Abstract
Preterm birth (PTB) occurs among 1:11 U.S. white women and 1:7.5 African American women and is a significant driver of racial disparities in infant mortality. Maternal stress is the most common clinical phenotype underlying spontaneous PTB. Specific patterns of stress and biological mediators driving PTB remain unclear. We examined the effect of childhood stress on birth timing among African American women and evaluated maternal cortisol elevation as a biological mediator. A prospective observational design was employed, with a single study visit at 28–32 weeks gestation and medical record review. The Stress and Adversity Inventory was administered, which provides a comprehensive estimate of childhood stress, stress in adulthood, and five core characteristic subscales (interpersonal loss, physical danger, humiliation, entrapment, role disruption). Venipuncture was performed between 11:00am and 4:00pm and plasma cortisol quantified by ELISA. Analyses controlled for stress in adulthood. Among a final sample of 89, cumulative childhood stress predicted birth timing (p = .01). The association was driven by stress related to interpersonal loss and physical danger, with support for maternal cortisol as a biological mediator (ab = 0.02, 95% CI [0.001, 0.045]; ab = 0.02, 95% CI [0.001, 0.043], respectively). Results were similar, overall, in sub-group analyses among spontaneously laboring women (n = 53); however, role disruption arose as an additional predictor, as mediated by cortisol elevations (ab = 0.03, 95% CI [0.005, 0.074]). Of note, cortisol was no longer supported as a mediator linking physical danger to birth timing after adjusting for sleep quality and hours awake prior to venipuncture (ab = 0.02, 95% CI [−0.0001, 0.046]). We provide preliminary evidence that, independent of stress in adulthood, childhood stress of specific core characteristics may shape birth timing, with cortisol elevation as a biological mediator. Further investigation is warranted and may bolster the development of biologically-informed screening tools for the prediction and targeted prevention of stress-related PTB.
Keywords: African Americans, Cohort Studies, Cortisol, Pregnancy, Premature Birth, Stress, Psychological
1. Introduction
Preterm birth (PTB) is a unique condition in that its diagnosis is based entirely on the unexpected timing of an expected event (i.e., birth before 37 weeks of pregnancy). The syndrome is quite common, with 1 in 11 United States (U.S.) white women and 1 in 7.5 African American women giving birth preterm (Martin et al., 2017). Racial disparities in birth timing are also the most significant driver of racial disparities in infant mortality (MacDorman and Mathews, 2011). Of African Americans born before 37 weeks, 5% die in the first year of life (Matthews et al., 2015). When birth occurs before 34 weeks, the African American infant mortality rate climbs to 12.3% (Matthews et al., 2015). As such, there is considerable interest in identifying biological aberrations driving the development of PTB, particularly among African Americans.
In order to isolate the factors driving PTB, it has been critical to conceptualize PTB as a syndrome with diverse phenotypes (i.e., underlying characteristics occurring alone or in combination). A recent study examining nine diverse potential PTB phenotypes (e.g., inflammation, decidual hemorrhage) among 1,025 women identified maternal psychosocial stress as the most common phenotype underlying spontaneously-initiated PTB (Manuck et al., 2015). In fact, some form of maternal stress or distress (i.e., stressor exposure, financial strain, perceived stress, symptoms of depression or anxiety, maladaptive coping behaviors) was present during the current pregnancy among 56.6% of women giving birth before 34 weeks (Manuck et al., 2015). Findings highlight the importance of identifying risk for and combatting stress-related PTB.
At present, clinical approaches to stress-related PTB prevention focus entirely on alleviating stress and distress proximal to pregnancy (American College of Obstetricians and Gynecologists, 2010; Moyer and U.S. Preventive Services Task Force, 2013). There is growing evidence that childhood stress also increases risk for health conditions in adulthood (Murphy et al., 2017; Nusslock and Miller, 2016), including complications of pregnancy such as PTB (Blackmore et al., 2016; Christiaens et al., 2015; Hillis et al., 2004; Smith et al., 2016). What remains unclear is whether childhood stress exerts independent effects on the health of the pregnancy or if stress in adulthood, a noted correlate of childhood stress (Kingston et al., 2012), drives stress-related PTB. Cumulative estimates of childhood stress also often include stress associated with particularly traumatic events (e.g., death or imprisonment of a parent, physical or sexual abuse), including in the aforementioned PTB studies. A more complete picture of the effects of various forms of commonly experienced childhood stress (i.e., loss, danger, humiliation, entrapment, role disruption) on birth timing would also be informative.
Mechanisms underlying the effects of stress on birth timing also require further attention. Per the ecobiodevelopmental framework, robust, repeated, or extended activation of stress systems in childhood lead to lasting neurobiological changes, with both chronic activation of stress systems and exaggerated responsivity to adult stressors implicated in adult disease (Shonkoff et al., 2012). In pregnancy, a popular theory proposed to explain stress-related PTB is that of the accelerated “placental clock,” which posits that stress-induced hypothalamic-pituitary-adrenal (HPA) activation hastens the expected pregnancy-associated rise in maternal cortisol, upregulating placental corticotropin-releasing hormone (CRH) production via feed-forward mechanisms (Sandman et al., 2006). This pathway has been of greatest interest in development of spontaneous PTB, as CRH is known to promote labor, including through immunomodulatory effects on myometrial contractility and fetal membrane integrity (e.g., Li and Challis, 2005; You et al., 2014). HPA hyperactivity may also underlie overall risk for PTB, as some studies have identified a potential role in placental pathology (e.g., in preeclampsia; Harville et al., 2008; Petsas et al., 2012), which increases risk for medically-indicated PTB.
As such, childhood stress and stress in adulthood may contribute to PTB via HPA activation. However, there has been considerable variability in methods employed to test this pathway. To our knowledge, only three studies have examined the effect of childhood stress on prenatal HPA activity, with a potential role of lasting biological effects supported (n = 295; Moog et al., 2016) and contested (n = 123; Noll et al., 2007; n = 58; Karakash et al., 2016). Stress in adulthood was not accounted for in these studies. Of studies assessing effects of stress in adulthood on HPA activity and birth timing, formal, prospective testing of biological mediation is rare (Hoffman et al., 2016; Mancuso et al., 2004). As such, it is not surprising that some (Guendelman et al., 2008; Hobel et al., 1999; Hoffman et al., 2016; Mancuso et al., 2004; Moog et al., 2016), but not all (Himes and Simhan, 2011; Karakash et al., 2016; Kramer et al., 2009; Kramer et al., 2013; Noll et al., 2007; Owen et al., 2017), studies provide support for the “placental clock” theory. The two studies formally testing mediation are in favor of the proposed pathway.
Also, as noted, advanced understanding of PTB is of particular interest among African American women, a population at increased risk for PTB (Martin et al., 2017). Of additional importance is the fact that African American women are significantly more likely to show evidence of maternal stress in the context of PTB as well as pregnancy associated maternal cortisol patterns that differ significantly from white women (Christian et al., 2016; Glynn et al., 2007). As such, it is critical that studies assessing biological pathways to expedited birth be powered to examine effects among African American women.
The current study sought to address these critical gaps in knowledge by examining, among African American women, the effect of childhood stress on birth timing, independent of adult stressor exposure, and determining whether childhood stress involving specific core characteristics (i.e., interpersonal loss, physical danger, humiliation, entrapment, or role disruption) were of particular importance. To test the theory of the stress hormone-driven accelerated “placental clock”, we also examined the mediational role of maternal cortisol elevation during pregnancy in the relationship between childhood stress and birth timing. First, we examined the overall effect of childhood stress on birth timing among the full sample. We then focused on women giving spontaneously-initiated birth, a sub-group of primary interest for this work and enriched through enrollment criteria. We hypothesized that, controlling for stress in adulthood, greater childhood stress would be associated with earlier birth as mediated by maternal cortisol elevations. We anticipated that interpersonal loss and physical danger subscales would play an important role based on prior literature and that associations would present in the full cohort and spontaneous labor sub-group.
