes. 28
Normal Auricular Architecture
Achieving proper orientation and positioning of the reconstructed ear is critical for a successful outcome. Understanding anthropometric data and normal aesthetic relationships can assist in preoperative planning and assessment, particularly if both ears are injured.
The average adult ear is 55 to 65 mm long and has a width approximately 55% of its length ( Fig. 4 ). 29 The superior level of the ear should match the level of the superior tarsal crease, and the most inferior point of the lobule should align with the subnasale. 30 31 Up to 10% of the population has evidence of asymmetry of the ear level, but this is often too subtle to detect visually. 32 The helical rim protrudes 1 to 2 cm from the scalp 33 with the angle of protrusion averaging 21 to 25 degrees. 34 The degree of protrusion can vary up to 5 degrees in 25% of people. 35 Lastly, the long axis of the ear is tilted posteriorly at an angle of approximately 20 degrees from the vertical.
Fig. 4.
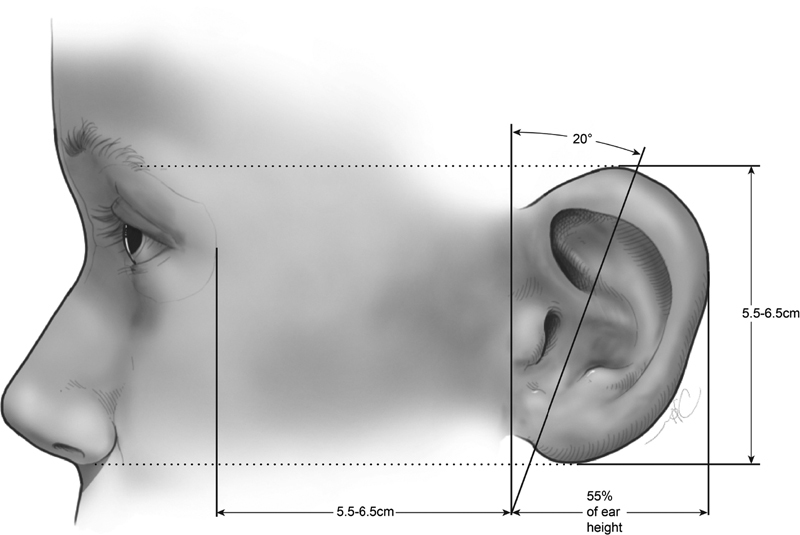
Anthropometric measure of the external ear. (Reprinted with permission from Texas Children's Hospital.)
General Reconstructive Principles
The fundamental goals of partial auricular reconstruction are to achieve symmetry with the contralateral ear and establish a stable construct to support eyewear and certain hearing devices. Successful repair requires both careful analyses of the defect and surrounding tissue and a systematic approach to the reconstruction. 36 The location, size, and vascularity of the defect will significantly influence the operative plan and reconstructive options. Three-dimensional considerations must also be accounted for to reconstruct an ear that matches the unaffected contralateral ear in size, shape, and orientation.
Prior to reconstruction, patient-specific factors should be identified and addressed. The surgeon should elicit the patient's expectations and any comorbidities that may preclude lengthy or multistaged operations. Some patients, particularly the elderly or critically ill, may be best treated conservatively out of necessity or patient preference. Levin et al 37 evaluated secondary intention healing of full-thickness auricular defects after Mohs surgery and found all wounds to heal within 10 weeks and reported that exposed cartilage was not a contraindication to healing by secondary intention.
Although classification systems for microtia are well-described, partial traumatic auricular defects are less well-categorized. 38 Some authors divide traumatic injuries into defects of cutaneous coverage, with or without intact cartilage, and full-thickness defects. 30 The most popular classification for full-thickness traumatic ear defects is anatomical. 39
Upper-Third Defects
Anatomically, the upper third of the auricle is delineated by the line drawn parallel to the Frankfort horizontal line through the superior edge of the concha cymba. The visibility of the superior third of the ear makes it a particularly noticeable area for both marginal and nonmarginal defects. For patients that wear eyeglasses, the structural integrity of the superior portion of the ear is critical to be re-established. The specific technique chosen in each case depends on the size and depth of the defect and how much local skin remains for the reconstruction.
