Abstract
A patient-derived orthotopic xenograft (PDOX) model of undifferentiated pleomorphic sarcoma (UPS) was previously established that acquired red fluorescent protein (RFP)-expressing stroma by growth in an RFP transgenic nude mouse. In the present study, an imageable PDOX model (iPDOX) of UPS was established by orthotopic implantation in the biceps femoris of transgenic RFP nude mice. After the tumors grew to a diameter of 10 mm, they were harvested and the brightest portion of the tumors were subsequently orthotopically transplanted to both RFP and non-colored nude mice. The UPS PDOX tumor was again transplanted to RFP transgenic and non-colored nude mice and finally a 3rd passage was made in the same manner. Five UPS tumors from each passage in both RFP and non-colored mouse models were harvested. The FV1000 confocal microscope was used to visualize and quantitate the RFP area of the resected tumors. The average percent fluorescent area in the first passage of RFP mice was 34 ± 22%; in the second passage, 34 ± 20%; and 36 ± 11% in the third passage of RFP transgenic nude mice. The average tumor RFP area in the first passage from RFP mice to non-colored mice was 20±7%; in the second passage, 28±11%; in the third passage was 27±13%. The present results demonstrate the extensive and stable acquisition of stroma by the UPS-tumor growing orthotopically in transgenic RFP nude mice (iPDOX). This model can be used for screening for effective drugs for individual patients and drug discovery.
Keywords: Soft tissue sarcoma, patient-derived orthotopic xenograft (PDOX), red fluorescent protein (RFP), transgenic nude mouse, passaging, stroma labeling, imaging
Introduction
Kiyuna, T., Murakami, T., Tome, Y., Igarashi, K., Kawaguchi, K., Russell, T., Eckhardt, M.A., Crompton, J., Singh, A., Bernthal, N., Bukata, S., Federman, N., Kanaya, F., Eilber, F.C., and Hoffman, R.M. Labeling the stroma of a patient-derived orthotopic xenograft (PDOX) mouse models of undifferentiated pleomorphic soft-tissue sarcoma with red fluorescent protein for rapid non-invasive drug screening. J. Cell. Biochem. 118, 361–365, 2017.
Appropriate mouse models of cancer that accurately represent patient cancer behavior, most importantly, metastasis [Hoffman, 2015]. Therefore, our laboratory has established patient-derived orthotopic xenograft (PDOX) nude-mouse models of the major cancers: colon [Fu et al., 1991; Metildi et al., 2014; Hiroshima et al., 2014a]; pancreas [Fu et al., 1992; Hiroshima et al., 2014b,c,d,e, 2015a,b; Yano et al., 2015]; lung [Wang et al., 1992]; ovarian [Fu and Hoffman, 1993]; breast [Fu et al., 1993]; stomach [Furukawa et al., 1993]; mesothelioma [Astoul et al., 1996]; soft tissue sarcoma [Hiroshima et al., 2015c,2015d; Murakami et al., 2016a, b; Kiyuna et al., 2017]; follicular dendritic-cell sarcoma [Kiyuna et al., 2016]; Ewing’s sarcoma [Murakami et al., 2016a, b] and melanoma [Yamamoto et al., 2016; Kawaguchi et al., 2016a, b].
We previously developed an imageable PDOX model for pancreatic cancer [Suetsugu et al., 2012a] by passaging the tumor in fluorescent-protein-expressing mice. The pancreatic PDOX acquired and maintained fluorescent stroma for each mouse [Suetsugu et al., 2012b]. The labeled PDOX tumors were then orthotopically passaged to non-transgenic non-colored nude mice where they could be non-invasively imaged [Suetsugu et al., 2012c].
We previously established an undifferentiated pleomorphic sarcoma (UPS) PDOX model [Murakami et al., 2016] and subsequently showed it acquired bright red fluorescent protein (RFP)-expressing stroma through one passage in RFP transgenic mice. Upon passage to non-colored nude mice, the RFP-expressing UPS PDOX was non-invasively imageable [Kiyuna et al., 2017].
The present report describes the extent of acquisition of RFP stroma in the UPS iPDOX after multiple serial passages in RFP mice.