2. Methods
2.1. Participants
The Pathways to shortened gestation among African American women (PATH) study was conducted among a convenience sample of 96 pregnant women with the primary aims of examining genetic and stress-related biological pathways to early birth via immune dysregulation among African American women (for results regarding the gene-immune pathway, see Gillespie et al., 2017). The potential role of HPA hyperactivity was also examined in secondary analyses. Women were recruited from two hospital-based obstetrics and gynecology clinics as well as the surrounding community of Columbus, Ohio. Eligible participants were African American, non-Hispanic, born and raised in the U.S., and adults (i.e., aged 18–34 at the time of conception for the current pregnancy). Eligible participants were also required to have completed both dating and anatomy ultrasounds lacking diagnosis of fetal anomaly, intrauterine grown restriction, incompetent cervix, oligohydramnios, or polyhydramnios. Women were excluded if they reported height and pre-pregnancy weight that placed them in the underweight (< 18.5) or obese class III (> 40) range according to the standard of body mass index (BMI; kg/m2; World Health Organization, 2000). Additional exclusion criteria included diagnosis of gestational diabetes mellitus, gestational hypertension, or preeclampsia or supplementation with progesterone or cervical cerclage placement prior to enrollment. Report of a chronic condition (e.g., human immunodeficiency virus, diabetes mellitus, disordered sleep) or regular use of a medication (e.g., corticosteroids) with immune or endocrine implications as well as tobacco, alcohol, or illicit drug use after the first trimester were also grounds for exclusion. Enrollment criteria were chosen to maximize the number of participants initiating the birth process through spontaneous labor or rupture, the primary outcome of interest. Seven enrolled participants were excluded from analyses for the following reasons: venipuncture outside of study visit window (n = 1), unsuccessful venipuncture (n = 2), tobacco use after the first trimester (n = 1), loss to follow-up (n = 1), and outlying maternal cortisol value >3 SD above the mean (n = 2). Therefore, hypotheses were tested among a final sample of 89 women.
2.2. Procedure
The PATH study employed a prospective observational design. A single study visit was completed at 28 weeks 0 days – 32 weeks 6 days gestation. In addition to demographic and clinical interviews, stress experiences over the life course were assessed. Antecubital venipuncture was performed. Participants were followed prospectively and birth outcomes determined through a comprehensive post-birth medical record review. The study protocol was approved by the Institutional Review Boards of The Ohio State University (Biomedical; #2013H0022) and OhioHealth (#OH1–13–00478). Informed consent and HIPAA authorizations were obtained from all study participants prior to completion of the study visit.
2.3. Stress assessment
The Stress and Adversity Inventory (STRAIN) was administered by interview to assess exposure to 96 acute and chronic stressors over the life course. Timing, duration, frequency, and severity (on a scale of 1 – 5, with 5 representing the greatest severity) of each endorsed stressor exposure is also measured. Information gained using this method allows for calculation of various cumulative stress scores by adding severity ratings of stressors endorsed, including total stress, acute stress, chronic stress, childhood stress (before age 18) as well as stress in adulthood (> age 18). Given the gaps in the literature presented above, current analyses focused on childhood stress, controlling for stress in adulthood.
Subscale scores can also be calculated across five core characteristics by adding the severity ratings for stressors endorsed within each category. These characteristics include interpersonal loss (e.g., maternal death, parental separation, suicide attempt of loved one, perceived social isolation), physical danger (e.g., physical abuse, sexual abuse, unsafe neighborhood, hospitalization), humiliation (e.g., harsh parental discipline, bullying, workplace exclusion or discrimination, unfaithful partner), entrapment (e.g., parental relationship problems, overcrowded living conditions, job with overwhelming demands, chronic caregiving), and role disruption (e.g., maternal mental illness, unstable living situation, abuse of loved one, infertility).
Development of the STRAIN was guided by well-validated, gold-standard life stress interview systems, including the Life Events and Difficulties Schedule (LEDS; Brown and Harris, 1978). While the LEDS requires extensive staff training, 1–2 hours per subject for administration, and 1–2 hours to produce expert panel contextual ratings, including loss, danger, humiliation, entrapment, and role conflict ratings (Brown et al., 1987; Brown et al., 1995), the STRAIN uses an online system with extensive intelligent logic to produce comprehensive estimates of life stress and automated ratings in 18 – 30 minutes. In prior work, STRAIN indices correlated positively with negative affect, correlated negatively with forgiveness, and were linked to greater fatigue (in agreement with prediction per childhood neglect [Childhood Trauma Questionnaire]), greater metabolic risk (in agreement with prediction per pessimism [Life Orientation Test-Revised]), more mental health symptoms, and more physical health symptoms (Bower et al., 2014; Dooley et al., 2017; Kurtzman et al., 2012; Shields et al., 2017; Toussaint et al., 2016).
2.4. Maternal cortisol measurement
To minimize external, diurnal, and awakening effects on plasma cortisol values while also integrating sample collection into typical patterns of prenatal care, participants were asked to refrain from vigorous exercise or caffeine use prior to the study visit and venipuncture was standardized to occur between the hours of 11:00am and 4:00pm and at least 2.5 hours removed from awakening (Bessinger et al., 2002; Miller et al., 2016; Tsubouchi et al., 2006). Mean time of venipuncture was 1:03pm (SD 1 hour 32 minutes), with women awake for an average 5 hours 45 minutes prior to venipuncture (SD 1 hour 54 minutes).
Following collection, heparinized whole blood was stored on ice until centrifuged at 3000 RPMI for 10 minutes at 15°C. Plasma was aspirated and stored at −80°C. In batches, plasma underwent a single thaw for all participants and total cortisol was quantified in duplicate by solid phase competitive enzyme-linked immunosorbent assay (Calbiotech, Spring Valley, CA) and spectrophotometry (BioTek PowerWave Microplate Spectrophotometer, Winooski, VT) per manufacturer instructions. Briefly, plasma of unknown cortisol concentration and cortisol enzyme conjugate were added to anti-cortisol monoclonal antibody-coated wells and mixed thoroughly. Following a 60 minute incubation at room temperature, each well was washed three times with 1X wash buffer and TMB substrate was added. Following an additional 15 minute incubation at room temperature, stop solution was added and absorbance read at 450 nm within 20 minutes. Intra- and inter-assay coefficients of variation were 3.7% and 9.7%, respectively. The lower limit of detection was 23.3 ng/ml. The distribution of plasma cortisol level for the final sample met normality assumptions by visualization and D’Agostino K2 testing (χ2 = 3.16, p = 0.21). No transformations were required to meet modeling assumptions.
2.5. Determination of birth timing
Birth timing was operationalized as a days gestation at birth continuous variable and calculated according to obstetric estimate of date of delivery and actual date of delivery extracted from the prenatal and labor and delivery records. In order to minimize the potential for error in estimation of birth timing, eligible participants were required to report a dating ultrasound at < 15 weeks gestation, at which time crown-rump length or composite fetal biometric measurements are expected to predict estimated date of delivery within 7 days (American College of Obstetricians and Gynecologists, 2014). A dating ultrasound could be confirmed in 94.4% of the prenatal records secured for enrolled participants, with an average gestational age at dating ultrasound of 10 weeks 0 days (SD 3 weeks 1 day). For descriptive purposes, births were also classified as occurring following spontaneous labor, spontaneous rupture of membranes, induction of labor, or non-laboring cesarean section. This information was extracted from a detailed review of the labor and delivery record.
2.6 Measurement of potential confounders
During the study visit, participants completed demographic and clinical interviews. Height was measured and, in combination with self-reported pre-pregnancy weight, used to calculate pre-pregnancy BMI (World Health Organization, 2000). Sleep quality over the past month was assessed using the Pittsburgh Sleep Quality Index (PSQI), with a range of 0–21 and higher scores indicating poorer sleep quality (Buysse et al., 1989). The PSQI is validated for use among pregnant women of varying socioeconomic statuses (Skouteris et al., 2009). Awakening times, draw times, and gestational ages at draw were also recorded.