Small full-thickness defects (< 1.5 cm) of the helix and antihelix in this area can be repaired by conversion to a wedge-shaped resection followed by primary layered closure. The wedge excision is best suited for rim defects less than 15% of the auricular height. 40 Burow's triangles of skin and cartilage on either side of the wedge may need to be excised to permit closure without subsequent ear distortion. Although wedge excision does decrease the vertical height of the ear, the difference is often inconspicuous. 30 The unilateral or bilateral Antia-Buch chondrocutaneous advancement flap 41 is also particularly well-suited for small helical rim upper-third defects ( Fig. 5 ). 42 In this single-stage technique, flaps are created by mobilizing the helix from the scapha through an incision in the helical sulcus that extends through the anterior skin and cartilage. The posterior auricular skin superficial to the perichondrium is undermined until the entire helix is hanging as a chondrocutaneous flap on the posterior skin. The flap is perfused via the posterior arterial network. Benefits of the Antia-Buch technique include superior cosmesis, technical simplicity, preserved auricular anatomical landmarks, and a low risk of flap necrosis. 43
Fig. 5.
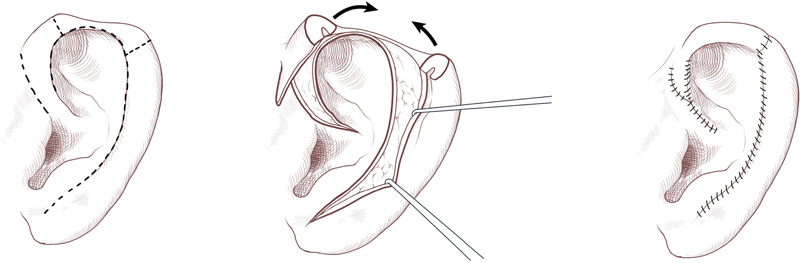
Antia-Buch procedure. (Reprinted with permission from Texas Children's Hospital.)
For reconstruction of defects up to 2.5 cm, crescentic or star excisions can be used to allow for distribution of tension throughout the auricle ( Fig. 6 ). 8 To further avoid ear distortion, the apex of the wedge resection may be extended into the concha and point to the root of the helix, if possible. Composite grafts from the contralateral ear are another option for helical rim defects of this size. A full-thickness wedge of skin and cartilage is harvested from the contralateral ear and is sewn into the defect by reapproximating all three layers. To minimize the risk of graft necrosis, the harvested wedge should be one-half the size of the defect and should not be larger than 1.5 cm. This technique allows reduction of the contralateral ear to maintain some symmetry. 11 The Antia-Buch chondrocutaneous advancement flap may also be utilized for medium-sized helical rim defects, with the additional advancement of the flaps achieved by using a V-Y island advancement of the root of the helix. 30 Although initially described for helical rim defects up to 2 cm, one study reported good results in defects up to 2.8 cm with the Antia-Buch technique. 43 Several additional modifications of the Antia-Buch method have been described. 11 44 45 46 47 48 49 When the defect extends from the helical rim into the hollow of the scapha, the Antia-Buch technique can be combined with crescent-shaped chondrocutaneous excisions on either side of the defect, preserving posterior perichondrium and skin, 48 or with a postauricular pull-through flap. 49 50
Fig. 6.
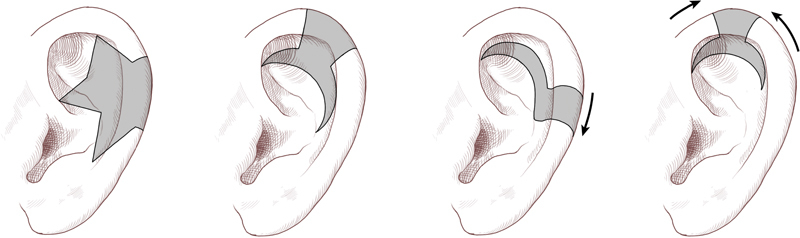
Varied techniques for crescentic and star excision depending on the exact helical defect location. (Reprinted with permission from Texas Children's Hospital.)