Materials and Methods
Mice
Athymic nu/nu nude mice and transgenic RFP-expressing athymic nu/nu mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. Animals were housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All surgical procedures and imaging were performed with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. In order to minimize any suffering of the animals, the use of anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation and if they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop.
Patient-derived tumor
The patient was previously diagnosed with UPS of the thigh received tumor resection. The disease recurred locally a few months later and was surgical resected by F.C.E., Division of Surgical Oncology, University of California, Los Angeles (UCLA). Written informed consent was obtained from the patient as a part of UCLA Institutional Review Board approved protocol [Murakami et al., 2016a].
Establishment of a fluorescent PDOX model of UPS by surgical orthotopic implantation (SOI)
The UPS was previously established in nude mice orthotopically in the biceps femoris [Murakami et al., 2016a]. Subsequently we established an imageable PDOX (iPDOX) of UPS in RFP transgenic nude mice [Kiyuna et al., 2017]. In the present study, the iPDOX tumors in the RFP transgenic mouse grew to 10 mm in diameter, the tumors were harvested and the brightest portion of the tumors were subsequently serially transplanted orthotopically to both RFP and non-colored nude mice for a total of 3 times (Fig. 1).
Figure 1. Experimental design.
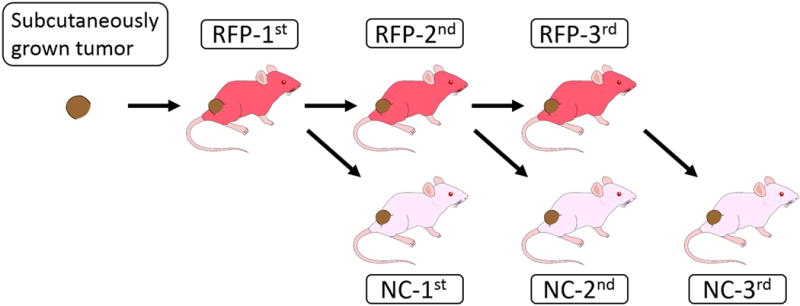
N = 5 tumor harvested at each passage. NC = non-colored.
Frozen section imaging of the fluorescent UPS PDOX model
The OV100 Small Animal Fluorescence Imaging System (Olympus, Tokyo, Japan) [Yamauchi et al., 2006] was used to visualize RFP-expression in the UPS stroma by whole-body imaging. Five UPS tumors at each passage of both RFP and non-colored nude mice were harvested at each passage. The center portion of each tumor was selected for frozen sectioning. The frozen blocks of tumors were sliced at 10 μm thickness. Three different images of field per one slide were observed with the FV1000 confocal laser microscope (Olympus) [Uchugonova et al., 2011]. The percentage of RFP-expressing tumor stroma area was analyzed with Image J v1.440 (National Institutes of Health).
Statistical analysis
Statistical analysis was performed with JMP pro version 12. The percentage of RFP-expressing tumor stroma area was expressed as mean ± SD. The two-tailed Student’s t-test was used to compare continuous variables between 3 groups. A P value of < 0.05 was considered statistically significant.
Results and Discussion
Whole body imaging of the UPS iPDOX model during multiple passages
The tumor grew orthotopically in the right biceps femoris of RFP-expressing nude mice (Fig. 2). The iPDOX tumors had sufficient shows RFP expression to be imaged through the skin in the first passage in RFP and non-colored nude mice (Fig. 2A). The tumors harvested from RFP mice and after subsequent passage in non-colored nude mice had acquired sufficient bright RFP stroma to be very brightly imaged.
Figure 2. Imaging of UPS iPDOX tumor in first passage in an RFP transgenic mouse and after first passage to a non-colored nude mouse.

Left panels: Noninvasive image of the UPS iPDOX in transgenic RFP and non-colored nude mice. Right panel: Excised tumors from RFP and non-colored nude mice. The UPS tumor was passaged from an RFP to a non-colored nude mouse.
Confocal imaging of the RFP stroma in the UPS iPDOX model during multiple passages
Images obtained with the FV1000 confocal laser microscope showed RFP-expressing stroma in the UPS tumors harvested from each passage of RFP and non-colored nude mice (Fig 3). The images were obtained from frozen sections of the central tumor area containing bright tumor stroma (Fig 3). Tumors from each passage of RFP and non-colored mice contained bright red fluorescent stroma.