2.7 Statistical analyses
Data were examined visually and according to mean/standard deviation or count/frequency. First, unadjusted models were fit by applying the framework and process of Hayes (2013), which addresses three questions by building three consecutive models (here, multivariate linear regression models). Specifically, is there an effect of a predictor on a criterion: 1) without consideration of a potential mediator (i.e., total effect), 2) controlling for a potential mediator (i.e., direct effect), and/or 3) through a potential mediator (i.e., indirect effect)? In the present analyses, the first model (Equation 1) tested the effect of childhood stress (X1) on days gestation at birth (Y), controlling for stress in adulthood (X2). The second model (Equation 2) tested the effect of childhood stress (X1) on maternal cortisol level (M), controlling for stress in adulthood (X2). The third model (Equation 3) tested the effect of maternal cortisol level (M) on days gestation at birth (Y), controlling for both childhood stress (X1) and stress in adulthood (X2).
| (1) |
| (2) |
| (3) |
Here, b0 represents the intercept, c, a, c′, b, and b2 are the coefficients assigned to each predictor in the estimation of each respective outcome, and ei is the error in estimation of each respective outcome. The total effect is represented by c, the direct effect is represented by c′, and the indirect effect is represented by the product of a and b (ab). Therefore, the hypotheses can be considered as follows for testing the total, direct, and indirect effects, respectively: H0: tc = 0, H1: tc ≠ 0; H0: tc′ = 0, H1: tc′ ≠ 0; and H0: tatb = 0, H1: tatb ≠ 0, where t is the true value of the estimate. For the indirect effect, statistical inference involves the construction of 10,000 bootstrap confidence intervals, bias-corrected and with replacement. A confidence interval lacking zero warrants rejection of the null hypothesis of no indirect effect.
To determine whether childhood stress of particular core characteristics plays a particularly strong role in the relationship among childhood stress and birth timing and assess the potential mediational role of maternal cortisol level within the characteristic-specific pathways, we also repeated the Hayes process with interpersonal loss-related, physical danger-related, humiliation-related, entrapment-related, and role disruption-related childhood stress subscale scores serving as predictors. Further, given our particular interest in births that occur following the spontaneous initiation of labor processes, we performed sub-group analyses, repeating the steps described above among women giving birth following spontaneous initiation of labor.
A significance level of α = .05 was used to evaluate all tests. STATA 12.0 (College Station, TX) was used for preliminary analyses and generation of figures. SPSS 22.0 (New York, NY) was used to apply the Hayes process. We performed post-estimation diagnostics to review assumption satisfaction for each model. As expected, days gestation at birth was significantly negatively skewed (χ2 = 12.8, p < 0.01), resulting in non-normality and heteroskedasticity of error terms when birth timing served as the criterion variable. To correct this violation, days gestation at birth was reflected (i.e., -(days gestation at birth) + 288 + 1) and a square root transformation applied, with this transformed variable used in all analyses (termed √(reflected days gestation) in the remainder of the manuscript).
Given our interest in the effects of childhood stress independent of stress in adulthood, cumulative stress in adulthood was included as an a priori covariate in all models. To address potential for confounding, we also performed a series of bivariate analyses using Spearman correlations, t-tests, and Kruskal-Wallis tests, as appropriate, to determine whether demographic (i.e., maternal age, education, employment status), clinical (i.e., parity, pre-pregnancy BMI, sleep quality), or behavioral (i.e., maternal tobacco use in early pregnancy) characteristics were associated with childhood stress, maternal cortisol level, or birth timing. Given diurnal and gestational effects on cortisol levels, hours awake prior to venipuncture and gestational age at venipuncture were also examined. An augmented backward elimination approach, which has been found to reduce bias in variable selection, was used to identify variables for the final adjusted models (Dunkler et al., 2014). Briefly, childhood stress effects were modeled using equations 1–3 above and including all variables in the working set of potential confounders. Each potential confounder with a p value > .15, in descending order, was individually removed from the model and the change in estimate for associations of interest examined. A potential confounder producing a change > 15% was retained whereas a potential confounder producing a change < 15% was removed. This process was repeated until no potential confounders fit defined thresholds. In that three consecutive models are used for mediational analyses, a confounder identified for any of the three models was retained for adjustment in all models.
3. Results
3.1 Descriptive Statistics
Participant characteristics are summarized in Table 1. The sample was primarily of lower socioeconomic status. Of the 89 participants, the majority of women (73%) had not completed a bachelor’s degree and 6 (6.7%) described their family as upper class or upper middle class, 43 (48.3%) as middle class, and 40 (44.9%) as working or lower class. In addition, 28 (31.4%) reported often or sometimes feeling worried that their family’s food would run out before getting money to buy more. As expected per the enrollment criteria, women also generally experienced healthy pregnancies, with only 11 (12.4%) women developing one of the major complications of gestational diabetes mellitus, gestational hypertension, or preeclampsia.
Table 1.
Participant characteristics (N = 89)
| Participant characteristic | Count (%) | Mean + SD |
|---|---|---|
| Maternal age | 26.5 + 4.5 | |
| Maternal education | ||
| < High school or general equivalency degree | 26 (29.2%) | |
| Some college, associate’s/technical degree, or diploma | 39 (43.8%) | |
| > Bachelor’s degree | 24 (27.0%) | |
| Unemployed (yes) | 22 (24.7%) | |
| Pre-pregnancy body mass index | 28.3 + 5.7 | |
| Sleep quality (PSQI global score) | 7.6 + 3.4 | |
| Hours awake prior to venipuncture | 5.8 + 1.9 | |
| Gestational age at venipuncture (weeks gestation) | 30.5 + 1.5 | |
| Maternal tobacco use 6 months before pregnancy (yes) | 22 (24.7%) | |
| Quit before pregnancya | 2 (9.1%) | |
| Quit when pregnancy known, In first trimestera | 18 (81.8%) | |
| Quit after pregnancy known, In first trimestera | 2 (9.1%) | |
| Nulliparous converting to primiparous at birth (yes) | 28 (31.5%) | |
| Prior preterm birth (> 1) | 9 (10.1%) | |
| Gestational diabetes in current pregnancy (yes) | 0 (0%) | |
| Gestational hypertension in current pregnancy (yes) | 9 (10.1%) | |
| Preeclampsia in current pregnancy (yes) | 2 (2.3%) | |
| Oligo/polyhydramnios in current pregnancy (yes) | 8 (9.0%) |
Percentages calculated among tobacco users; PSQI = Pittsburgh Sleep Quality Index
Descriptive statistics for the primary variables are presented in Table 2 and Spearman correlations presented in Table 3. Participants’ experiences of childhood stress and stress in adulthood varied widely, with distributions positively skewed for all stress variables. Notably, 36 (40%) participants gave birth as a result of induction of labor or pre-labor cesarean section. However, the majority (28/36) of these women reached 39 weeks of pregnancy (i.e., full term) prior to induction of labor/pre-labor cesarean section. Cumulative childhood stress and cumulative stress in adulthood (rs = .36, p < .01) as well as stress per adult year (rs = .42, p < .01) showed positive rank order correlations of moderate strength. Some prior work has noted no association between age and maternal cortisol, (e.g., Wikenius et al. 2016) and a positive association between BMI and maternal cortisol (e.g., Berglund et al. 2016). However, similar to work from Finegood et al. (2016) and Stiratt et al. (2016), maternal cortisol level was negatively associated with maternal age (rs = −.26, p = .01) and pre-pregnancy BMI (rs = −.22, p = .04) among our participants. Bivariate associations among standardized stress scores (i.e., z-scores) and maternal cortisol level as well as stress z-scores and birth timing are also depicted in Figures 1 and 2, respectively.
Table 2.