For defects > 2.5 cm and confined to the helical rim, multistaged pre- or postauricular tubed flaps can be considered. In the first (delay) stage, a tube is created from either pre- or postauricular skin and remains attached to the superior and inferior poles for approximately 3 weeks. In the second stage, there is a division of either the superior or inferior pedicle with attachment to one end of the helical defect. In the third stage, there is a division of the remaining pedicle and flap inset with the opening of the tube. In situations where there is a skin deficit, composite pedicled flaps can be used such as those described by Davis 51 and Orticochea. 52 The Davis flap is based at the root of the helix and is transposed to the marginal defect. The anterior skin and cartilage of the conchal bowl are dissected away from the posterior skin, and the rotated superiorly. A full-thickness postauricular skin graft is required to fill the donor site. In comparison, the Orticochea flap is based on the caudal part of the helix. Yotsuyanagi et al devised an alternative reconstructive technique that uses a combination of several flaps. 53 A chondrocutaneous flap is raised from the conchal bowl and is advanced into the defect to provide cartilage support and anterior skin coverage. A postauricular flap is transposed to provide posterior skin coverage of the wound. A full-thickness skin graft is then used to cover the postauricular defect.
Major losses in the superior third will require a new cartilaginous framework and utilize many of the principles applied in microtia reconstruction. Donor cartilage can be obtained from the contralateral conchal bowl, nasal septum, or rib. 11 After the autologous cartilage has been shaped, temporoparietal fascia (TPF) can be harvested from the ipsilateral scalp, tunneled into the superior auricular sulcus, and then draped over the cartilage framework. 11 Alternatively, the TPF can be harvested from the contralateral scalp and transferred as a free flap. The TPF is thin, pliable, and highly vascular; it is most frequently based on the posterior branch of the STA. 11 A split- or full-thickness skin graft is then applied over the fascia to complete the reconstruction.
Middle-Third Defects
Reconstruction of middle-third defects relies on similar principles to those of the upper third. The structural support of the middle third is not as functionally critical for eyeglass support; thus, reconstructive options are often fewer and more straightforward. Smaller defects (< 1.5 cm) can be managed with wedge excision and direct closure. Some authors have advocated for the inclusion of accessory triangle excisions in the scaphoid fossa and cavum conchae to prevent subsequent contour deformity following reconstruction.
Middle-third defects too large (2.0–2.5 cm) to be amenable to wedge excision and direct closure can be addressed via chondrocutaneous advancement flaps similar to those employed in upper-ear defects. 41 Alternatively, large middle-third defects can be reconstructed with a two-stage posteriorly based retroauricular flap and autologous cartilage graft ( Fig. 7 ). The comparatively thinner and more pliable postauricular skin in the middle third of the ear allows for greater applicability in retroauricular flap coverage. In the first stage, a cutaneous advancement flap is created from the posterior scalp and advanced over the lateral aspect of the defect. 30 Septal or conchal cartilage is implanted beneath the advancement flap during this initial procedure. During the second stage, the flap is divided from the scalp and then folded to cover the medial aspect of the cartilage graft. 30 If unable to be closed primarily, a skin graft is used to cover the postauricular donor site.
Fig. 7.
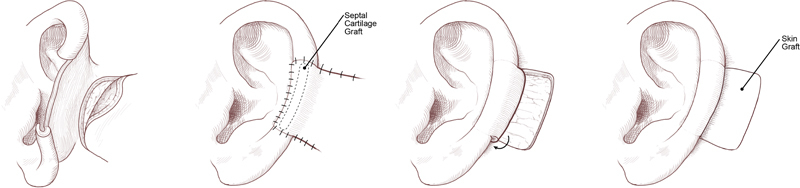
Staged postauricular and autologous cartilage graft reconstruction for large middle-third defects. (Reprinted with permission from Texas Children's Hospital.)