Figure 3.
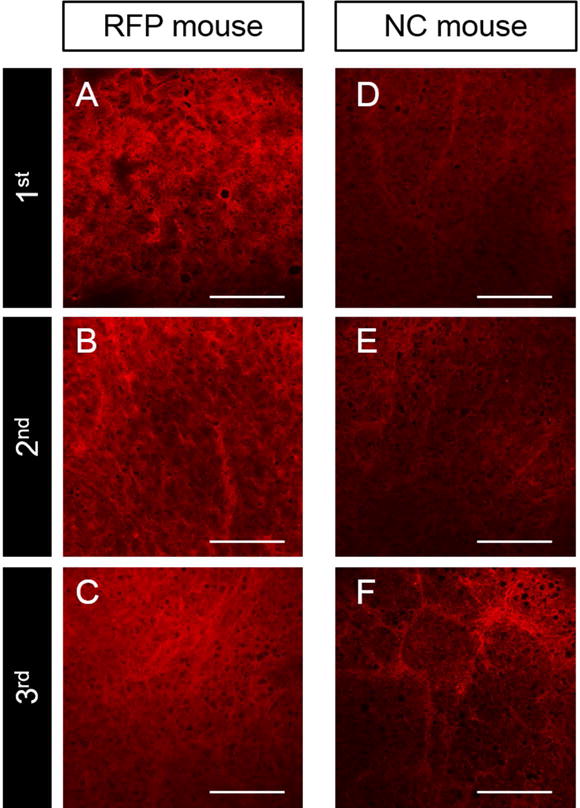
Frozen section images of PDOX tumors with RFP-expressing stroma visualized with the FV1000 confocal laser microscope from 3 successive passages as shown in Figure 1.
Quantitation of RFP-expressing tumor stroma area at each passage of the UPS iPDOX
The average tumor RFP area in the first passage of RFP mice was 34 ± 22%; in the second passage was 34 ± 20%; and in the third passage was 36 ± 11%. The average tumor RFP area in the first passage from RFP mice to non-colored mice was 20±7%; in the second passage was 28±11%; and in the third passage was 27±13%. The average tumor RFP area after all passages in RFP mice and to non-colored nude mice did not significantly differ.
The present results demonstrate the extensive and stable acquisition of stroma by the UPS-tumor growing orthotopically in transgenic RFP nude mice (iPDOX). This is shown by the bright stroma in the UPS iPDOX after a single passage in RFP mice, which is not diluted after passage in non-colored nude mice. Serial passage of UPS iPDOX did not increase the extent of acquired RFP-expressing stroma which was probably maximal in the first passage in RFP nude mice.
Figure 4. Bar graphs indicate percentage fluorescent area in UPS iPDOX growing in RFP transgenic nude mice at 1st, 2nd, and 3rd passage.

Error bars: +1 SD. n.s.: not significant. RFP: red fluorescent protein
Figure 5. Bar graph indicate percentage fluorescent area in UPS iPDOX growing in non-colored nude mice at 1st, 2nd, and 3rd passage.

Error bars: +1 SD. n.s.: not significant. NC: non-colored.
Acknowledgments
This study was supported in part by National Cancer Institute grant CA213649.