Primary variable descriptive statistics (N = 89)
| Variable | No stress exposure (%) | Count (%) | Median (Range) |
|---|---|---|---|
| Cumulative childhood stress | 9 (10.1%) | −- | 10 (0 – 69) |
| Interpersonal loss subscale | 23 (25.8%) | −- | 3 (0 – 15) |
| Physical danger subscale | 47 (52.8%) | −- | 0 (0 – 20) |
| Humiliation subscale | 41 (46.1%) | −- | 2 (0 – 11) |
| Entrapment subscale | 52 (58.4%) | −- | 0 (0 – 14) |
| Role disruption subscale | 65 (73.0%) | −- | 0 (0 – 17) |
| Cumulative stress in adulthood | 1 (1.1%) | −- | 34 (0 – 127) |
| Stress per adult year | 1 (1.1%) | −- | 4.8 (0 – 29) |
| Maternal cortisol level (ng/ml) | −- | −- | 115.3 (55.1 – 205.6) |
| Birth timing (weeks gestation) | −- | −- | 39.3 (34.1 – 41.1) |
| Late preterm (34weeks 0days – 36weeks 6days) | −- | 7 (7.9%) | −- |
| Early term (37weeks 0days – 38weeks 6days) | −- | 26 (29.2%) | −- |
| Full term (39weeks 0days – 40weeks 6days) | −- | 51 (57.3%) | −- |
| Late term (41weeks 0days – 41weeks 6days) | −- | 5 (5.6%) | −- |
| Presentation to labor and delivery suite | −- | −- | −- |
| In labor | −- | 53 (60.0%) | −- |
| For induction/pre-labor cesarean section after full term | −- | 28 (31.0%) | −- |
| For induction/pre-labor cesarean section before full terma | −- | 8 (9.0%) | −- |
Indications: Hypertensive disorder (n = 5), non-reassuring fetal wellbeing (n = 2), oligohydramnios (n = 1)
Table 3.
Spearman correlations (N = 89)
| 1 | 1a | 1b | 1c | 1d | 1e | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cumulative childhood stress | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 1a. Interpersonal loss subscale | .68** | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 1b. Physical danger subscale | .62** | .21* | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 1c. Humiliation subscale | .70** | .36** | .35** | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 1d. Entrapment subscale | .59** | .28** | .26* | .37** | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 1e. Role disruption subscale | .48** | .36** | .25* | .23* | .12 | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- | −- |
| 2. Cumulative stress in adulthood | .36** | .22* | .18 | .31** | .36** | −.01 | 1.00 | −- | −- | −- | −- | −- | −- | −- | −- |
| 3. Stress per adult year | .42** | .27** | .22* | .36** | .22* | .17 | .69** | 1.00 | −- | −- | −- | −- | −- | −- | −- |
| 4. Maternal age | −.12 | −.05 | −.02 | −.14 | .08 | −.19 | .09 | −.59** | 1.00 | −- | −- | −- | −- | −- | −- |
| 5. Pre-pregnancy body mass index | −.13 | .003 | −.08 | −.13 | −.03 | −.11 | .08 | −.09 | .21* | 1.00 | −- | −- | −- | −- | −- |
| 6. Sleep quality (PSQI global score) | .07 | .11 | −.003 | .04 | .10 | −.10 | .32** | .29** | −.04 | .05 | 1.00 | −- | −- | −- | −- |
| 7. Hours awake prior to venipuncture | −.09 | −.10 | −.09 | −.01 | .07 | −.24* | .23* | .004 | .22* | .11 | .16 | 1.00 | −- | −- | −- |
| 8. Gestational age at venipuncture | −.07 | .01 | .03 | −.20 | −.07 | .11 | −.07 | .09 | −.16 | .08 | .05 | −.03 | 1.00 | −- | −- |
| 9. Maternal cortisol level | .27** | .22* | .22* | .12 | .05 | .37** | −.05 | .16 | −.26** | −.22* | .01 | −.43** | −.002 | 1.00 | −- |
| 10. Birth timing | −.24* | −.23* | −.20 | −.10 | −.11 | −.18 | −.03 | −.08 | .07 | .03 | −.22* | .01 | .10 | −.22* | 1.00 |
p < .05;
p < .01
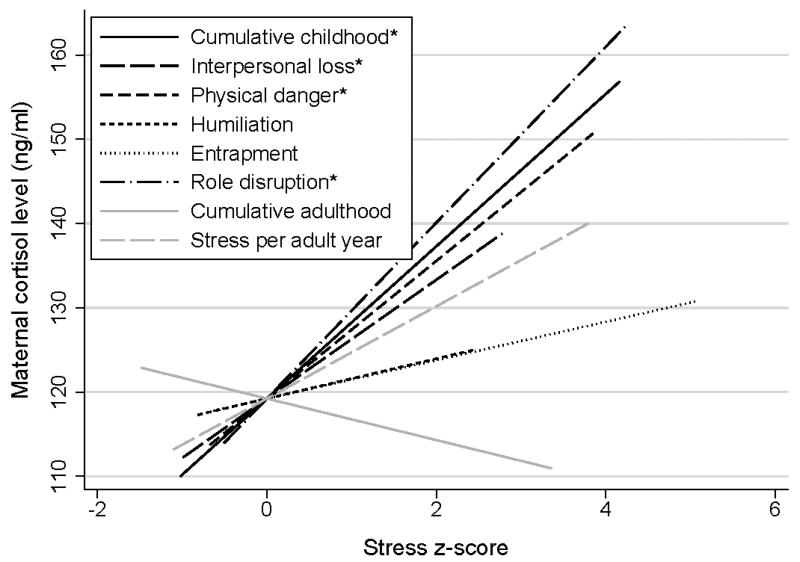
Figure 1.
Associations among stress z-scores and maternal cortisol level. Greater cumulative childhood stress (rs = .27), childhood interpersonal loss-related stress (rs = .22), childhood physical danger-related stress (rs = .22), and childhood role disruption-related stress (rs = .37) were each associated with greater maternal cortisol level per Spearman rank correlation. Note: The interpersonal loss, physical danger, humiliation, entrapment, and role disruption subscale scores are each specific to childhood. *p < .05
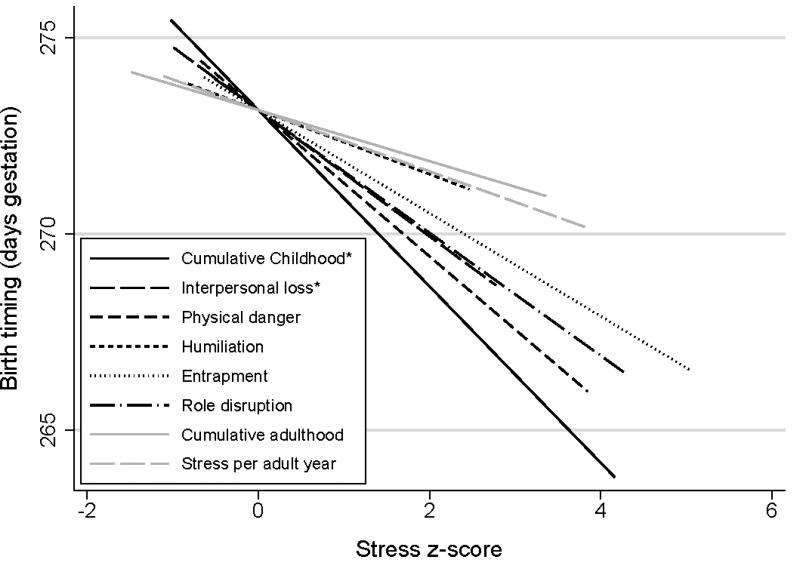
Figure 2.
Associations among stress z-scores and birth timing. Greater cumulative childhood stress (rs = −.24) and childhood interpersonal loss-related stress (rs = −.23) were each associated with earlier birth timing per Spearman rank correlation. Physical danger (rs = −.20, p = .06) and role disruption (rs = −.18, p = .09) showed marginal but non-statistically significant associations. Note: The interpersonal loss, physical danger, humiliation, entrapment, and role disruption subscale scores are each specific to childhood. Birth timing is untransformed. *p < .05
3.2 Cumulative Childhood Stress and Birth Timing
Controlling for stress in adulthood (b2 = −0.002, t(86) = −0.43, p = .67), there was a total effect of childhood stress on birth timing without consideration of maternal cortisol level (c = 0.03, t(86) = 2.50, p = .01). In applying the derived equation, correcting for the birth timing transformation and holding stress in adulthood to average levels, birth would be expected to occur 2.8 days earlier among women with a score one standard deviation above the mean and 5.8 days earlier among women with a score two standard deviations above the mean as compared to women with childhood stress at the mean. Before proceeding with analyses, we aimed to confirm that the effect of childhood stress and lack of effect of stress in adulthood on birth timing was not driven by differential length of time available to encounter stressors in childhood (18 years) versus adulthood (1–17 years, depending on maternal age). Controlling for stress in adulthood per year of possible exposure (b2 = −0.003, t(86) = −0.11, p = .91), a total effect of childhood stress on birth timing remained (c = 0.03, t(86) = 2.41, p = .02).