Inferior-Third Defects
The lobule comprises the lower third of the auricle and is uniquely devoid of a cartilage framework. The lobule constitutes an important aesthetic landmark and, as in other ear defects, defect size often dictates the most appropriate method of reconstruction. Lobular defects can arise from trauma, neoplasms, congenital deformities, keloids, burns, or gauge defects, and are most frequently seen among patients who wear earrings ( Fig. 8 ).
Fig. 8.
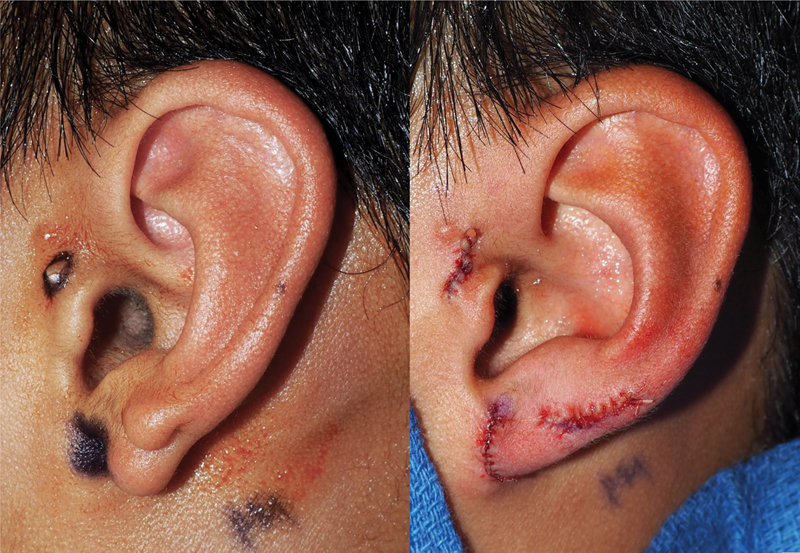
Congenital earlobe deformity.
The relative soft tissue mobility and redundancy of the lower ear can often allow for direct closure of small, soft tissue only defects in this region. Defects comprising nearly 50% of the lobule may be closed directly with minimal resultant deformity. 30 Most lobule defects can be comfortably performed under local anesthesia in the office setting or emergency department.
A special note should be made regarding split earlobe defects resulting from earring trauma. Although this represents a small defect in the overall scheme of auricular reconstruction, it is one of the most common referrals to the otolaryngologist or plastic surgeon. 54 Partial tears in the body of the lobule itself can often be excised and closed directly. Others advocate for conversion to a complete tear to avoid a pointed lobule after repair. Complete tears through the lobular border can be repaired in a variety of ways, including z-, l-, or v-plasty with wedge resection. 55 56 Typically, de-epithelialization of the cleft border and primary approximation will provide a durable and cosmetically acceptable repair without lobular distortion.
For larger or total lobular defects, many authors recommend staged reconstruction with autologous cartilage grafting using either conchal or septal cartilage. Cartilage helps to resist wound contracture and prevent contour deformity of the reconstructed lobule. 44 Using a 2-stage approach, a shaped piece of cartilage is first buried in a subcutaneous pocket below the auricle. About 6 weeks later, the segment is then typically elevated with its overlying skin as a composite graft and advanced into the lobule location. The posterior side of the graft is then skin grafted.
Fat grafting for lobular augmentation is very useful for volume asymmetry.
Conclusion
Reconstruction of partial ear defects remains a challenging, but rewarding aspect of facial reconstructive surgery. Consideration must be given not only to the defect size and location, but also to the quality and availability of the surrounding tissues. The principles of microtia reconstruction should be applied in situations of significant auricular loss. Successful outcomes in partial auricular reconstruction rely on an appreciation for the uniqueness of each defect and thorough knowledge of the anatomy of reconstructive options.