References
- Astoul P, Wang X, Colt HG, Boutin C, Hoffman RM. A patient-like human malignant pleural mesothelioma nude-mouse model. Oncol Rep. 1996;3:483–487. [PubMed] [Google Scholar]
- Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically from histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically intact patient specimens. Anticancer Res. 1993;13:283–286. [PubMed] [Google Scholar]
- Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nud emouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. [PubMed] [Google Scholar]
- Furukawa T, Kubota T, Watanabe M, Kitajima M, Fu X, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi: 10.1002/ijc.2910530414. [DOI] [PubMed] [Google Scholar]
- Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, Miwa S, Yano S, Sato S, Murakami T, Momiyama M, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014a;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming BJ, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) J Cell Biochem. 2014b;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- Hiroshima Y, Maawy A, Sato S, Murakami T, Uehara F, Miwa S, Yano S, Momiyama M, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM. Hand-held high-resolution fluorescence imaging system for fluorescence guided surgery of patient and cell-line pancreatic tumors growing orthotopically in nude mice. J Surg Res. 2014c;187:510–517. doi: 10.1016/j.jss.2013.11.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, Tanaka K, Ichikawa Y, Endo I, Hoffman RM, Bouvet M. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mousemodel is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19-9-conjugated fluorophore. PLoS ONE. 2014d;9:e114310. doi: 10.1371/journal.pone.0114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Zhao M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with antiangiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014e;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Maawy AA, Katz MH, Fleming JB, Bouvet M, Endo I, Hoffman RM. Selective efficacy of zoledronic acid on metastasis in a patient derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015a;111:311–315. doi: 10.1002/jso.23816. [DOI] [PubMed] [Google Scholar]
- Hiroshima Y, Maawy A, Zhan Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Endo I, Hoffman RM, Bouvet M. Fluorescence-guided surgery, but not bright-light surgery, prevents local recurrence in a pancreatic cancer patient-derived orthotopic xenograft (PDOX) model resistant to neoadjuvant chemotherapy (NAC) Pancreatology. 2015b;15:295–301. doi: 10.1016/j.pan.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS ONE. 2015c;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshima Y, Zhang Y, Zhang N, Uehara F, Maawy A, Murakami T, Mii S, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, Bouvet M, Endo I, Hoffman RM. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015d;35:697–701. [PubMed] [Google Scholar]
- Hoffman RM. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, Eilber FC, Hoffman RM. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016a;7:71737–71743. doi: 10.18632/oncotarget.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, Eilber FC, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016b;7:85929–85936. doi: 10.18632/oncotarget.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, Li Y, Bouvet M, Kanaya F, Singh A, Dry S, Eilber FC, Hoffman RM. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054. doi: 10.18632/oncotarget.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyuna T, Murakami T, Tome Y, Igarashi K, Kawaguchi K, Russell T, Eckhardt MA, Crompton J, Singh A, Bernthal N, Bukata S, Federman N, Kanaya F, Eilber FC, Hoffman RM. Labeling the stroma of a patient-derived orthotopic xenograft (PDOX) mouse models of undifferentiated pleomorphic soft-tissue sarcoma with red fluorescent protein for rapid non-invasive drug screening. J Cell Biochem. 2017;118:361–365. doi: 10.1002/jcb.25643. [DOI] [PubMed] [Google Scholar]
- Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, Quan C, Hiroshima Y, Matsuyama R, Chishima T, Tanaka K, Bouvet M, Chawla S, Endo I, Hoffman RM. Tumor targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016a;7:12783–12790. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, Hiroshima Y, Russell T, Eckardt MA, Yanagawa J, Federman N, Matsuyama R, Chishima T, Tanaka K, Bouvet M, Endo I, Eilber FC, Hoffman RM. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016b;7:47556–47564. doi: 10.18632/oncotarget.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Moriwaki H, Bouvet M, Saji S, Hoffman RM. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. J Cell Biochem. 2012a;113:2290–2295. doi: 10.1002/jcb.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, Moriwaki H, Bouvet M, Hoffman RM. Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with human patient primary pancreatic cancer specimens. Anticancer Res. 2012b;32:1175–1180. [PubMed] [Google Scholar]
- Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, Moriwaki H, Bouvet M, Hoffman RM. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res. 2012c;32:3063–3067. [PubMed] [Google Scholar]
- Uchugonova A, Duong J, Zhang N, Konig K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]
- Yano S, Hiroshima Y, Maawy A, Kishimoto H, Suetsugu A, Miwa S, Toneri M, Yamamoto M, Katz MHG, Fleming JB, Urata Y, Tazawa H, Kagawa S, Bouvet M, Fujiwara T, Hoffman RM. Color-coding cancer and stromal cells with genetic reporters in a patient-derived orthotopic xenograft (PDOX) model of pancreatic cancer enhances fluorescence-guided surgery. Cancer Gene Ther. 2015;22:344–350. doi: 10.1038/cgt.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]