We also examined direct and indirect effects of childhood stress on birth timing to estimate effects of childhood stress on birth timing controlling for as well as through maternal cortisol, respectively (Table 4). The direct effect of childhood stress on birth timing, controlling for stress in adulthood, approached but did not reach statistical significance (c′ = 0.02, t(85) = 1.86, p = .07). The indirect effect of childhood stress on birth timing through maternal cortisol level also failed to meet criteria for significance (ab = 0.006, 95% CI [−0.001, 0.014]).
Table 4.
Mediation analyses of childhood stress on birth timing through maternal cortisol level (N = 89)
| M: Maternal cortisol level
|
Y: Birth timinga
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Coeff. | (SE) | p | Coeff. | (SE) | p | |||
| Cumulative childhood stress models | ||||||||
|
| ||||||||
| X: Childhood stress (cumulative) | a: | 0.89 | (0.30) | < .01** | c′: | 0.02 | (0.01) | .07 |
| M: Maternal cortisol level | −-- | −-- | −-- | b: | 0.01 | (0.004) | .08 | |
| Covariate: Stress in adulthood | b2: | −0.27 | (0.15) | .07 | b2: | −0.0005 | (0.005) | .93 |
| Constant | b0: | 117.71 | (6.73) | < .01** | b0: | 2.71 | (0.51) | < .01** |
|
| ||||||||
| Total effect: Effect = 0.03, Boot SE = 0.01, p = .01**; Indirect effect through cortisol: Effect = 0.006, Boot SE = 0.004, 95%CI −0.001, 0.01 | ||||||||
|
| ||||||||
| Childhood interpersonal loss-related stress subscale models | ||||||||
|
| ||||||||
| X: Childhood interpersonal loss-related stress | a: | 2.03 | (0.96) | .04* | c′: | 0.05 | (0.03) | .12 |
| M: Maternal cortisol level | −-- | −-- | −-- | b: | 0.01 | (0.004) | .05* | |
| Covariate: Stress in adulthood | b2: | −0.17 | (0.15) | .26 | b2: | 0.002 | (0.005) | .72 |
| Constant | b0: | 117.74 | (7.10) | < .01** | b0: | 2.60 | (0.50) | < .01** |
|
| ||||||||
| Total effect: Effect = 0.07, Boot SE = 0.03, p = .05*; Indirect effect through cortisol: Effect = 0.02, Boot SE = 0.01, 95%CI 0.001, 0.05* | ||||||||
|
| ||||||||
| Childhood physical danger-related stress subscale models | ||||||||
|
| ||||||||
| X: Childhood physical danger-related stress | a: | 2.18 | (0.87) | .01** | c′: | 0.04 | (0.03) | .18 |
| M: Maternal cortisol level | −-- | −-- | −-- | b: | 0.01 | (0.004) | .05* | |
| Covariate: Stress in adulthood | b2: | −0.20 | (0.15) | .18 | b2: | 0.002 | (0.005) | .74 |
| Constant | b0: | 120.43 | (6.67) | < .01** | b0: | 2.69 | (0.51) | < .01** |
| Total effect: Effect = 0.06, Boot SE = 0.03, p = .06*; Indirect effect through cortisol: Effect = 0.02, Boot SE = 0.01, 95%CI 0.001, 0.04* | ||||||||
Note. Coeff. = coefficient; SE = standard error; X = predictor; M = mediator; Y = criterion.
√(reflected days gestation).
p < .05;
p < .01
3.3 Childhood Stress Core Characteristic Subscales and Birth Timing
In examining the childhood stress subscales separately, a total effect for interpersonal loss-related childhood stress on birth timing was revealed, again controlling for stress in adulthood and without consideration of maternal cortisol level (c = 0.07, t(86) = 2.02, p = .05). In examining the direct and indirect effects (Table 4), there was no direct effect of interpersonal loss-related childhood stress on birth timing controlling for stress in adulthood and maternal cortisol level (c′ = 0.05, t(85) = 1.56, p = .12). However, there was an indirect effect of interpersonal loss-related childhood stress on birth timing through maternal cortisol level, which provides support for maternal cortisol as a biological mediator (ab = 0.02, 95% CI [0.001, 0.045]). The total effect of physical danger-related childhood stress on birth timing approached significance, controlling for stress in adulthood and without consideration of maternal cortisol level (c = 0.06, t(86) = 1.86, p = .06). Evaluation of the direct and indirect effects (Table 4) revealed no direct effect of physical danger-related childhood stress on birth timing controlling for maternal cortisol level (c’ = 0.04, t(85) = 1.35, p = .18) but a significant indirect effect of physical danger-related childhood stress on birth timing through the maternal cortisol mediational pathway (ab = 0.02, 95% CI [0.001, 0.043]). In examining the additional childhood stress subscales (i.e., humiliation, entrapment, and role disruption) and controlling for stress in adulthood, there was no evidence of a total effect, direct effect, or indirect effect on birth timing (p values > .13).
3.4 Sub-group analyses among women giving birth following spontaneous labor onset
Fifty-three (60.0%) participants gave birth following the spontaneous onset of labor processes. We repeated the Hayes process for assessing mediation among this sub-group, again controlling for cumulative stress in adulthood in all analyses. Among these women, there was a total effect of cumulative childhood stress (c = 0.03, t(50) = 2.11, p = .04) but not cumulative stress in adulthood (c = −0.004, t(50) = −0.48, p = .63) on birth timing. According to the derived equation, spontaneous birth would be expected to occur 3.5 days earlier among women with a score one standard deviation above the mean and 7.5 days earlier among women with a score two standard deviations above the mean as compared to women with a childhood stress score at the mean. There was no direct effect of cumulative childhood stress on birth timing controlling for maternal cortisol level (c′ = 0.02, t(49) = 1.38, p = .17) but a significant indirect effect of cumulative childhood stress on birth timing through maternal cortisol level (ab = 0.01, 95% CI [0.002, 0.021]), which was not appreciable in the full cohort.
Similar to the full cohort, a total effect (here, marginal) for interpersonal loss-related childhood stress on birth timing (c = 0.08, t(50) = 1.90, p = .06) and lack of a direct effect of interpersonal loss-related childhood stress on birth timing (c′ = 0.06, t(49) = 1.50, p = .14) was noted. However, maternal cortisol was no longer statistically supported as a biological mediator (ab = 0.02, 95% CI [−0.0002, 0.061]). As before, there was no statistically significant total effect (c = 0.06, t(50) = 1.48, p = .14) or direct effect (c = 0.03, t(49) = 0.64, p = .52) of physical danger-related childhood stress on birth timing but a significant indirect effect through the maternal cortisol mediational pathway (ab = 0.03, 95% CI [0.007, 0.075]). Also congruent with the full cohort, there was no evidence of a total effect, direct effect, or indirect effect of humiliation- or entrapment-related childhood stress on birth timing (p values > .48). However, not present in primary analyses but emerging in sub-group analyses was a marginal total effect (c′ = 0.08, t(50) = 1.88, p = .07) and significant indirect effect (ab = 0.03, 95% CI [0.005, 0.074]) of role disruption-related childhood stress on birth timing.
3.4 Sensitivity analyses
Through bivariate analyses, maternal age, pre-pregnancy BMI, and sleep quality were identified as potential confounders and assessed alongside hours awake prior to venipuncture and gestational age at venipuncture for inclusion in the final adjusted model. Through the process of augmented backward elimination, with a confounder defined as a variable producing a p value < .15 or a change in estimate > 15%, maternal age (p values > .33; change in estimates < 3.8%), pre-pregnancy BMI (p values > .17; change in estimates < 8.6%), and gestational age at venipuncture (p values > .19; change in estimates < 4.1%) did not show evidence of confounding for any model and were removed. Therefore, the final adjusted model controlled for sleep quality and hours awake prior to venipuncture.