References
- 1.Horlock N, Vögelin E, Bradbury E T, Grobbelaar A O, Gault D T. Psychosocial outcome of patients after ear reconstruction: a retrospective study of 62 patients. Ann Plast Surg. 2005;54(05):517–524. doi: 10.1097/01.sap.0000155284.96308.32. [DOI] [PubMed] [Google Scholar]
- 2.Steffen A, Klaiber S, Katzbach R, Nitsch S, König I R, Frenzel H. The psychosocial consequences of reconstruction of severe ear defects or third-degree microtia with rib cartilage. Aesthet Surg J. 2008;28(04):404–411. doi: 10.1016/j.asj.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Harris P A, Ladhani K, Das-Gupta R, Gault D T. Reconstruction of acquired sub-total ear defects with autologous costal cartilage. Br J Plast Surg. 1999;52(04):268–275. doi: 10.1054/bjps.1998.3053. [DOI] [PubMed] [Google Scholar]
- 4.Steffen A, Katzbach R, Klaiber S. A comparison of ear reattachment methods: a review of 25 years since Pennington. Plast Reconstr Surg. 2006;118(06):1358–1364. doi: 10.1097/01.prs.0000239539.98956.b0. [DOI] [PubMed] [Google Scholar]
- 5.Pearl R A, Sabbagh W. Reconstruction following traumatic partial amputation of the ear. Plast Reconstr Surg. 2011;127(02):621–629. doi: 10.1097/PRS.0b013e318200a948. [DOI] [PubMed] [Google Scholar]
- 6.Elsahy N I.Acquired ear defects Clin Plast Surg 20022902175–186., v–vi [DOI] [PubMed] [Google Scholar]
- 7.Talan D A, Citron D M, Abrahamian F M, Moran G J, Goldstein E J; Emergency Medicine Animal Bite Infection Study Group.Bacteriologic analysis of infected dog and cat bites N Engl J Med 19993400285–92. [DOI] [PubMed] [Google Scholar]
- 8.Janis J E. Boca Raton, FL: CRC Press; 2014. Essentials of Plastic Surgery. 2nd ed. [Google Scholar]
- 9.Anniko M, Bernal-Sprekelsen M, Bonkowsky V, Bradley P J, Iurato S. Heidelberg, Germany: Springer; 2010. Otorhynolaryngology, Head and Neck Surgery. [Google Scholar]
- 10.Crumley R L, Abemayor E. Burn of the auricle. Head Neck. 1992;14(03):243–246. doi: 10.1002/hed.2880140313. [DOI] [PubMed] [Google Scholar]
- 11.Shonka D C, Jr, Park S S. Ear defects. Facial Plast Surg Clin North Am. 2009;17(03):429–443. doi: 10.1016/j.fsc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Sand M, Sand D, Brors D, Altmeyer P, Mann B, Bechara F G. Cutaneous lesions of the external ear. Head Face Med. 2008;4:2. doi: 10.1186/1746-160X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson D A, Krige J E, Strover R M, King H S. Malignant melanoma of the external ear. Br J Plast Surg. 1990;43(05):608–611. doi: 10.1016/0007-1226(90)90129-n. [DOI] [PubMed] [Google Scholar]
- 14.Blake G B, Wilson J S. Malignant tumours of the ear and their treatment. I. Tumours of the auricle. Br J Plast Surg. 1974;27(01):67–76. doi: 10.1016/0007-1226(74)90065-4. [DOI] [PubMed] [Google Scholar]
- 15.Sadler T W. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. Langman's Medical Embryology. 12th ed. [Google Scholar]
- 16.Kelley P E, Scholes M A.Microtia and congenital aural atresia Otolaryngol Clin North Am 2007400161–80., vi v i [DOI] [PubMed] [Google Scholar]
- 17.Netter F H. Philadelphia, PA: Saunders; 2014. Atlas of Human Anatomy. 6th ed. [Google Scholar]
- 18.Imanishi N, Nakajima H, Aiso S. Arterial anatomy of the ear. Okajimas Folia Anat Jpn. 1997;73(06):313–323. doi: 10.2535/ofaj1936.73.6_313. [DOI] [PubMed] [Google Scholar]
- 19.Zilinsky I, Erdmann D, Weissman O et al. Reevaluation of the arterial blood supply of the auricle. J Anat. 2017;230(02):315–324. doi: 10.1111/joa.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C, Roh T S.Anatomy and embryology of the external ear and their clinical correlation Clin Plast Surg 20022902155–174., v [DOI] [PubMed] [Google Scholar]