Differences in results among the full cohort are as follows: the direct effect of cumulative childhood stress on birth timing progressed from statistically marginal (c′ = 0.02, t(85) = 1.86, p = .07) to statistically significant (c′ = 0.02, t(83) = 2.11, p = .04); the total effect of physical danger-related childhood stress on birth timing progressed from statistically marginal (c′ = 0.06, t(86) = 1.90, p = .06) to statistically significant (c′ = 0.06, t(84) = 1.96, p = .05); the indirect effect of physical danger-related childhood stress on birth timing fell out of statistical significance (from ab = 0.02, 95% CI [0.001, 0.043] to ab = 0.02, 95% CI [−0.0001, 0.046]). Among the spontaneous labor subgroup, all findings held with the exception of a progression of the total effect of role disruption-related childhood stress on birth timing from statistically marginal (c′ = 0.08, t(50) = 1.89, p = .07) to statistically significant (c′ = 0.10, t(48) = 2.31, p = .03).
4. Discussion
In the present study, we administered the Stress and Adversity Inventory (STRAIN), a computer assisted stress assessment system, to quantify participant exposures to 96 acute and chronic stressors across the life course. As such, we were able to provide data linking a comprehensive estimate of dose and severity of childhood stress to birth timing in adulthood. Findings lend support to a large literature linking various forms of stress and distress to birth timing (e.g., Manuck et al., 2015) and add to a small literature implicating childhood stress in particular (Christiaens et al., 2015; Smith et al., 2016). We also isolated the effects of childhood stress on adult pregnancy independent of dose and severity of stress in adulthood. Given that childhood stress and stress in adulthood exhibit covariation (Kingston et al., 2012), which we also witnessed in our sample, this is a critical step toward understanding the independent effects of each in the development of stress-related PTB.
We also examined five childhood stress core characteristic subscales (i.e., interpersonal loss, physical danger, humiliation, entrapment, and role disruption). Childhood stress is commonly assessed by producing a cumulative score of exposures to a limited set of experiences generally considered traumatic (Kalmakis and Chandler, 2015). For example, the widely used Adverse Childhood Experiences Study Questionnaire is composed of 17 items assessing personal exposure to or close contact with psychological, physical, sexual, or substance abuse, mental illness, and criminal behavior, with no exposure to any of the assessed traumatic experiences reported among 47.9% of respondents (Felitti et al., 1998). Our findings suggest that a more nuanced depiction of various childhood stress exposures may be informative. Only 10.1% of our participants reported no childhood stress exposure. Childhood interpersonal loss, including death of a loved one, parental separation, and social isolation, was particularly common (lack of exposure among 25.8%) and, in our sample, had important implications for maternal HPA activity and birth timing. Physical danger-, humiliation-, entrapment-, and role disruption-related childhood stress were less commonly experienced (lack of exposure among 52.8%, 46.1%, 58.4%, and 73.0%, respectively). We were able to detect birth timing effects of physical danger-related stress (e.g., physical and sexual abuse, perceptions of neighborhood danger) and role disruption-related stress (e.g., housing instability, parental mental illness); however, it must be considered that minimal variability may have prevented detection of effects for humiliation- and entrapment-related stress. These findings do lend support to previous work linking, for example, childhood parental death and childhood forced sexual activity to PTB (Selk et al., 2016; Vagero and Rajaleid, 2017) and, to our knowledge, provides new evidence implicating role disruption in expedited spontaneous birth. Overall, these data provide preliminary support of differential health impact of childhood stress dependent upon stressor core characteristic.
Our findings also suggest that childhood stress may promote long-term activation of the HPA axis, including during pregnancy, which is consistent with the finding of an association between childhood trauma and peripheral CRH (Moog et al., 2016). However, childhood stress has also been linked with differential stress responsivity (e.g., Kuras et al., 2017) during stressful exposures in adulthood. While we controlled for stress in adulthood, it is possible that pregnancy itself serves as a “stressor.” This is an interesting concept that may be best addressed through inclusion of non-pregnant comparison groups in future studies. We also provide preliminary support for the accelerated “placental clock” theory, which is consistent with some (Guendelman et al., 2008; Hobel et al., 1999; Hoffman et al., 2016; Mancuso et al., 2004; Moog et al., 2016), but not all (Himes and Simhan, 2011; Karakash et al., 2016; Kramer et al., 2009; Kramer et al., 2013; Noll et al., 2007; Owen et al., 2017), prior studies. It may be that inconsistencies are largely related to the considerable variability in the timing, duration, and nature of the stressors assessed. Specifically, in adulthood, as time passes following the first emergence of a stressor, HPA activity tends to rebound to subnormal levels, particularly if the stressor is no longer present (Miller et al., 2007). However, a growing body of literature supports that robust, repeated, or extended stress exposure during the sensitive period of childhood has enduring neurobiological consequences, including the potential for chronic HPA activation (Shonkoff et al., 2012). We are among the first to simultaneously model the effects of childhood stress and stress in adulthood on birth timing through the HPA pathway, highlighting the need for replication, ideally through longitudinal work, to determine whether childhood stress is uniquely capable of promoting early birth via this pathway.
Results must also be interpreted in consideration of several important strengths and limitations. First, the STRAIN offers significant advantages over alternative stress measures in terms of the breadth of information gained. However, in comparison to systems such as the LEDS, the instrument is relatively new and estimates of childhood stress and stress in adulthood are dependent upon participant recall. Evidence does suggest that autobiographical memory tends to be quite accurate even following a significant time-lapse, with no difference among traumatized and non-traumatized individuals (Wittekind et al., 2017). Stress assessment was also standardized to occur during healthy pregnancy to minimize the systematic effect of pregnancy outcome on recall. However, the potential for recall bias cannot be fully excluded. Further, the enrolled sample was primarily of low socioeconomic status, with a number of women reporting significant exposure to stress across the life course. This may have increased our ability to detect effects of childhood stress in a relatively small sample but also impacts generalizability.
In addition, the current study aimed to provide preliminary data regarding the potential of maternal HPA hyperactivity to promote early birth, with plasma cortisol level our chosen marker. However, HPA output can be measured in numerous ways (e.g., CRH, ACTH, plasma cortisol, salivary cortisol, hair cortisol). Markers tend to correlate well during pregnancy (e.g., Glynn et al., 2007; O’Keane et al., 2011), with each linked to early birth in prior work (i.e., serum and plasma CRH (Guendelman et al., 2008; Hobel et al., 1999; Mancuso et al., 2004; Moog et al., 2016) plasma ACTH (Hobel et al., 1999), plasma cortisol (Hobel et al., 1999), diurnal salivary cortisol pattern (Patacchioli et al., 2013), hair cortisol (Hoffman et al., 2016)). Given our interest in the peripheral actions of cortisol, use of plasma as opposed to saliva may also be considered a strength given the notable individual variability in passive salivary diffusion and rate of conversion of active cortisol to inactive cortisone by type II 11β-hydroxysteroid dehydrogenase (Perogamvros et al., 2010). However, it would be informative to assess the full axis in future work. Also, our data relies on cortisol assessed at a single time point. While cortisol rises progressively across pregnancy, the most pronounced rise occurs during the early 2nd trimester, with a relative stabilization across late 2nd and early 3rd trimesters (D’Anna-Hernandez et al., 2011; Smy et al., 2016). With a 28–32 week sampling window, we witnessed a negligible effect of gestational age on plasma cortisol, which may have increased our ability to link individual variation at a single time point to birth timing. In longitudinal studies, various HPA markers in the 2nd and 3rd trimesters provide some predictive power for birth timing (e.g., Hobel et al., 1999; Hoffman et al., 2016). Additional work is required to determine which HPA markers and at which time points, including potentially the rate of rise, are most useful in risk prediction.