- 21.Park C, Lineaweaver W C, Rumly T O, Buncke H J. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992;90(01):38–44. doi: 10.1097/00006534-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Zilinsky I, Cotofana S, Hammer N et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg. 2015;68(01):56–62. doi: 10.1016/j.bjps.2014.08.062. [DOI] [PubMed] [Google Scholar]
- 23.McKinney P, Katrana D J. Prevention of injury to the great auricular nerve during rhytidectomy. Plast Reconstr Surg. 1980;66(05):675–679. doi: 10.1097/00006534-198011000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sharma V S, Stephens R E, Wright B W, Surek C C. What is the lobular branch of the great auricular nerve? Anatomical description and significance in rhytidectomy. Plast Reconstr Surg. 2017;139(02):371e–378e. doi: 10.1097/PRS.0000000000002980. [DOI] [PubMed] [Google Scholar]
- 25.Janis J E, Hatef D A, Ducic I et al. Anatomy of the auriculotemporal nerve: variations in its relationship to the superficial temporal artery and implications for the treatment of migraine headaches. Plast Reconstr Surg. 2010;125(05):1422–1428. doi: 10.1097/PRS.0b013e3181d4fb05. [DOI] [PubMed] [Google Scholar]
- 26.Tubbs R S, Salter E G, Wellons J C, Blount J P, Oakes W J. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20(03):235–238. doi: 10.1002/ca.20297. [DOI] [PubMed] [Google Scholar]
- 27.Bumsted R M, Ceilley R I. Local anesthesia of the auricle. J Dermatol Surg Oncol. 1979;5(06):448–449. doi: 10.1111/j.1524-4725.1979.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 28.Brent B. Philadelphia, PA: WB Saunders; 1990. Reconstruction of the auricle; pp. 2094–2152. [Google Scholar]
- 29.Farkas L G. Anthropometry of the normal and defective ear. Clin Plast Surg. 1990;17(02):213–221. [PubMed] [Google Scholar]
- 30.Cheney M L, Hadlock T A, Quatela V C. Edinburgh: Elsevier Mosby; 2007. Reconstruction of the auricle; pp. 581–624. [Google Scholar]
- 31.Posnick J C, al-Qattan M M, Whitaker L A.Assessment of the preferred vertical position of the ear Plast Reconstr Surg 199391071198–1203., discussion 1204–1207 [PubMed] [Google Scholar]
- 32.Farkas L G.Vertical location of the ear, assessed by the Leiber test, in healthy North American Caucasians 6--19 years of age Arch Otorhinolaryngol 1978220(1-2):9–13. [DOI] [PubMed] [Google Scholar]
- 33.McDowell A J. Goals in otoplasty for protruding ears. Plast Reconstr Surg. 1968;41(01):17–27. doi: 10.1097/00006534-196801000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Farkas L G. Anthropometry of normal and anomalous ears. Clin Plast Surg. 1978;5(03):401–412. [PubMed] [Google Scholar]
- 35.Farkas L G. New York, NY: Raven Press; 1994. Anthropometry of the Head and Face. 2nd ed. [Google Scholar]
- 36.Brent B. The acquired auricular deformity. A systematic approach to its analysis and reconstruction. Plast Reconstr Surg. 1977;59(04):475–485. [PubMed] [Google Scholar]
- 37.Levin B C, Adams L A, Becker G D.Healing by secondary intention of auricular defects after Mohs surgery Arch Otolaryngol Head Neck Surg 19961220159–66., discussion 67 [DOI] [PubMed] [Google Scholar]
- 38.Li D, Xu F, Zhang R et al. Surgical reconstruction of traumatic partial ear defects based on a novel classification of defect sizes and surrounding skin conditions. Plast Reconstr Surg. 2016;138(02):307e–316e. doi: 10.1097/PRS.0000000000002408. [DOI] [PubMed] [Google Scholar]