The presented work was also interested in furthering understanding of the effects of stress on birth timing among U.S.-born African American women, a considerable contribution to a literature generally lacking racial stratification. We provide data on generalized risk among the full sample (n = 89), as it is biologically plausible that stress-induced endocrine dysregulation may expedite spontaneous birth as well as medically-indicated birth. We also provide data among the spontaneously-initiated birth sub-group (n = 53), the primary population of interest. However, medically-initiated birth occurred in few women (n = 36) and for varied reasons (e.g., hypertensive disorders, non-reassuring fetal wellbeing, oligohydramnios), prohibiting examination of these individual pathways. As such, our enrollment criteria, which targeted seemingly healthy pregnancy, did increase our ability to observe spontaneously-initiated birth, as the rate of induction of labor/pre-labor cesarean was 40% in our sample compared to an overall U.S. rate of greater than 50% (Laughon et al., 2012; Zhang et al., 2010). However, it remains to be determined whether the associations we witnessed in the full sample were driven entirely by spontaneous labor pathways or were also affected by expedited medically-indicated birth. Results, particularly those regarding biological mediation, must be interpreted accordingly.
Finally, multiple comparisons were made among a relatively small sample. The null hypothesis was rejected more frequently than expected by chance; however, the potential for Type I error is noted, highlighting the preliminary nature of this work. The sample size may have resulted in null findings when small effects were indeed present and also prohibited examination of differential effects of childhood stress according to age, which would be informative for future work. Relatedly, in reanalyzing models with sleep quality and hours awake prior to venipuncture included as covariates, the indirect effect of physical danger-related childhood stress on birth timing through maternal cortisol level fell slightly out of significance. It is difficult to determine whether this change is indicative of confounding or diminished power. Replication in a larger sample would, of course, help to address this question. The potential role of poor sleep quality, in particular, in the development of stress-related PTB would also be an interesting avenue of further inquiry. In our sample, poor sleep quality was not associated with greater childhood stress but was associated with greater stress in adulthood as well as birth timing.
Taken together, the current study and a building literature suggest that childhood stress, particularly related to interpersonal loss, physical danger, and role disruption, may be capable of leaving a lasting imprint on stress response systems, including the HPA axis. Further, effects may occur independent of stress in adulthood and, during pregnancy, HPA hyperactivity may be a notable biological mediator in the development of childhood stress-related PTB. This holds significant clinical implications. First, while maternal stress assessment is considered an important component of prenatal care, recommendations for screening cover only intimate partner violence and maternal depression (American College of Obstetricians and Gynecologists, 2010; Moyer and U.S. Preventive Services Task Force, 2013). While important, this approach may only be scratching the surface in terms of identifying women at risk for stress-related PTB. Further, among women screening positive using these limited methods, intervention focuses only on alleviating the current situation or symptoms, with no attention paid to correcting the biological systems that may be driving the development of stress-related PTB. The development of screening tools that account for the critical nuances in stress-related PTB risk prediction and identify the biological mediators that must be targeted in the prevention of stress-related PTB would help to usher in a new era of precision prenatal care, which is critically needed.
Supplementary Material
Highlights.
Childhood stress shapes birth timing, independent of stress in adulthood.
Childhood interpersonal loss, physical danger, and role disruption play important roles.
Maternal cortisol elevation appears to be a key biological mediator.
Acknowledgments
We appreciate the assistance of Dr. George Slavich and Grant Shields to our work with the STRAIN and the contributions of our Undergraduate Research Assistants, Amy Kole and Patricia Do, to data collection. We appreciated the technical assistance of Brent Sullenbarger to laboratory procedures. We would like to thank our study participants and the leadership and staff of The Ohio State University Wexner Medical Center Obstetrics & Gynecology Clinic and OhioHealth Riverside OB/GYN Community Care.
Funding
This work was supported by the National Institute of Nursing Research (grant number F31NR01460) and the National Center for Advancing Translational Sciences mechanism (grant number UL1TR001070) of the National Institutes of Health; Association of Women’s Health, Obstetric and Neonatal Nurses; Midwest Nursing Research Society; Sigma Theta Tau International, Epsilon Chapter; Cola-Cola Critical Difference for Women and the Department of Women’s, Gender and Sexuality Studies, Graduate School, and Office of Diversity and Inclusion of The Ohio State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Gillespie, Christian, and Salsberry contributed to the design of the described study. Drs. Gillespie and Alston contributed to the acquisition of data. Dr. Gillespie performed data analyses and led formulation of research questions, interpretation of results, and drafting of the article, with input and critical revision from each co-author. All authors approved the final version of the submitted article.
Conflicts of Interest: none.
Preliminary results, in part, were presented at the Council for the Advancement of Nursing Science 2016 State of the Science Congress.
References
- American College of Obstetricians and Gynecologists. Committee opinion no 611: Method for estimating due date. Obstet Gynecol. 2014;124(4):863–6. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Committee opinion no. 453: Screening for depression during and after pregnancy. Obstet Gynecol. 2010;115(2 Pt 1):394–5. doi: 10.1097/AOG.0b013e3181d035aa. [DOI] [PubMed] [Google Scholar]
- Berglund SK, Garcia-Valdes L, Torres-Espinola FJ, Segura MT, Martinez-Zaldivar C, Aguilar MJ, Agil A, Lorente JA, Florido J, Padilla C, et al. Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: An observational cohort study (PREOBE) BMC Public Health. 2016;16:207–218. doi: 10.1186/s12889-016-2809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessinger RC, McMurray RG, Hackney AC. Substrate utilization and hormonal responses to moderate intensity exercise during pregnancy and after delivery. Am J Obstet Gynecol. 2002;186(4):757–64. doi: 10.1067/mob.2002.122093. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Putnam FW, Pressman EK, Rubinow DR, Putnam KT, Matthieu MM, Gilchrist MA, Jones I, O’Connor TG. The effects of trauma history and prenatal affective symptoms on obstetric outcomes. J Trauma Stress. 2016;29(3):245–52. doi: 10.1002/jts.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress: Risk factors for cancer-related fatigue. Clin Psychol Sci. 2014;2(1):108–115. doi: 10.1177/2167702613496243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. Free Press; New York, NY: 1978. [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: A patient and non-patient comparison. Psychol Med. 1995;25(1):7–21. doi: 10.1017/s003329170002804x. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: Some refinements. Br J Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Christiaens I, Hegadoren K, Olson DM. Adverse childhood experiences are associated with spontaneous preterm birth: A case-control study. BMC Med. 2015;13:124. doi: 10.1186/s12916-015-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Mitchell AM, Gillespie SL, Palettas M. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology. 2016;74:69–76. doi: 10.1016/j.psyneuen.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiol Behav. 2011;104(2):348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley LN, Slavich GM, Moreno PI, Bower JE. Strength through adversity: Moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors. Stress Health. Advance online publication. 2017 doi: 10.1002/smi.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkler D, Plischke M, Leffondre K, Heinze G. Augmented backward elimination: A pragmatic and purposeful way to develop statistical models. PLoS One. 2014;9(11):e113677. doi: 10.1371/journal.pone.0113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14(4):245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Finegood ED, Blair C, Granger DA, Hibel LC, Mills-Koonce R Family Life Project Key Investigators. Psychobiological influences on maternal sensitivity in the context of adversity. Dev Psychol. 2016;52(7):1073–87. doi: 10.1037/dev0000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Neal JL, Christian LM, Szalacha LA, McCarthy DO, Salsberry PJ. Interleukin-1 receptor antagonist polymorphism and birth timing: Pathway analysis among african american women. Nurs Res. 2017;66(2):95–104. doi: 10.1097/NNR.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28(6):1155–61. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Kosa JL, Pearl M, Graham S, Kharrazi M. Exploring the relationship of second-trimester corticotropin releasing hormone, chronic stress and preterm delivery. J Matern Fetal Neonatal Med. 2008;21(11):788–95. doi: 10.1080/14767050802379031. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, Light KC. Stress and placental resistance measured by doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet Gynecol. 2008;32(1):23–30. doi: 10.1002/uog.5344. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. The Guildford Press; New York, NY: 2013. [Google Scholar]
- Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics. 2004;113(2):320–7. doi: 10.1542/peds.113.2.320. [DOI] [PubMed] [Google Scholar]
- Himes KP, Simhan HN. Plasma corticotropin-releasing hormone and cortisol concentrations and perceived stress among pregnant women with preterm and term birth. Am J Perinatol. 2011;28(6):443–8. doi: 10.1055/s-0030-1270119. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG. Measures of maternal stress and mood in relation to preterm birth. Obstet Gynecol. 2016;127(3):545–52. doi: 10.1097/AOG.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: A systematic review. J Am Assoc Nurse Pract. 2015;27(8):457–65. doi: 10.1002/2327-6924.12215. [DOI] [PubMed] [Google Scholar]
- Karakash SD, Tschankoshvili N, Weedon J, Schwartz RM, Kirschbaum C, Minkoff H. Hypocortisolism and preterm birth. J Neonatal Perinatal Med. 2016;9(4):333–9. doi: 10.3233/NPM-161640. [DOI] [PubMed] [Google Scholar]
- Kingston D, Sword W, Krueger P, Hanna S, Markle-Reid M. Life course pathways to prenatal maternal stress. J Obstet Gynecol Neonatal Nurs. 2012;41(5):609–26. doi: 10.1111/j.1552-6909.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Goulet L, Kahn S, Dahhou M, Platt RW, Sharma S, Meaney MJ, Seguin L. Maternal stress/distress, hormonal pathways and spontaneous preterm birth. Paediatr Perinat Epidemiol. 2013;27(3):237–46. doi: 10.1111/ppe.12042. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, et al. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169(11):1319–26. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Kuras YI, McInnis CM, Thoma MV, Chen X, Hanlin L, Gianferante D, Rohleder N. Increased alpha-amylase response to an acute psychosocial stress challenge in healthy adults with childhood adversity. Dev Psychobiol. 2017;59(1):91–8. doi: 10.1002/dev.21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman L, O’Donovan A, Koslov K, Arendander J, Epel ES, Slavich GM. Sweating the big stuff: Dispositional pessimism exacerbates the deleterious effects of life stress on metabolic health. European J. of Psychotraumatology. 2012;3:19557. [Google Scholar]
- Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206(6):486. doi: 10.1016/j.ajog.2012.03.014. e1,486.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Challis JR. Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J Clin Endocrinol Metab. 2005;90(12):6569–74. doi: 10.1210/jc.2005-1445. [DOI] [PubMed] [Google Scholar]
- MacDorman MF, Mathews TJ. Understanding racial and ethnic disparities in U.S. infant mortality rates. NCHS Data Brief. 2011;74:1–8. [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004;66(5):762–9. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, Huang H, Varner MW, Andrews W, Saade G, et al. The phenotype of spontaneous preterm birth: Application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(4) doi: 10.1016/j.ajog.2015.02.010. 487.e1–487.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. [PubMed] [Google Scholar]
- Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. 2015;64(9):1–30. [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller R, Stalder T, Jarczok M, Almeida DM, Badrick E, Bartels M, Boomsma DI, Coe CL, Dekker MC, Donzella B, et al. The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology. 2016;73:16–23. doi: 10.1016/j.psyneuen.2016.07.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biol Psychiatry. 2016;79(10):831–9. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer VA and U.S. Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;158(6):478–86. doi: 10.7326/0003-4819-158-6-201303190-00588. [DOI] [PubMed] [Google Scholar]
- Murphy MO, Cohn DM, Loria AS. Developmental origins of cardiovascular disease: Impact of early life stress in humans and rodents. Neurosci Biobehav Rev. 2017;74(Pt B):453–65. doi: 10.1016/j.neubiorev.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll JG, Schulkin J, Trickett PK, Susman EJ, Breech L, Putnam FW. Differential pathways to preterm delivery for sexually abused and comparison women. J Pediatr Psychol. 2007;32(10):1238–48. doi: 10.1093/jpepsy/jsm046. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: A pilot study. J Affect Disord. 2011;130(1–2):300–5. doi: 10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Wood L, Tomenson B, Creed F, Neilson JP. Social stress predicts preterm birth in twin pregnancies. J Psychosom Obstet Gynaecol. 2017;38(1):63–72. doi: 10.1080/0167482X.2016.1235146. [DOI] [PubMed] [Google Scholar]
- Patacchioli FR, Perrone G, Merlino L, Simeoni S, Bevilacqua E, Capri O, Galoppi P, Brunelli R. Dysregulation of diurnal salivary cortisol production is associated with spontaneous preterm delivery: A pilot study. Gynecol Obstet Invest. 2013;76(1):69–73. doi: 10.1159/000351873. [DOI] [PubMed] [Google Scholar]
- Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95(11):4951–8. doi: 10.1210/jc.2010-1215. [DOI] [PubMed] [Google Scholar]
- Petsas G, Jeschke U, Richter DU, Minas V, Hammer A, Kalantaridou S, Toth B, Tsatsanis C, Friese K, Makrigiannakis A. Aberrant expression of corticotropin-releasing hormone in pre-eclampsia induces expression of FasL in maternal macrophages and extravillous trophoblast apoptosis. Mol Hum Reprod. 2012;18(11):535–45. doi: 10.1093/molehr/gas027. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27(6):1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Selk SC, Rich-Edwards JW, Koenen K, Kubzansky LD. An observational study of type, timing, and severity of childhood maltreatment and preterm birth. J Epidemiol Community Health. 2016;70(6):589–95. doi: 10.1136/jech-2015-206304. [DOI] [PubMed] [Google Scholar]
- Shields GS, Moons WG, Slavich GM. Better executive function under stress mitigates the effects of recent life stress exposure on health in young adults. Stress. 2017;20(1):75–85. doi: 10.1080/10253890.2017.1286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–46. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: A study across two time points examining the pittsburgh sleep quality index and associations with depressive symptoms. Womens Health Issues. 2009;19(1):45–51. doi: 10.1016/j.whi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20(4):790–8. doi: 10.1007/s10995-015-1909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smy L, Shaw K, Amstutz U, Smith A, Berger H, Carleton B, Koren G. Hair cortisol as a hypothalamic-pituitary-adrenal axis biomarker in pregnant women with asthma: A retrospective observational study. BMC Pregnancy Childbirth. 2016;16(1):176. doi: 10.1186/s12884-016-0962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirrat LI, O’Reilly JR, Barr SM, Andrew R, Riley SC, Howie AF, Bowman M, Smith R, Lewis JG, Denison FC, et al. Decreased maternal hypothalamic-pituitary-adrenal axis activity in very severely obese pregnancy: Associations with birthweight and gestation at delivery. Psychoneuroendocrinology. 2016;63:135–43. doi: 10.1016/j.psyneuen.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, Slavich GM. Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. J Health Psychol. 2016;21(6):1004–14. doi: 10.1177/1359105314544132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, Shimoya K, Hayashi S, Toda M, Morimoto K, Murata Y. Effect of coffee intake on blood flow and maternal stress during the third trimester of pregnancy. Int J Gynaecol Obstet. 2006;92(1):19–22. doi: 10.1016/j.ijgo.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Vagero D, Rajaleid K. Does childhood trauma influence offspring’s birth characteristics? Int J Epidemiol. 2017;46(1):219–229. doi: 10.1093/ije/dyw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenius E, Moe V, Kjellevold M, Smith L, Lyle R, Waagbo R, Page CM, Myhre AM. The association between hair cortisol and self-reported symptoms of depression in pregnant women. PLoS One. 2016;11(9):e0161804. doi: 10.1371/journal.pone.0161804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind CE, Jelinek L, Kleim B, Muhtz C, Moritz S, Berna F. Age effect on autobiographical memory specificity: A study on autobiographical memory specificity in elderly survivors of childhood trauma. J Behav Ther Exp Psychiatry. 2017;54:247–53. doi: 10.1016/j.jbtep.2016.09.002. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: Preventing and managing the global epidemic. report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- You X, Liu J, Xu C, Liu W, Zhu X, Li Y, Sun Q, Gu H, Ni X. Corticotropin-releasing hormone (CRH) promotes inflammation in human pregnant myometrium: The evidence of CRH initiating parturition? J Clin Endocrinol Metab. 2014;99(2):e199–208. doi: 10.1210/jc.2013-3366. [DOI] [PubMed] [Google Scholar]
- Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, Landy HJ, Hibbard JU, Haberman S, Ramirez MM, et al. Contemporary cesarean delivery practice in the united states. Am J Obstet Gynecol. 2010;203326(4) doi: 10.1016/j.ajog.2010.06.058. e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.