- 39.Stephen J. Philadelphia, PA: Elsevier; 2006. Plastic Surgery. 2nd ed. [Google Scholar]
- 40.Radonich M A, Zaher M, Bisaccia E, Scarborough D. Auricular reconstruction of helical rim defects: wedge resection revisited. Dermatol Surg. 2002;28(01):62–65. doi: 10.1046/j.1524-4725.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- 41.Antia N H, Buch V I. Chondrocutaneous advancement flap for the marginal defect of the ear. Plast Reconstr Surg. 1967;39(05):472–477. doi: 10.1097/00006534-196705000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Calhoun K H, Slaughter D, Kassir R, Seikaly H, Hokanson J A. Biomechanics of the helical rim advancement flap. Arch Otolaryngol Head Neck Surg. 1996;122(10):1119–1123. doi: 10.1001/archotol.1996.01890220083014. [DOI] [PubMed] [Google Scholar]
- 43.Al-Shaham A. Helical advancement: Pearls and pitfalls. Can J Plast Surg. 2012;20(02):e28–e31. [PMC free article] [PubMed] [Google Scholar]
- 44.Fukuzumi S, Tai Y, Kubota J et al. A new method of earlobe reconstruction. J Jpn Plast Reconstr Surg. 1985;28:482–489. [Google Scholar]
- 45.Butler C E. Reconstruction of marginal ear defects with modified chondrocutaneous helical rim advancement flaps. Plast Reconstr Surg. 2003;111(06):2009–2013. doi: 10.1097/01.PRS.0000056834.94472.4C. [DOI] [PubMed] [Google Scholar]
- 46.Majumdar A, Townend J. Helix rim advancement for reconstruction of marginal defects of the pinna. Br J Oral Maxillofac Surg. 2000;38(01):3–7. doi: 10.1054/bjom.1998.0009. [DOI] [PubMed] [Google Scholar]
- 47.Argamaso R V, Lewin M L. Repair of partial ear loss with local composite flap. Plast Reconstr Surg. 1968;42(05):437–441. doi: 10.1097/00006534-196811000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Bialostocki A, Tan S T. Modified Antia-Buch repair for full-thickness upper pole auricular defects. Plast Reconstr Surg. 1999;103(05):1476–1479. doi: 10.1097/00006534-199904050-00019. [DOI] [PubMed] [Google Scholar]
- 49.Zenn M R. Helical advancement with a posterior auricular pull-through flap: A technique for reconstruction of combined helical and scaphal defects. Aesthetic Plast Surg. 1999;23(02):131–133. doi: 10.1007/s002669900255. [DOI] [PubMed] [Google Scholar]
- 50.Ha R Y, Trovato M J. Plastic Surgery of the Ear. Select Read Plast Surg. 2011;11(03):1–46. [Google Scholar]
- 51.Davis J, Tanzer R C, Edgerton M T.Reconstruction of the upper third of the ear with a chondrocutaneous composite flap based on the crus helixIn:, eds.Symposium on Reconstruction of the Auricle St. Louis, MO: CV Mosby; 1974247
- 52.Orticochea M. Reconstruction of partial loss of the auricle. Plast Reconstr Surg. 1970;46(04):403–405. doi: 10.1097/00006534-197010000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Yotsuyanagi T, Nihei Y, Sawada Y. Reconstruction of defects involving the upper one-third of the auricle. Plast Reconstr Surg. 1998;102(04):988–992. doi: 10.1097/00006534-199809040-00008. [DOI] [PubMed] [Google Scholar]
- 54.Niamtu J., III Eleven pearls for cosmetic earlobe repair. Dermatol Surg. 2002;28(02):180–185. doi: 10.1046/j.1524-4725.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 55.Sharma R, Krishnan S, Kumar S, Verma M. Rotation flap lobuloplasty: technique and experience with 24 partially torn earlobes. Int J Oral Maxillofac Surg. 2014;43(10):1206–1210. doi: 10.1016/j.ijom.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Narasimhan J K, Jackson I T. A long-term review of Z-plasty technique for repair of split earlobes. Eur J Plast Surg. 2010;33(03):125–128. [Google Scholar]


